Hormonal Signaling in the Progamic Phase of Fertilization in Plants
Abstract
1. Introduction
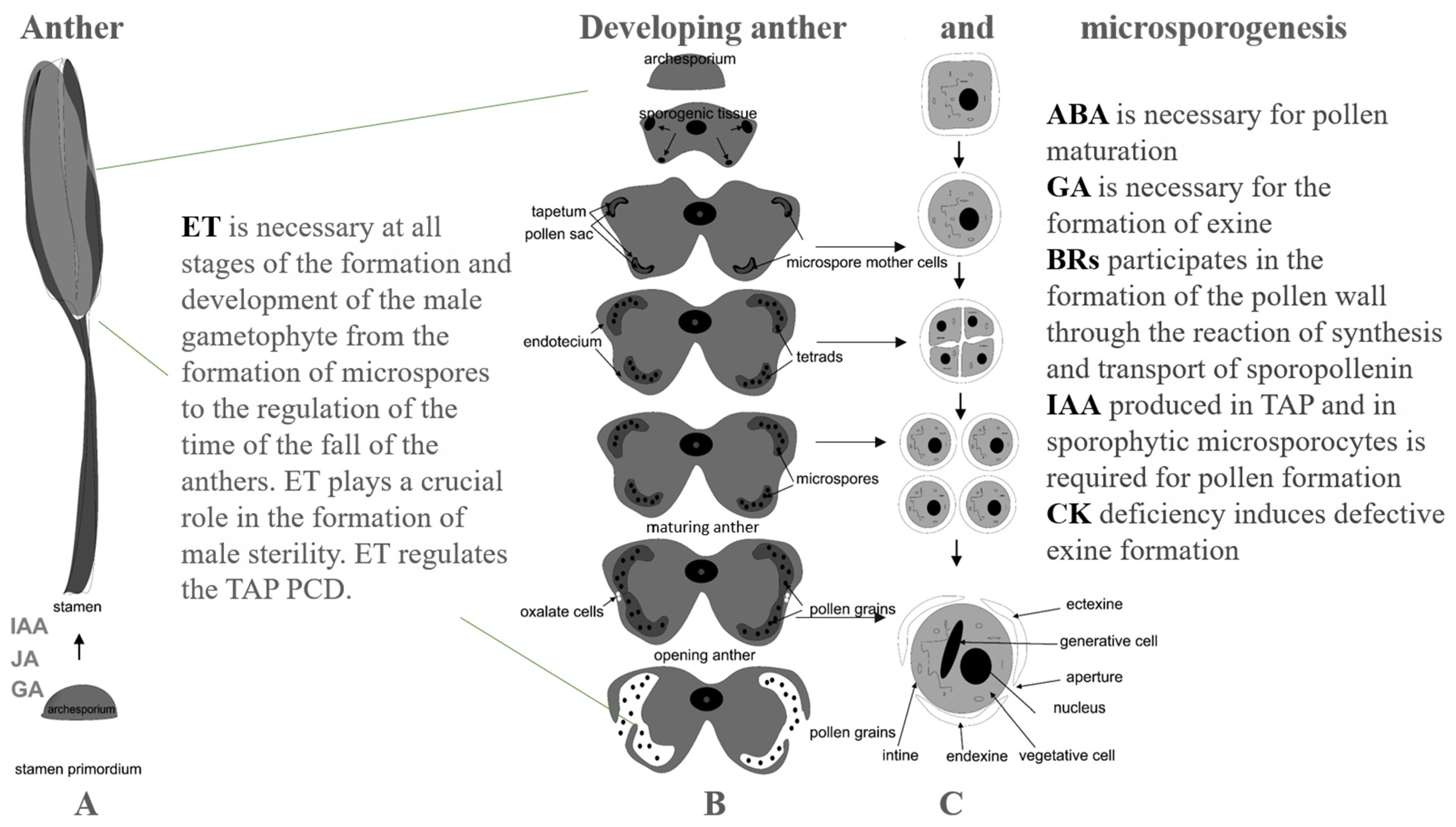
2. Phytohormones in Tapetum (TAP) and Pollen Wall Development Programs
2.1. Auxin (IAA)
2.2. GA and JA Are Indispensable for Stamen Development
2.3. ABA
2.4. ET Signaling in Male Gametophyte Development
2.5. CK Signaling Is Involved in TAP and Pollen Development
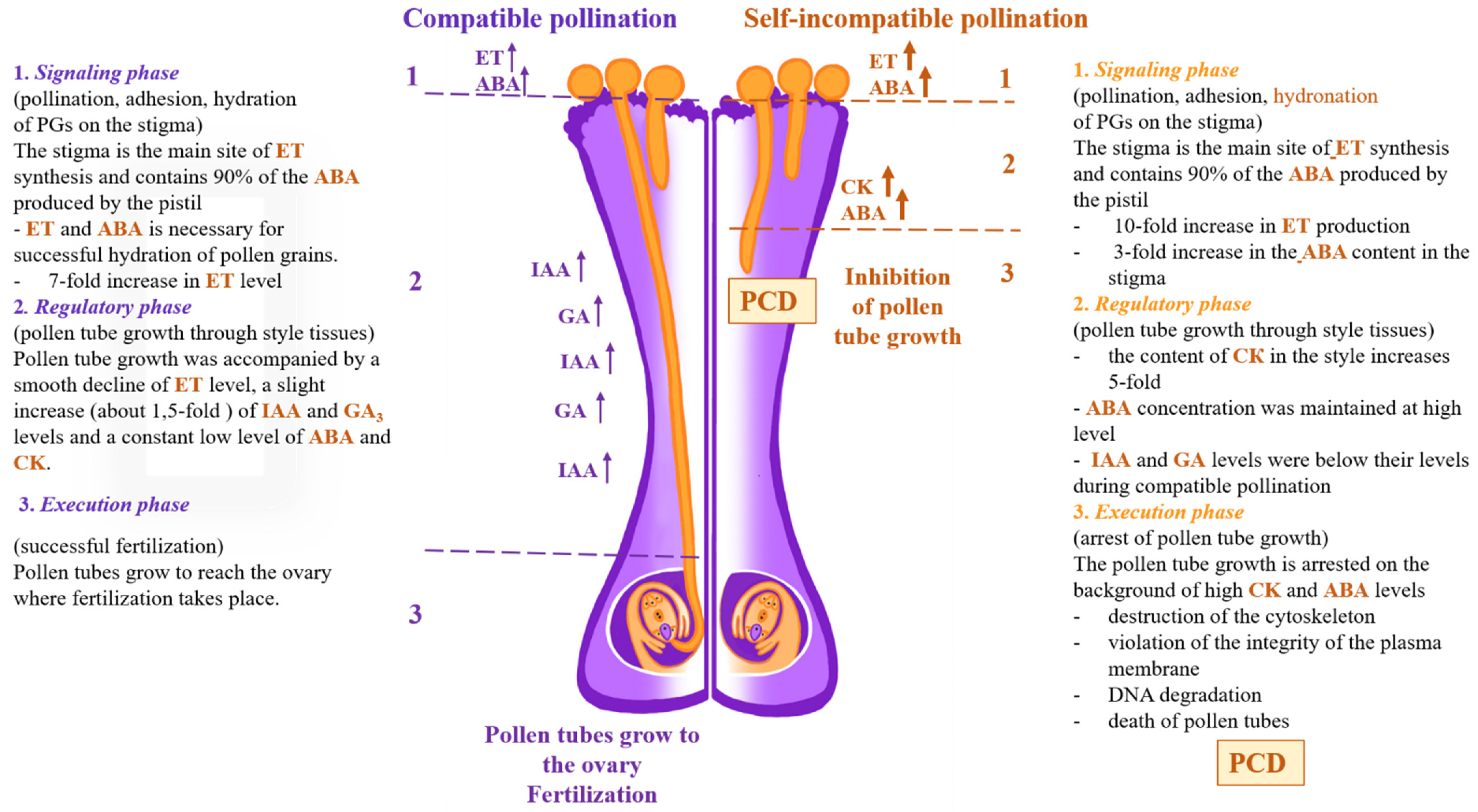
3. Pollen–Pistil Interactions in the Progamic Phase of Fertilization
3.1. Growth
3.2. Pollen–Pistil Interaction
3.3. BRs Are Essential for Male Fertility
3.4. ET Signaling in Pollen–Pistil System
3.5. ET as a Regulator of Hormonal Interplay in Pollen–Pistil System
3.6. ET–IAA Interplay
3.7. ET-ABA Interplay
3.8. IAA-CK Interplay
3.9. ET-IAA-CK Interplay
3.10. BRs and PT Growth
4. Self-Incompatibility (SI)-Induced PCD
4.1. Papaver SI System
4.2. S-RNase–Based SI
4.3. ET, ABA, and CK as the Factors of SI-Induced PCD in P. hybrida L.
4.3.1. ET and ABA
4.3.2. CK as a Factor of SI-Induced PCD
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lord, E.M.; Russell, S.D. The mechanisms of pollination and fertilization in plants. Annu. Rev. Cell Dev. Biol. 2002, 18, 81–105. [Google Scholar] [CrossRef] [PubMed]
- Franklin-Tong, V.E. Signaling and the modulation of pollen tube growth. Plant Cell 1999, 11, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Lord, E.M. Adhesion and guidance in compatible pollination. J. Exp. Bot. 2003, 54, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lin, Y.; Heath, R.M.; Zhu, M.X.; Yang, Z. Control of pollen tube tip growth by a Rop GTPase-dependent pathway that leads to tip-localized calcium influx. Plant Cell 1999, 11, 1731–1742. [Google Scholar] [PubMed]
- Pruitt, R.E.; Vielle-Calzada, J.P.; Ploense, S.E.; Grossniklaus, U.; Lolle, S.J. FIDDLEHEAD, a gene required to suppress epidermal cell interactions in Arabidopsis, encodes a putative lipid biosynthetic enzyme. Proc. Nat. Acad. Sci. USA 2000, 97, 1311–1316. [Google Scholar] [CrossRef]
- Nasrallah, M.E.; Kandasamy, M.K.; Chang, M.-C.; Stadler, Z.; Lim, S.; Nasrallah, J.B. Identifying genes for pollen-stigma recognition in crucifers. Ann. Bot. 2000, 85 (Suppl. A), 125–132. [Google Scholar] [CrossRef]
- Geitmann, A.; Emons, A.M.C. The cytoskeleton in plant and fungal cell tip growth. J. Microsc. 2000, 198, 218–245. [Google Scholar] [CrossRef]
