The Phenolic Composition of Hops (Humulus lupulus L.) Was Highly Influenced by Cultivar and Year and Little by Soil Liming or Foliar Spray Rich in Nutrients or Algae
Abstract
:1. Introduction
2. Materials and Methods
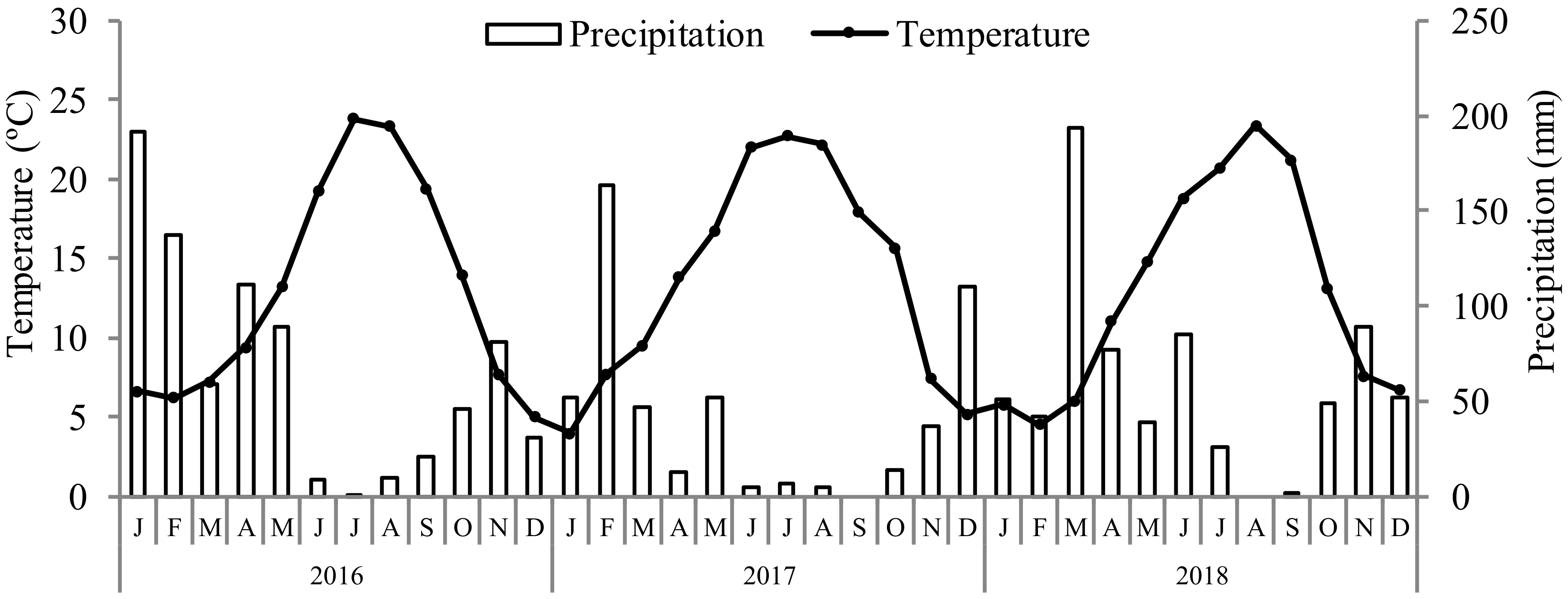
2.1. Experimental Conditions
2.2. Experimental Designs
2.3. Plant Sampling and Tissue Analysis for Elemental Composition
2.4. Analysis of Total Phenolics
2.5. HPLC Analysis
2.6. Statistical Analysis
3. Results
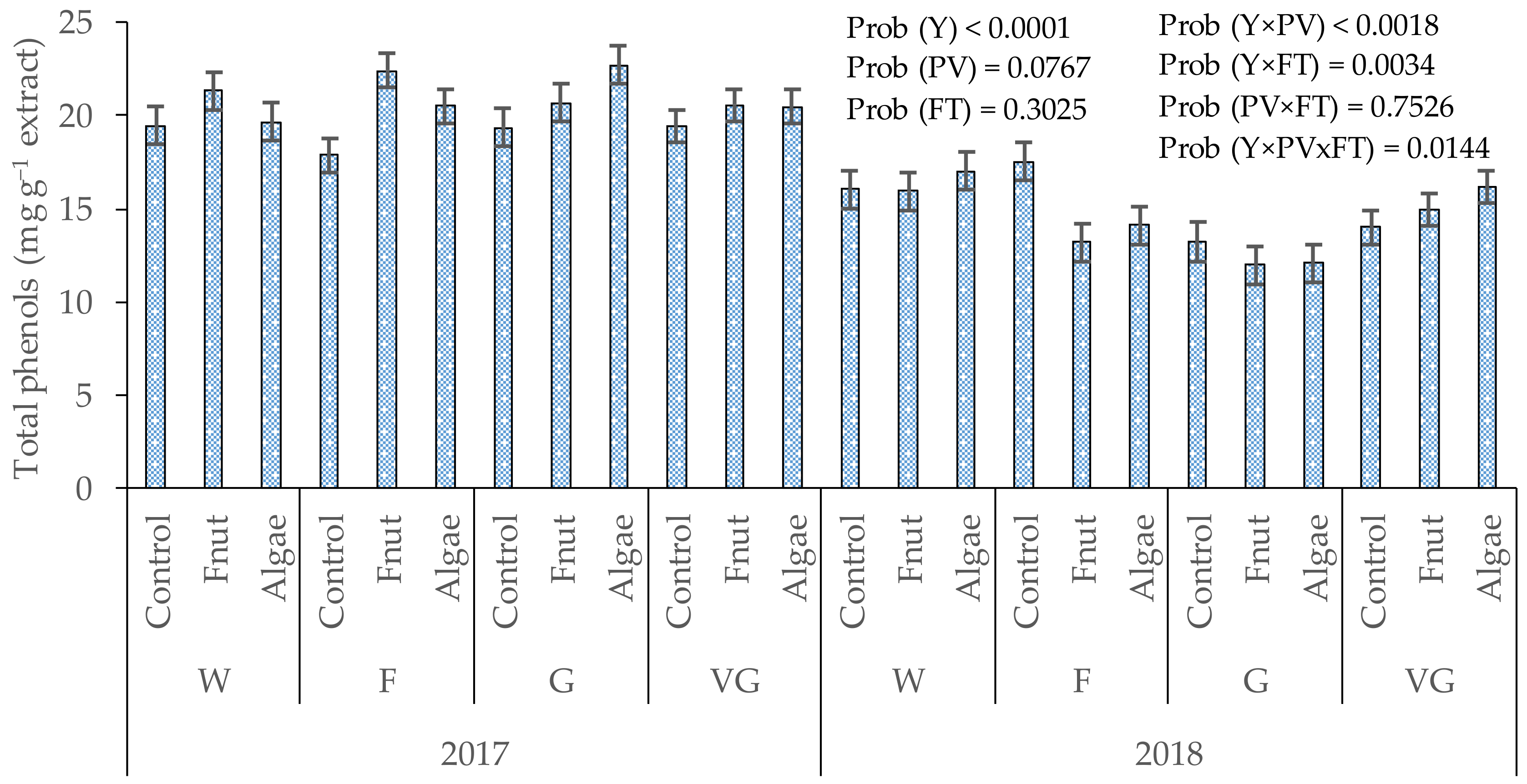
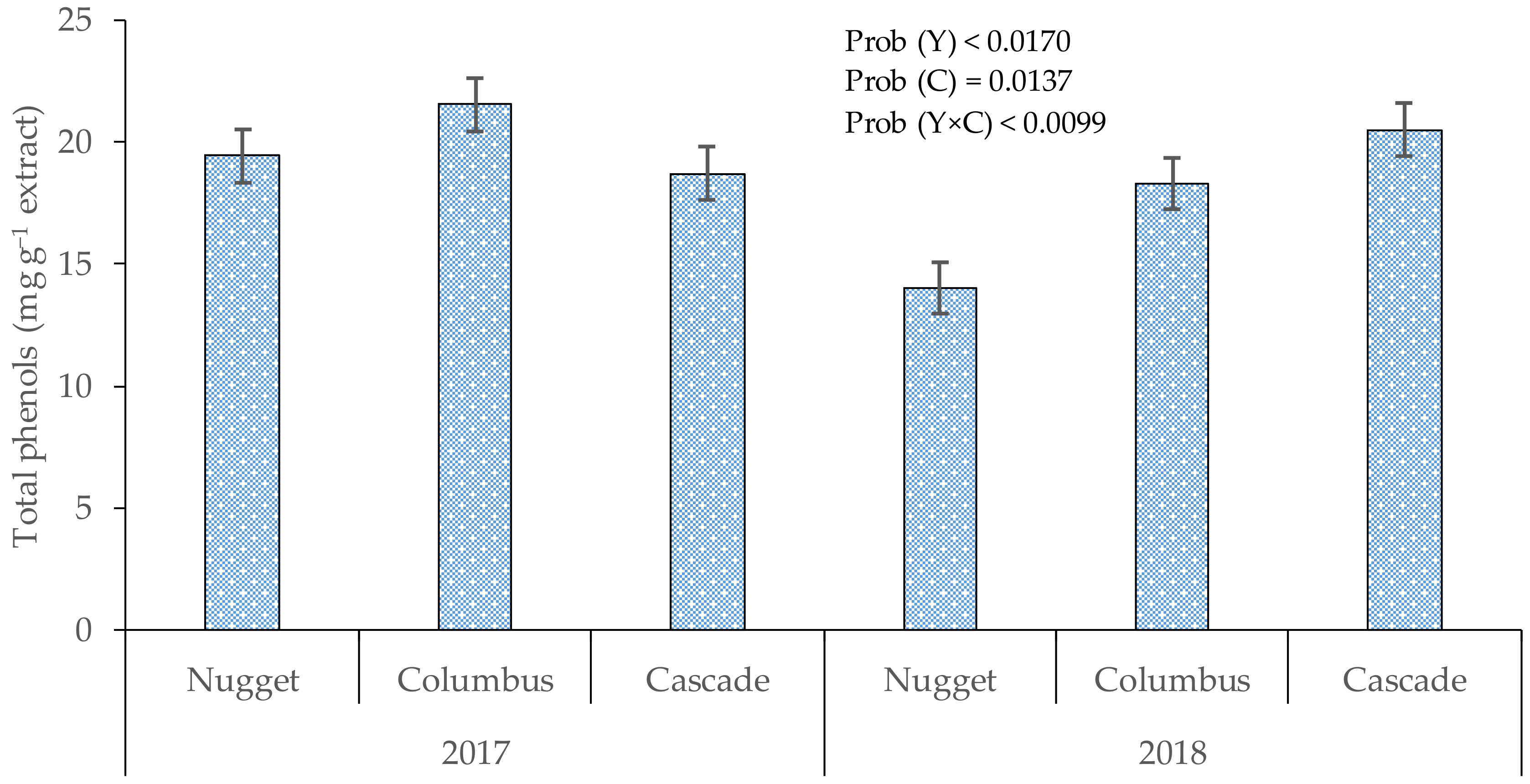
3.1. Total Phenols in Cones
3.2. Principal Component Analysis
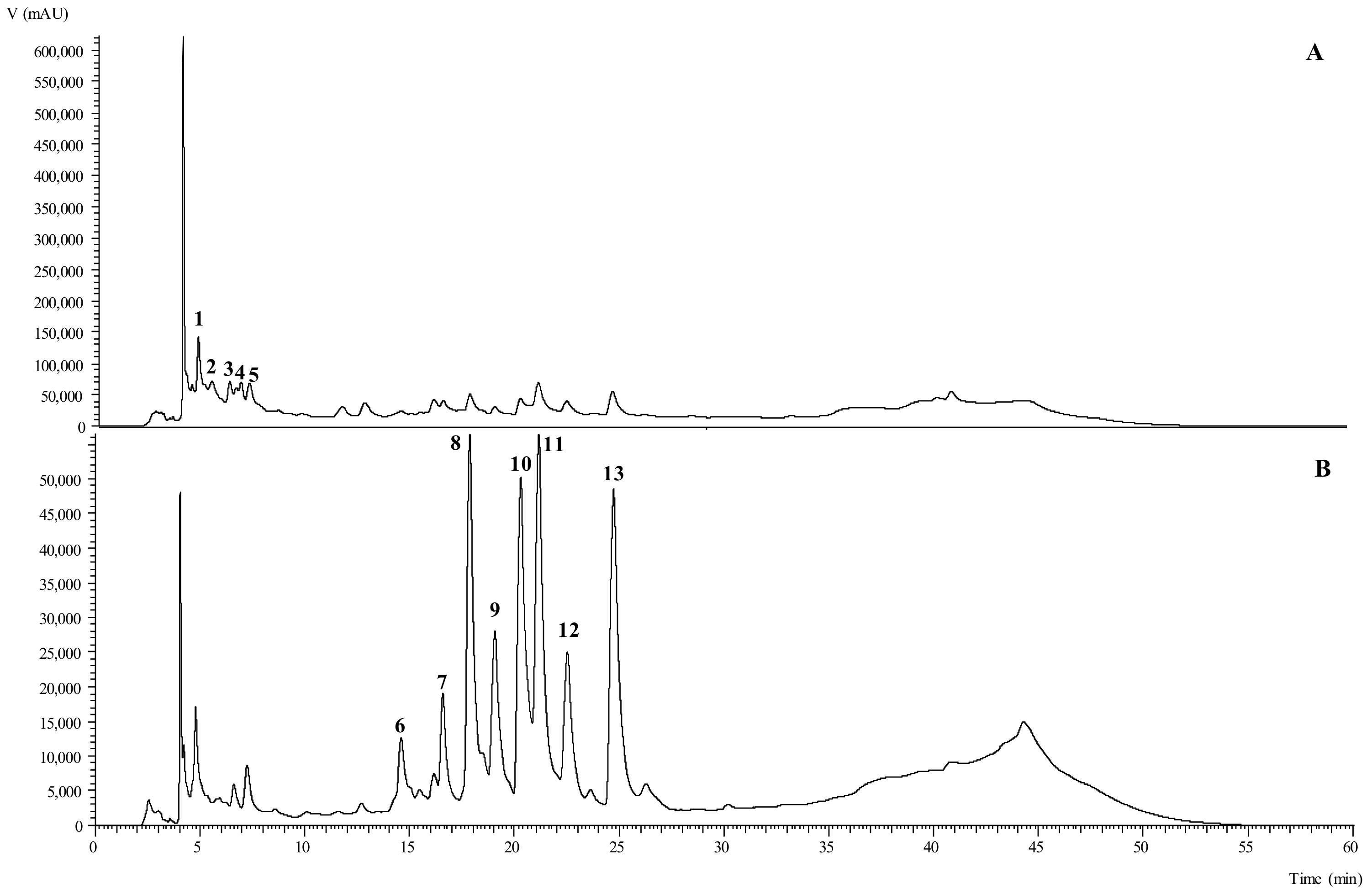
3.3. Phenolic Compounds Identification and Quantification
4. Discussion
4.1. Total Phenols in Hop Cones
4.2. PCA and Correlation Analysis
4.3. Phenolic Compounds Identification and Quantification