- Blasiak, J.; Mulcahy, D.L.; Musgrave, M.E. Oxytropism: A new twist in pollen tube orientation. Planta 2001, 213, 318–322. [Google Scholar] [CrossRef]
- Geitmann, A.; Snowman, B.; Franklin-Tong, V.E.; Emons, A.M.C. Alterations in the actin cytoskeleton of the pollen tube are induced by the self-incompatibility reaction in Papaver rhoeas. Plant Cell 2000, 12, 1239–1251. [Google Scholar] [CrossRef]
- de Graaf, B.H.; Derksen, J.W.M.; Mariani, C. Pollen and pistil in the progamic phase. Sex. Plant Reprod. 2001, 14, 41–55. [Google Scholar] [CrossRef]
- Cheung, A.Y.; Wu, H.-M. Over-expression of an Arabidopsis formin stimulates supernumerary actin cable formation from pollen tube cell membrane. Plant Cell 2004, 16, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.J.; Franklin-Tong, V.E.; Franklin, F.C.H. The molecular and genetic basis of pollen-pistil interactions. New Phytol. 2001, 151, 565–584. [Google Scholar] [CrossRef] [PubMed]
- Dumas, C.; Knox, R.B. Callose and determination of pistil viability and incompatibility. Theor. Appl. Genet. 1983, 67, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kovaleva, L.V.; Zakharova, E.V.; Minkina, Y.V.; Voronkov, A.S. Effects of flavonols and phytohormones on germination and growth of petunia male gametophyte. Allelopath. J. 2009, 23, 51–62. [Google Scholar]
- Barendse, G.W.M.; Pereira, A.S.R.; Berkers, P.A.; Driessen, F.M.; van Eyden-Emons, A.; Linskens, H.F. Growth hormons in pollen, styles and ovaries of Petunia hybrida and Lilium species. Acta Bot. Neerl. 1970, 19, 175–185. [Google Scholar] [CrossRef]
- Sondheimer, E.; Linskens, H.F. Control of in vitro germination and tube extension of Petunia hybrida pollen. Proc. Konin. Neder. Akad. Wetensch. 1974, C77, 116–124. [Google Scholar]
- Liang, X.; Abel, S.; Keller, J.A.; Shen, N.F.; Theologis, A. The 1-aminocyclopropane-1-carboxylate synthase gene family of Arabidopsis thaliana. Proc. Nat. Acad. Sci. USA 1992, 89, 11046–11050. [Google Scholar] [CrossRef]
- Theologis, A.; Zarembinski, T.I.; Oeller, P.W.; Liang, X.; Abel, S. Modification of fruit ripening by suppressing gene expression. Plant Physiol. 1992, 100, 549–551. [Google Scholar] [CrossRef]
- Nemhauser, J.L.; Hong, F.; Chory, J. Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell 2006, 126, 467–475. [Google Scholar] [CrossRef]