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bocquet, L.; Sahpaz, S.; Hilbert, J.L.; Rambaud, C.; Rivière, C. Humulus lupulus L., a very popular beer ingredient and medicinal plant: Overview of its phytochemistry, its bioactivity, and its biotechnology. Phytochem. Rev. 2018, 17, 1047–1090. [Google Scholar] [CrossRef]
- Korpelainen, H.; Pietiläinen, M. Hop (Humulus lupulus L.): Traditional and present use, and future potential. Econ. Bot. 2021, 75, 302–322. [Google Scholar] [CrossRef]
- De Keukeleire, D. Fundamentals of beer and hop chemistry. Quim. Nova 2000, 23, 108–112. [Google Scholar] [CrossRef]
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus—A story that begs to be told: A review. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar] [CrossRef]
- Biendl, M. Hops and Health. Master Brew. Assoc. Am. Tech. Q. 2009, 46, 1–7. [Google Scholar] [CrossRef]
- Olsovska, J.; Kolar, M. Humulus lupulus L. (hops)—A valuable source of compounds with bioactive effects for future therapies. Mil. Med. Sci. Lett. 2016, 85, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Yanagida, A.; Komeya, S.; Kawana, M.; Honma, D.; Tagashira, M.; Kanda, T.; Shibusawa, Y. Comprehensive separation and structural analyses of polyphenols and related compounds from bracts of hops (Humulus lupulus L.). J. Agric. Food Chem. 2014, 62, 2198–2206. [Google Scholar] [CrossRef]
- Rodrigues, M.Â.; Morais, J.; Castro, J.P. O lúpulo: Da cultura ao extrato. Técnica cultural tradicional. In Livro de Atas Das Jornadas do Lúpulo e da Cerveja: Novas Oportunidades de Negócio; Instituto Politécnico: Bragança, Portugal, 2015; pp. 1–10. [Google Scholar]
- Euromonitor. Beer in Portugal. Available online: https://www.euromonitor.com/beer-in-portugal/report (accessed on 15 August 2020).