- Brault, M.; Amiar, Z.; Pennarun, A.M.; Monestiez, M.; Zhang, Z.; Cornel, D.; Dellis, O.; Knight, H.; Bouteau, F.; Rona, J.-P. Plasma membrane depolarization induced by abscisic acid in Arabidopsis suspension cells involves reduction of proton pumping in addition to anion channel activation, which are both Ca2+ dependent. Plant Physiol. 2004, 135, 231–243. [Google Scholar] [CrossRef]
- He, X.-J.; Mu, R.-L.; Cao, W.-H.; Zhang, Z.-G.; Zhang, J.-S.; Chen, S.-Y. AtNAC2, a transcription factor downstream of ethylene and auxin signaling pathways, is involved in salt stress response and lateral root development. Plant J. 2005, 44, 903–916. [Google Scholar] [CrossRef] [PubMed]
- Zalejski, C.; Zhang, Z.; Quettier, A.L.; Maldiney, R.; Bonnet, M.; Brault, M.; Demandre, C.; Miginiac, E.; Rona, J.-P.; Sotta, B.; et al. Diacylglycerol pyrophosphate is a second messenger of abscisic acid signaling in Arabidopsis thaliana suspension cells. Plant J. 2005, 42, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Bögre, L.; Henriques, R.; Magyar, Z. TOR tour to auxin. EMBO J. 2013, 32, 1069–1071. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, J.P. The male gametophyte of flowering plants. Plant Cell 1989, 1, 657–664. [Google Scholar] [CrossRef]
- McCormick, S. Male gametophyte development. Plant Cell 1993, 5, 1265–1275. [Google Scholar] [CrossRef]
- Liu, L.; Wang, T. Male gametophyte development in flowering plants: A story of quarantine and sacrifice. J. Plant Physiol. 2021, 258, 153365. [Google Scholar] [CrossRef]
- Huang, J.; Dong, J.; Qu, L.-J. From birth to function: Male gametophyte development in flowering plants. Curr. Opin. Plant Biol. 2021, 63, 102118. [Google Scholar] [CrossRef]
- Bedinger, P. The remarkable biology of pollen. The Plant Cell 1992, 4, 879–887. [Google Scholar]
- Hafidh, S.; Fíla, J.; Honys, D. Male gametophyte development and function in angiosperms: A general concept. Plant Reprod. 2016, 29, 31–51. [Google Scholar] [CrossRef]
- Dukowic-Schulze, S.; van der Linde, K. Oxygen, secreted proteins and small RNAs: Mobile elements that govern anther development. Plant Reprod. 2021, 34, 1–19. [Google Scholar] [CrossRef]
- Wilson, Z.A.; Zhang, D.B. From Arabidopsis to rice: Pathways in pollen development. J. Exp. Bot. 2009, 60, 1479–1492. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.D.; Dai, L.F.; Wang, S.B.; Wolukau, J.N.; Jahn, M.; Chen, J.F. Male gamete development and early tapetal degeneration in cytoplasmic male-sterile pepper investigated by meiotic, anatomical and ultrastructural analyses. Plant Breed. 2006, 125, 395–399. [Google Scholar] [CrossRef]
- Varnier, A.L.; Mazeyrat-Gourbeyre, F.; Sangwan, R.S.; Clément, C. Programmed cell death progressively models the development of anther sporophytic tissues from the tapetum and is triggered in pollen grains during maturation. J. Struct. Biol. 2005, 152, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lou, Y.; Xu, X.; Yang, Z.N. A genetic pathway for tapetum development and function in Arabidopsis. J. Integr. Plant Biol. 2011, 53, 892–900. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, L. Specification of tapetum and microsporocyte cells within the anther. Curr. Opin. Plant Biol. 2014, 17, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Aya, K.; Hobo, T.; Sakakibara, H.; Kojima, M.; Shim, R.A.; Hasegawa, Y.; Ueguchi-Tanaka, M.; Matsuoka, M. Comprehensive transcriptome analysis of phytohormone biosynthesis and signaling genes in microspore/pollen and tapetum of rice. Plant Cell Physiol. 2008, 49, 1429–1450. [Google Scholar] [CrossRef]
- Heslop-Harrison, J. Pollen: Development and Physiology; Butterworth: London, UK, 2013; p. 337. [Google Scholar]
- Mutasa-Göttgens, E.; Hedden, P. Gibberellin as a factor in floral regulatory networks. J. Exp. Bot. 2009, 60, 1979–1989. [Google Scholar] [CrossRef]
- Wang, M.; Hoekstra, S.; van Bergen, S.; Lamers, G.E.; Oppedijk, B.J.; van der Heijden, M.W.; de Priester, W.; Schilperoort, R.A. Apoptosis in developing anthers and the role of ABA in this process during androgenesis in Hordeum vulgare L. Plant Mol. Biol. 1999, 39, 489–501. [Google Scholar] [CrossRef]
- Kovaleva, L.V.; Voronkov, A.C.; Zakharova, E.V.; Andreev, I.M. ABA and IAA control microsporogenesis in Petunia hybrida. Protoplasma 2018, 255, 751–759. [Google Scholar] [CrossRef]
- Huang, H.; Liu, B.; Liu, L.; Song, S. Jasmonate action in plant growth and development. J. Exp. Bot. 2017, 68, 1349–1359. [Google Scholar] [CrossRef]
- Qian, Q.; Yang, Y.; Zhang, W.; Hu, Y.; Li, Y.; Yu, H.; Hou, X. A novel Arabidopsis gene RGAT1 is required for GA-mediated tapetum and pollen development. New Phytol. 2021, 231, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Avanci, N.C.; Luche, D.D.; Goldman, G.H.; Goldman, M.H.S. Jasmonates are phytohormones with multiple functions, including plant defense and reproduction. Genet. Mol. Res. 2010, 9, 484–505. [Google Scholar] [CrossRef] [PubMed]