- Small, E. Hop (Humulus lupulus)—A bitter crop with sweet prospects. Biodiver 2016, 17, 115–127. [Google Scholar] [CrossRef]
- Marceddu, R.; Carrubba, A.; Sarno, M. Cultivation trials of hop (Humulus lupulus L.) in semi-arid environments. Heliyon 2020, 6, e05114. [Google Scholar] [CrossRef]
- Ruggeri, R.; Loreti, P.; Rossini, F. Exploring the potential of hop as a dual purpose crop in the Mediterranean environment: Shoot and cone yield from nine commercial cultivars. Eur. J. Agron. 2018, 93, 11–17. [Google Scholar] [CrossRef]
- Rossini, F.; Virga, G.; Loreti, P.; Provenzano, M.E.; Danieli, P.P.; Roberto, R. Beyond beer: Hop shoot production and nutritional composition under Mediterranean climatic conditions. Agronomy 2020, 10, 1547. [Google Scholar] [CrossRef]
- Vidmar, M.; Abram, V.; Čeh, B.; Demšar, L.; Ulrih, N. White hop shoot production in Slovenia: Total phenolic, microelement and pesticide residue content in five commercial cultivars. Food Technol. Biotech. 2019, 57, 525–534. [Google Scholar] [CrossRef] [Green Version]
- Sirrine, J.R.; Rothwell, N.; Lizotte, E.; Goldy, R.; Marquie, S.; Brown-Rytlewski, D. Sustainable hop production in the Great Lakes region. Michigan State University Extension Bulletin E-3083. 2010. Available online: https://www.uvm.edu/sites/default/files/media/Sirrine-Sustainable-Hop-Production-in-the-Great-Lakes-Region.pdf (accessed on 12 February 2022).
- Čeh, B.; Čremožnik, B. Soil pH and hop (Humulus lupulus) yield related to liming material rate. Hmelj. Bilt. 2015, 22, 49–57. [Google Scholar]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Bindraban, P.S.; Dimkpa, C.; Nagarajan, L.; Roy, A.; Rabbinge, R. Revisiting fertilisers and fertilisation strategies for improved nutrient uptake by plants. Biol. Fertil. Soils 2015, 51, 897–911. [Google Scholar] [CrossRef] [Green Version]
- Balogun, D.A.; Oke, M.A.; Rocha-Meneses, L.; Fawole, O.B.; Omojasola, P.F. Phosphate solubilization potential of indigenous rhizosphere fungi and their biofertilizer formulations. Agron. Res. 2021, 19. [Google Scholar] [CrossRef]
- Pascual, P.; Carabio, D.; Abello, N.; Remedios, E.; Pascual, V. Enhanced assimilation rate due to seaweed biostimulant improves growth and yield of rice bean (Vigna umbellata). Agron. Res. 2021, 19, 1863–1872. [Google Scholar] [CrossRef]
- Afonso, S.; Arrobas, M.; Rodrigues, M.Â. Soil and plant analyses to diagnose hop fields irregular growth. J. Soil Sci. Plant Nut. 2020, 20, 1999–2013. [Google Scholar] [CrossRef]
- Afonso, S.; Arrobas, M.; Morais, J.S.; Rodrigues, M.Â. Agronomic and chemical evaluation of hop cultivars grown under Mediterranean conditions. Span. J. Agric. Res. 2021, 19, e0904. [Google Scholar] [CrossRef]
- Afonso, S.; Arrobas, M.; Rodrigues, M.Â. Twenty-years of hop irrigation by flooding the inter-row did not cause a gradient along the row in soil properties, plant elemental composition and dry matter yield. Horticulturae 2021, 7, 194. [Google Scholar] [CrossRef]
- Afonso, S.; Arrobas, M.; Rodrigues, M.Â. Response of hops to algae-based and nutrient-rich foliar sprays. Agriculture 2021, 11, 798. [Google Scholar] [CrossRef]
- IPMA. Normais Climatológicas. Instituto Português do Mar e da Atmosfera. Available online: http://www.ipma.pt/pt/oclima/normais.clima/ (accessed on 20 July 2020).
- Van Reeuwijk, L. Procedures for Soil Analysis; Technical Paper 9; International Soil Reference and Information Centre: Wageningen, The Netherlands, 2002. [Google Scholar]
- Walinga, I.; Van Vark, W.; Houba, V.; Van der Lee, J. Soil and Plant Analysis, Part 7: Plant Analysis Procedures; Wageningen Agricultural University: Wageningen, The Netherlands, 1989. [Google Scholar]
- Bessada, S.M.F.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb.f.: An underexploited and highly disseminated species. Ind. Crop. Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Abram, V.; Čeh, B.; Vidmar, M.; Hercezi, M.; Lazić, N.; Bucik, V.; Možina, S.S.; Košir, I.J.; Kač, M.; Demšar, L.; et al. A comparison of antioxidant and antimicrobial activity between hop leaves and hop cones. Ind. Crop. Prod. 2015, 64, 124–134. [Google Scholar] [CrossRef]
- Smoleń, S. Foliar nutrition: Current state of knowledge and opportunities. In Advances in Citrus Nutrition; Srivastava, A.K., Ed.; Springer: New York, NY, USA, 2012; pp. 41–58. [Google Scholar] [CrossRef]
- Čeh, B.; Naglič, B.; Luskar, M. Hop (Humulus lupulus L.) cones mass and length at cv. Savinjski golding. Hmelj. Bilt. 2012, 19, 5–16. [Google Scholar]
- Matsui, H.; Inui, T.; Oka, K.; Fukui, N. The influence of pruning and harvest timing on hop aroma, cone appearance, and yield. Food Chem. 2016, 202, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Rossini, F.; Loreti, P.; Provenzano, M.E.; De Santis, D.; Ruggeri, R. Agronomic performance and beer quality assessment of twenty hop cultivars grown in Central Italy. Ital. J. Agron. 2016, 11, 180. [Google Scholar] [CrossRef] [Green Version]
- MacKinnon, D.; Pavlovič, V.; Čeh, B.; Naglič, B.; Pavlovic, M. The impact of weather conditions on alpha-acid content in hop (Humulus lupulus L.) cv. Aurora. Plant Soil Environ. 2020, 66, 519–525. [Google Scholar] [CrossRef]
- Treutter, D. Managing phenol contents in crop plants by phytochemical farming and breeding-visions and constraints. Int. J. Mol. Sci. 2010, 11, 807–857. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Zu, Y.; Mei, X.; Li, B.; Li, T.; Li, Q.; Qin, L.; Yang, Z. Effects of calcium application on the yields of flavonoids and saponins in Panax notoginseng under cadmium stress. Int. J. Environ. Anal. Chem. 2020, 1–12. [Google Scholar] [CrossRef]
- Drobek, M.; Frac, M.; Cybulska, J. Plant biostimulants: Importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress-A review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef] [Green Version]
- Salvi, L.; Brunetti, C.; Cataldo, E.; Niccolai, A.; Centritto, M.; Ferrini, F.; Mattii, G.B. Effects of Ascophyllum nodosum extract on Vitis vinifera: Consequences on plant physiology, grape quality and secondary metabolism. Plant Physiol. Biochem. 2019, 139, 21–32. [Google Scholar] [CrossRef]
- Vasantharaja, R.; Abraham, L.S.; Inbakandan, D.; Thirugnanasambandam, R.; Senthilvelan, T.; Jabeen, S.K.A.; Prakash, P. Influence of seaweed extracts on growth, phytochemical contents and antioxidant capacity of cowpea (Vigna unguiculata L. Walp). Biocatal. Agric. Biotechnol. 2019, 17, 589–594. [Google Scholar] [CrossRef]
- Chouliaras, V.; Tasioula, M.; Chatzissavvidis, C.; Therios, I.; Tsabolatidou, E. The effects of a seaweed extract in addition to nitrogen and boron fertilization on productivity, fruit maturation, leaf nutritional status and oil quality of the olive (Olea europaea L.) cultivar Koroneiki. J. Sci. Food Agric. 2009, 89, 984–988. [Google Scholar] [CrossRef]
- Xu, C.P.; Leskovar, D.I. Effects of A. nodosum seaweed extracts on spinach growth, physiology and nutrition value under drought stress. Sci. Hortic. 2015, 183, 39–47. [Google Scholar] [CrossRef]
- Maliar, T.; Nemecek, P.; Urgeova, E.; Maliarova, M.; Nesvadba, V.; Krofta, K.; Vulganova, K.; Kroslak, E.; Kraic, J. Secondary metabolites, antioxidant and anti-proteinase activities of methanolic extracts from cones of hop (Humulus lupulus L.) cultivars. Chem. Pap. 2017, 71, 41–48. [Google Scholar] [CrossRef]
- Arruda, T.R.; Pinheiro, P.F.; Silva, P.I.; Bernardes, P.C. A new perspective of a well-recognized raw material: Phenolic content, antioxidant and antimicrobial activities and α- and β-acids profile of Brazilian hop (Humulus lupulus L.) extracts. LWT 2021, 141, 110905. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Świeca, M.; Cichocka, J.; Gawlik-Dziki, U. The phenolic content and antioxidant activity of the aqueous and hydroalcoholic extracts of hops and their pellets. J. Inst. Brew. 2013, 119, 103–110. [Google Scholar] [CrossRef]
- Keskin, S.; Sirin, Y.; Cakir, H.E.; Keskin, M. An investigation of Humulus lupulus L.: Phenolic composition, antioxidant capacity and inhibition properties of clinically important enzymes. S. Afr. J. Bot. 2019, 120, 170–174. [Google Scholar] [CrossRef]
- Gent, D.H.; Sirrine, J.R.; Darby, H.M. Nutrient management and imbalances. In Field Guide for Integrated Pest Management in Hops; Washington Hop Commission: Moxee, WA, USA, 2015; pp. 98–100. Available online: https://ipm.wsu.edu/field/pdf/hophandbook2009.pdf (accessed on 13 April 2019).