- Heslop-Harrison, J. The pollen wall: Structure and development. In Pollen; Butterworth-Heinemann: Oxford, WI, USA, 1971; pp. 75–98. [Google Scholar]
- Zhang, F.P.; Sussmilch, F.; Nichols, D.S.; Cardoso, A.A.; Brodribb, T.J.; McAdam, S.A. Leaves, not roots or floral tissue, are the main site of rapid, external pressure-induced ABA biosynthesis in angiosperms. J. Exp. Bot. 2018, 69, 1261–1267. [Google Scholar] [CrossRef]
- Terceros, G.C.; Resentini, F.; Cucinotta, M.; Manrique, S.; Colombo, L.; Mendes, M.A. The Importance of Cytokinins during Reproductive Development in Arabidopsis and Beyond. Int. J. Mol. Sci. 2020, 21, 8161. [Google Scholar] [CrossRef]
- Rieu, I.; Wolters-Arts, M.; Derksen, J.; Mariani, C.; Weterings, K. Ethylene regulates the timing of anther dehiscence in tobacco. Planta 2003, 217, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.J. Programmed cell death in floral organs: How and why do flowers die? Ann. Bot. 2006, 97, 309–315. [Google Scholar] [CrossRef]
- Wang, Y.; Kumar, P.P. Characterization of two ethylene receptors PhERS1 and PhETR2 from petunia: PhETR2 regulates timing of anther dehiscence. J. Exp. Bot. 2007, 58, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Kovaleva, L.V.; Dobrovolskaya, A.; Voronkov, A.; Rakitin, V. Ethylene is involved in the control of male gametophyte development and germination in petunia. J. Plant Growth Reg. 2011, 30, 64–73. [Google Scholar] [CrossRef]
- Hua, J.; Sakai, H.; Nourizadeh, S.; Chen, Q.G.; Bleecker, A.B.; Ecker, J.R.; Meyerowitz, E.M. EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. Plant Cell 1998, 10, 1321–1332. [Google Scholar] [CrossRef]
- Jegadeesan, S.; Beery, A.; Altahan, L.; Meir, S.; Pressman, E.; Firon, N. Ethylene production and signaling in tomato (Solanum lycopersicum) pollen grains is responsive to heat stress conditions. Plant Reprod. 2018, 31, 367–383. [Google Scholar] [CrossRef]
- An, J.; Almasaud, R.A.; Bouzayen, M.; Zouine, M.; Chervin, C. Auxin and ethylene regulation of fruit set. Plant Sci. 2020, 292, 110381. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Xu, X.F.; Zhu, J.; Gu, J.N.; Blackmore, S.; Yang, Z.N. The tapetal AHL family protein TEK determines nexine formation in the pollen wall. Nat. Comm. 2014, 5, 3855. [Google Scholar] [CrossRef] [PubMed]