- Golia, E.; Angelaki, A.; Giannoulis, K.; Skoufogianni, E.; Bartzialis, D.; Cavalaris, C.; Vleioras, S. Evaluation of soil properties, irrigation and solid waste application levels on Cu and Zn uptake by industrial hemp. Agron. Res. 2021, 19, 92–99. [Google Scholar] [CrossRef]
- Otalora, G.; Pinero, M.C.; Lopez-Marin, J.; Varo, P.; del Amor, F.M. Effects of foliar nitrogen fertilization on the phenolic, mineral, and amino acid composition of escarole (Cichorium endivia L. var. latifolium). Sci. Hortic. 2018, 239, 87–92. [Google Scholar] [CrossRef]
- Elhanafi, L.; Houhou, M.; Rais, C.; Mansouri, I.; Elghadraoui, L.; Greche, H. Impact of excessive nitrogen fertilization on the biochemical quality, phenolic compounds, and antioxidant power of Sesamum indicum L. Seeds. J. Food. Qual. 2019, 2019, 9428092. [Google Scholar] [CrossRef] [Green Version]
- Rejmánková, E. Phenolic content and growth of wetland macrophytes: Is the allocation to secondary compounds driven by nutrient availability? Folia Geobot. 2016, 51, 239–250. [Google Scholar] [CrossRef] [Green Version]
- Kite, G.; Veitch, N. Assigning glucose or galactose as the primary glycosidic sugar in 3-O-mono-, di- and triglycosides of kaempferol using negative ion electrospray and serial mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 3125–3132. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, P.J.; Vieira, J.S.; Gonçalves, L.M.; Pacheco, J.G.; Guido, L.F.; Barros, A.A. Isolation of phenolic compounds from hop extracts using polyvinylpolypyrrolidone: Characterization by high-performance liquid chromatography–diode array detection–electrospray tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 3258–3268. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Pagano, F.; Salviati, E.; Chieppa, M.; Bertamino, A.; Manfra, M.; Sala, M.; Novellino, E.; Campiglia, P. Chemical profiling of bioactive constituents in Hop cones and pellets extracts by online comprehensive two dimensional liquid chromatography-tandem mass spectrometry and direct infusion Fourier Transform Ion Cyclotron Resonance MS. J. Sep. Sci. 2017, 41, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Taye Desta, K.; Lee, S.; Kim, Y.-H.; Shin, S.; Kim, G.-S.; Lee, S.; Shim, J.H.; Hacımüftüo, A. LC-MS/MS profiling of polyphenol-enriched leaf, stem, and root extracts of Korean Humulus japonicus Siebold & Zucc and determination of their antioxidant effects. Biomed. Chromatogr. 2017, 32, e4171. [Google Scholar] [CrossRef]
- Clifford, M.; Johnston, K.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MS n Identification of chlorogenic acids. J. Agr. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Clifford, M.; Knight, S.; Kuhnert, N. Discriminating between the six isomers of dicaffeoylquinic acid by LC-MS n. J. Agr. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef]
- Galieni, A.; Di Mattia, C.; De Gregorio, M.; Speca, S.; Mastrocola, D.; Pisante, M.; Stagnari, F. Effects of nutrient deficiency and abiotic environmental stresses on yield, phenolic compounds and antiradical activity in lettuce (Lactuca sativa L.). Sci. Hortic. 2015, 187, 93–101. [Google Scholar] [CrossRef]
- Ngadze, E.; Coutinho, T.A.; Icishahayo, D.; van der Waals, J.E. Effect of calcium soil amendments on phenolic compounds and soft rot resistance in potato tubers. Crop. Prot. 2014, 62, 40–45. [Google Scholar] [CrossRef] [Green Version]
- Almeida, A.D.R.; Maciel, M.V.D.O.B.; Machado, M.H.; Bazzo, G.C.; de Armas, R.D.; Vitorino, V.B.; Vitali, L.; Block, J.M.; Barreto, P.L.M. Bioactive compounds and antioxidant activities of Brazilian hop (Humulus lupulus L.) extracts. Int. J. Food Sci. Technol. 2020, 55, 340–347. [Google Scholar] [CrossRef]
- Santagostini, L.; Caporali, E.; Giuliani, C.; Bottoni, M.; Ascrizzi, R.; Araneo, S.R.; Papini, A.; Flamini, G.; Fico, G. Humulus lupulus L. cv. Cascade grown in Northern Italy: Morphological and phytochemical characterization. Plant Biosyst. 2020, 154, 316–325. [Google Scholar] [CrossRef]