- Pacini, E.; Guarnieri, M.; Nepi, M. Pollen carbohydrates and water content during development, presentation, and dispersal: A short review. Protoplasma 2006, 228, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Daneva, A.; Gao, Z.; Van Durme, M.; Nowack, M.K. Functions and regulation of programmed cell death in plant development. Ann. Rev. Cell Dev. Biol. 2016, 32, 441–468. [Google Scholar] [CrossRef] [PubMed]
- Papini, A.; Mosti, S.; Brighigna, L. Programmed-cell-death events during tapetum development of angiosperms. Protoplasma 1999, 207, 213–221. [Google Scholar] [CrossRef]
- Uzair, M.; Xu, D.; Schreiber, L.; Shi, J.; Liang, W.; Jung, K.-H.; Chen, M.; Luo, Z.; Zhang, Y.; Yu, J.; et al. PERSISTENT TAPETAL CELL2 is required for normal tapetal programmed cell death and pollen wall patterning. Plant Physiol. 2020, 182, 962–976. [Google Scholar] [CrossRef]
- Parish, R.W.; Li, S.F. Death of a tapetum: A programme of developmental altruism. Plant Sci. 2010, 178, 73–89. [Google Scholar] [CrossRef]
- Solís, M.T.; Chakrabarti, N.; Corredor, E.; Cortés-Eslava, J.; Rodríguez-Serrano, M.; Biggiogera, M.; Risueño, E.M.; Testillano, P.S. Epigenetic changes accompany developmental programmed cell death in tapetum cells. Plant Cell Physiol. 2014, 55, 16–29. [Google Scholar] [CrossRef]
- Zheng, S.; Li, J.; Ma, L.; Wang, H.; Zhou, H.; Ni, E.; Jiang, D.; Liu, Z.; Zhuang, C. OsAGO2 controls ROS production and the initiation of tapetal PCD by epigenetically regulating OsHXK1 expression in rice anthers. Proc. Nat. Acad. Sci. USA 2019, 116, 7549–7558. [Google Scholar] [CrossRef]
- Huang, M.D.; Hsing, Y.I.C.; Huang, A.H. Transcriptomes of the anther sporophyte: Availability and uses. Plant Cell Physiol. 2011, 52, 1459–1466. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, H.; Bai, J.; Ren, F. The regulatory framework of developmentally programmed cell death in floral organs: A review. Plant Physiol. Biochem. 2021, 158, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Knox, R.B. The pollen grain. In Embryology of Angiosperms; Springer: Berlin/Heidelberg, Germany, 1984; pp. 197–271. [Google Scholar]