- McAdam, E.L.; Vaillancourt, R.E.; Koutoulis, A.; Whittock, S.P. Quantitative genetic parameters for yield, plant growth and cone chemical traits in hop (Humulus lupulus L.). BMC Genet. 2014, 15, 22. [Google Scholar] [CrossRef]



| Soil Properties | Plot 1 (Weak) | Plot 2 (Fair) | Plot 3 (Good) | Plot 4 (Very Good) | Plot 5 (Very Good) | Plot 6 (Very Good) |
|---|---|---|---|---|---|---|
| Clay (%) a | 27.0 ± 5.8 | 35.0 ± 4.6 | 22.1 ± 2.0 | 18.1 ± 1.8 | 17.7 ± 2.1 | 16.8 ± 0.9 |
| Silt (%) a | 21.6 ± 10.7 | 22.8 ± 4.4 | 5.1 ± 1.6 | 35.5 ± 5.7 | 24.7 ± 3.2 | 24.3 ± 3.1 |
| Sand (%) a | 51.4 ± 16.5 | 42.2 ± 2.4 | 72.8 ± 18.4 | 46.4 ± 6.9 | 57.6 ± 4.8 | 58.9 ± 8.5 |
| pHH2O b | 5.8 ± 0.12 | 5.8 ± 0.04 | 5.5 ± 0.10 | 5.1 ± 0.13 | 5.8 ± 0.03 | 5.3 ± 0.03 |
| Organic carbon (g kg−1) c | 13.4 ± 0.20 | 15.7 ± 0.10 | 7.6 ± 0.04 | 14.5 ± 0.20 | 17.2 ± 0.08 | 19.4 ± 0.07 |
| Extract. P (mg P2O5 kg−1) d | 283 ± 45 | 452 ± 34 | 191 ± 28 | 213 ± 28 | 296 ± 20 | 289 ± 16 |
| Extract. K (mg K2O kg−1) d | 116 ± 7 | 193 ± 9 | 111 ± 6 | 286 ± 5 | 332 ± 9 | 162 ± 6 |
| Exch. Ca (cmolc kg−1) e | 14.8 ± 1.84 | 23.3 ± 1.39 | 10.7 ± 0.17 | 2.7 ± 0.46 | 4.9 ± 0.24 | 4.6 ± 0.18 |
| Exch. Mg (cmolc kg−1) e | 4.8 ± 0.84 | 9.5 ± 1.22 | 2.7 ± 0.07 | 0.5 ± 0.04 | 0.7 ± 0.03 | 0.6 ± 0.02 |
| Exch. K (cmolc kg−1) e | 0.3 ± 0.02 | 0.5 ± 0.04 | 0.2 ± 0.01 | 0.5 ± 0.08 | 0.6 ± 0.03 | 0.3 ± 0.01 |
| Exch. Na (cmolc kg−1) e | 0.2 ± 0.05 | 0.6 ± 0.05 | 0.1 ± 0.01 | 0.3 ± 0.06 | 0.06 ± 0.01 | 0.05 ± 0.01 |
| Exch. acidity (cmolc kg−1) e | 0.3 ± 0.03 | 0.3 ± 0.02 | 0.2 ± 0.03 | 0.6 ± 0.13 | 0.2 ± 0.02 | 0.4 ± 0.03 |
| CEC (cmolc kg−1) e | 20.7 ± 2.64 | 34.4 ± 2.56 | 14.0 ± 0.21 | 5.1 ± 0.37 | 6.7 ± 0.28 | 6.5 ± 0.17 |
| PC1 | PC2 | PC3 | PC4 | |
|---|---|---|---|---|
| Eigenvalue | 2.569 | 2.028 | 1.811 | 1.294 |
| Cronbach’s Alpha | 0.672 | 0.558 | 0.492 | 0.250 |
| Explained variance | 23.35 | 18.44 | 16.46 | 11.77 |
| Cumulative variance | 23.35 | 41.79 | 58.25 | 70.02 |
| Variable Loadings | ||||
| Total phenols | −0.730 | −0.359 | −1.606 | 0.753 |
| Nitrogen | 1.391 | −1.195 | 0.369 | 0.618 |
| Phosphorus | 1.516 | −0.091 | −0.555 | −0.074 |
| Potassium | −1.206 | −0.867 | 0.216 | 1.134 |
| Calcium | 0.272 | −1.982 | −0.014 | 0.311 |
| Magnesium | 1.319 | −0.363 | −0.584 | 1.707 |
| Iron | 0.054 | 1.226 | 0.822 | 1.147 |
| Manganese | −0.868 | −0.572 | 1.136 | 1.238 |
| Copper | 0.361 | 1.265 | 1.071 | 1.363 |
| Zinc | 1.277 | −0.179 | 1.409 | −0.823 |
| Boron | −0.668 | −1.077 | 1.578 | −0.605 |
| TPH | N | P | K | Ca | Mg | Fe | Mn | Cu | Zn | B | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TPH | 1 | ||||||||||
| N | −0.223 ** | 1 | |||||||||
| P | −0.008 | 0.412 ** | 1 | ||||||||
| K | 0.114 | −0.052 | −0.266 ** | 1 | |||||||
| Ca | 0.138 | 0.393 ** | 0.103 | 0.078 | 1 | ||||||
| Mg | −0.051 | 0.513 ** | 0.295 ** | 0.032 | 0.147 | 1 | |||||
| Fe | −0.186 * | −0.077 | −0.069 | −0.126 | −0.220 ** | 0.232 ** | 1 | ||||
| Mn | 0.074 | −0.016 | −0.298 ** | 0.402 ** | 0.019 | −0.191 * | 0.096 | 1 | |||
| Cu | −0.295 ** | 0.028 | 0.047 | −0.139 | −0.356 ** | 0.294 ** | 0.520 ** | 0.116 | 1 | ||
| Zn | −0.456 ** | 0.345 ** | 0.371 ** | −0.269 ** | 0.138 | 0.005 | 0.100 | −0.071 | 0.197 * | 1 | |
| B | −0.105 | 0.074 | −0.176 * | 0.250 ** | 0.348 ** | −0.273 ** | −0.058 | 0.311 ** | −0.070 | 0.223 ** | 1 |
| Peak | Tentative Identification | Rt (min) | λmax (nm) | [M-H] (m/z) | MS2 |
|---|---|---|---|---|---|
| 1 | 3-CQA | 4.80 | 340 | 353 | 191(100), 179(47), 173(3), 135(7) |
| 2 | cis 3-p-CoQA | 5.46 | 310 | 337 | 191(10), 163(100), 119(10) |
| 3 | trans 3-p-CoAD | 6.31 | 310 | 337 | 191(53), 163(100), 119(12) |
| 4 | 4-CQA | 6.86 | 325 | 353 | 191(14), 179(53), 173(100), 135(2) |
| 5 | 5-CQA | 7.25 | 323 | 353 | 191(100), 179(15),173(5), 135(2) |
| 6 | Q-3-2Rh-Ru | 14.6 | 330 | 755 | 609(45), 591(94), 573(12), 489(70), 301(100) |
| 7 | K-3-2Rh-Ru | 16.59 | 330 | 739 | 593(26), 575(100), 393(8), 285(38) |
| 8 | Q-3-Ru | 17.86 | 353 | 609 | 301(100) |