- Jiang, J.; Zhang, Z.; Cao, J. Pollen wall development: The associated enzymes and metabolic pathways. Plant Biol. 2013, 15, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.B.; Beals, T.P.; Sanders, P.M. Anther development: Basic principles and practical applications. Plant Cell 1993, 5, 1217–1229. [Google Scholar] [PubMed]
- Dobritsa, A.A.; Shrestha, J.; Morant, M.; Pinot, F.; Matsuno, M.; Swanson, R.; Møller, B.L.; Preuss, D. CYP704B1 is a long-chain fatty acid ω-hydroxylase essential for sporopollenin synthesis in pollen of Arabidopsis. Plant Physiol. 2009, 151, 574–589. [Google Scholar] [CrossRef]
- Ariizumi, T.; Toriyama, K. Genetic regulation of sporopollenin synthesis and pollen exine development. Ann. Rev. Plant Biol. 2011, 62, 437–460. [Google Scholar] [CrossRef]
- Shi, J.; Cui, M.; Yang, L.; Kim, Y.J.; Zhang, D. Genetic and biochemical mechanisms of pollen wall development. Trends Plant Sci. 2015, 20, 741–753. [Google Scholar] [CrossRef]
- Quilichini, T.D.; Grienenberger, E.; Douglas, C.J. The biosynthesis, composition and assembly of the outer pollen wall: A tough case to crack. Phytochemistry 2015, 113, 170–182. [Google Scholar] [CrossRef]
- Stanley, R.G.; Linskens, H.F. Pollen: Biology Biochemistry and Management; Springer: Berlin, Germany, 1974. [Google Scholar]
- Navashin, S. Resultate einer Revision der Befruchtungsvorgange bei Lilium martagon und Fritillaria tenella. Bull. Acad. St. Petersbourg 1898, 9, 377–382. [Google Scholar]
- Kordyum, E.L. Double fertilization in flowering plants: 1898–2008. Cytol. Genet. 2008, 42, 147–158. [Google Scholar] [CrossRef]
- Hanamata, S.; Sawada, J.; Ono, S.; Ogawa, K.; Fukunaga, T.; Nonomura, K.I.; Kimura, S.; Kurusu, T.; Kuchitsu, K. Impact of autophagy on gene expression and tapetal programmed cell death during pollen development in rice. Front. Plant Sci. 2020, 11, 172. [Google Scholar] [CrossRef]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Brumos, J.; Robles, L.M.; Yun, J.; Vu, T.C.; Jackson, S.; Alonso, J.M.; Stepanova, A.N. Local auxin biosynthesis is a key regulator of plant development. Dev. Cell 2018, 47, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Hirano, V.; Altamura, M.M.; Falasca, G.; Costantino, P.; Cardarelli, M. Auxin regulates Arabidopsis anther dehiscence, pollen maturation, and filament elongation. Plant Cell 2008, 20, 1760–1774. [Google Scholar]
- Feng, X.L.; Ni, W.M.; Elge, S.; Mueller-Roeber, B.; Xu, Z.H.; Xue, H.W. Auxin flow in anther filaments is critical for pollen grain development through regulating pollen mitosis. Plant Mol. Biol. 2006, 61, 215–226. [Google Scholar] [CrossRef]