| 9 | Q-3-H | 19.06 | 351 | 463 | 301(100) |
| 10 | Q-3-6M-G | 20.29 | 353 | 549 | 505(100), 463(25), 301(50) |
| 11 | K-3-Ru | 21.15 | 347 | 593 | 285(100) |
| 12 | K-3-G | 22.52 | 345 | 447 | 285(100) |
| 13 | K-3-6M-G | 24.72 | 347 | 533 | 489(100), 285(20) |
| Phenolic Compounds (mg kg−1, dw) | Foliar Treatment | Limestone Treatment | |||
|---|---|---|---|---|---|
| Fnut | Algae | Control | Limed | Control | |
| 3-CQA | 37.1 ± 16.4a | 39.8 ± 15.7a | 40.5 ± 10.4a | 24.4 ± 7.0a | 21.5 ± 6.4a |
| cis 3-p-CoQA | 27.9 ± 3.0a | 23.8 ± 6.9a | 28.0 ± 3.9a | 5.7 ± 3.6a | 5.8 ± 7.1a |
| trans 3-p-CoAD | 23.6 ± 6.9a | 16.0 ± 6.0a | 19.0 ± 4.1a | 1.1 ± 2.2a | 4.2 ± 7.3a |
| 4-CQA | 24.4 ± 1.7a | 23.2 ± 1.0a | 25.3 ± 1.9a | 24.5 ± 4.8a | 7.6 ± 15.2a |
| 5-CQA | 31.1 ± 7.8a | 26.1 ± 3.5a | 28.7 ± 3.7a | 5.3 ± 10.7a | 5.6 ± 11.2a |
| Q-3-2Rh-Ru | 47.5 ± 0.2a | 47.0 ± 0.3a | 47.2 ± 0.2a | 46.7 ± 0.4a | 47.7 ± 1.4a |
| K-3-2Rh-Ru | 46.5 ± 0.1a | 46.5 ± 0.2a | 46.5 ± 0.1a | 46.5 ± 0.2a | 11.6 ± 23.2b |
| Q-3-Ru | 56.1 ± 0.8a | 54.5 ± 4.0a | 58.5 ± 1.5a | 50.6 ± 1.1a | 50.5 ± 2.1a |
| Q-3-H | 72.6 ± 5.1a | 64.6 ± 4.6a | 71.5 ± 1.5a | 54.6 ± 4.5a | 56.6 ± 6.7a |
| Q-3-6M-G | 93.1 ± 4.8a | 84.9 ± 9.0a | 96.5 ± 6.4a | 70.2 ± 3.9a | 66.1 ± 10.3a |
| K-3-Ru | 50.2 ± 1.3a | 49.6 ± 1.5a | 51.0 ± 0.3a | 47.5 ± 0.7a | 46.9 ± 0.5a |
| K-3-G | 52.1 ± 0.6a | 50.8 ± 1.1a | 52.3 ± 0.6a | 48.0 ± 0.4a | 36.2 ± 24.2a |
| K-3-6M-G | 59.1 ± 3.5a | 57.3 ± 3.3a | 62.3 ± 2.7a | 52.4 ± 1.2a | 51.3 ± 3.1a |
| Total phenolic compounds | 621.4 ± 32.8a | 584.1 ± 52.0a | 627.2 ± 13.6a | 477.6 ± 25.4a | 414.0 ± 81.0a |
| Phenolic Compounds (mg kg−1 Dry Matter) | Cultivar | ||
|---|---|---|---|
| Nugget | Columbus | Cascade | |
| 3-CQA | 40.5 ± 10.4a | 32.6 ± 5.9a | 40.7 ± 9.9a |
| cis 3-p-CoQA | 28.0 ± 3.9a | 24.6 ± 14.9a | 11.6 ± 6.0a |
| trans 3-p-CoAD | 19.0 ± 4.1a | 15.1 ± 4.9ab | 9.0 ± 2.5b |
| 4-CQA | 25.3 ± 1.9ab | 28.2 ± 7.9a | 14.7 ± 3.2b |
| 5-CQA | 28.7 ± 3.7ab | 22.1 ± 3.2b | 32.1 ± 2.9a |
| Q-3-2Rh-Ru | 47.2 ± 0.2b | 46.8 ± 0.2b | 50.3 ± 0.6a |
| K-3-2Rh-Ru | 46.5 ± 0.1b | 46.6 ± 0.1b | 54.5 ± 2.4a |
| Q-3-Ru | 58.5 ± 1.5b | 52.3 ± 1.4b | 72.4 ± 5.2a |
| Q-3-H | 71.5 ± 1.5a | 62.3 ± 4.9b | 56.9 ± 1.6b |
| Q-3-6M-G | 96.5 ± 6.4a | 77.2 ± 11.4ab | 71.5 ± 4.7b |
| K-3-Ru | 51.0 ± 0.3b | 51.5 ± 1.4b | 74.2 ± 3.1a |
| K-3-G | 52.3 ± 0.6b | 54.9 ± 2.9ab | 58.5 ± 2.2a |
| K-3-6M-G | 62.3 ± 2.7a | 71.3 ± 10.7a | 76.1 ± 4.8a |
| Total phenolic compounds | 627.2 ± 13.6a | 585.3 ± 61.8a | 622.6 ± 35.7a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afonso, S.; Dias, M.I.; Ferreira, I.C.F.R.; Arrobas, M.; Cunha, M.; Barros, L.; Rodrigues, M.Â. The Phenolic Composition of Hops (Humulus lupulus L.) Was Highly Influenced by Cultivar and Year and Little by Soil Liming or Foliar Spray Rich in Nutrients or Algae. Horticulturae 2022, 8, 385. https://doi.org/10.3390/horticulturae8050385
Afonso S, Dias MI, Ferreira ICFR, Arrobas M, Cunha M, Barros L, Rodrigues MÂ. The Phenolic Composition of Hops (Humulus lupulus L.) Was Highly Influenced by Cultivar and Year and Little by Soil Liming or Foliar Spray Rich in Nutrients or Algae. Horticulturae. 2022; 8(5):385. https://doi.org/10.3390/horticulturae8050385
Chicago/Turabian StyleAfonso, Sandra, Maria Inês Dias, Isabel C. F. R. Ferreira, Margarida Arrobas, Mário Cunha, Lillian Barros, and Manuel Ângelo Rodrigues. 2022. "The Phenolic Composition of Hops (Humulus lupulus L.) Was Highly Influenced by Cultivar and Year and Little by Soil Liming or Foliar Spray Rich in Nutrients or Algae" Horticulturae 8, no. 5: 385. https://doi.org/10.3390/horticulturae8050385
APA StyleAfonso, S., Dias, M. I., Ferreira, I. C. F. R., Arrobas, M., Cunha, M., Barros, L., & Rodrigues, M. Â. (2022). The Phenolic Composition of Hops (Humulus lupulus L.) Was Highly Influenced by Cultivar and Year and Little by Soil Liming or Foliar Spray Rich in Nutrients or Algae. Horticulturae, 8(5), 385. https://doi.org/10.3390/horticulturae8050385