- Alani, R.; Aloni, E.; Langhans, M.; Ullrich, C.I. Role of auxin in regulating Arabidopsis flower development. Planta 2006, 223, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Dai, X.; Zhao, Y. Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev. 2006, 20, 1790–1799. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, B.; Moreno, I.; Duplakova, N.; Simon, S.; Carraro, N.; Reemmer, J.; Pěnčík, A.; Chen, X.; Tejos, R.; et al. ER-localized auxin transporter PIN8 regulates auxin homeostasis and male gametophyte development in Arabidopsis. Nat. Comm. 2012, 3, 941. [Google Scholar] [CrossRef] [PubMed]
- Cecchetti, V.; Brunetti, P.; Napoli, N.; Fattorini, L.; Altamura, M.M.; Costantino, P.; Cardarelli, M. ABCB1 and ABCB19 auxin transporters have synergistic effects on early and late Arabidopsis anther development. J. Integr. Plant Biol. 2015, 57, 1089–1098. [Google Scholar] [CrossRef]
- Cecchetti, V.; Celebrin, D.; Napoli, N.; Ghelli, R.; Brunetti, P.; Costantino, P.; Cardarelli, M. An auxin maximum in the middle layer controls stamen development and pollen maturation in Arabidopsis. New Phytol. 2017, 213, 1194–1207. [Google Scholar] [CrossRef]
- Yao, X.; Tian, L.; Yang, J.; Zhao, Y.N.; Zhu, Y.X.; Dai, X.; Zhao, Y.; Yang, Z.N. Auxin production in diploid microsporocytes is necessary and sufficient for early stages of pollen development. PLoS Genet. 2018, 14, e1007397. [Google Scholar] [CrossRef] [PubMed]
- Cecchetti, V.; Altamura, M.M.; Brunetti, P.; Petrocelli, V.; Falasca, G.; Ljung, K.; Costantino, P.; Cardarelli, M. Auxin controls Arabidopsis anther dehiscence by regulating endothecium lignification and jasmonic acid biosynthesis. Plant J. 2013, 74, 411–422. [Google Scholar] [CrossRef]
- Yang, J.; Tian, L.; Sun, M.-X.; Huang, X.-Y.; Zhu, J.; Guan, Y.-F.; Jia, Q.-S.; Yang, Z.-N. AUXIN RESPONSE FACTOR17 is essential for pollen wall pattern formation in Arabidopsis. Plant Physiol. 2013, 162, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, K.; Przedniczek, K. Comprehensive insight into gibberellin-and jasmonate-mediated stamen development. Genes 2019, 10, 811. [Google Scholar] [CrossRef] [PubMed]
- Plackett, A.R.; Thomas, S.G.; Wilson, Z.A.; Hedden, P. Gibberellin control of stamen development: A fertile field. Trends Plant Sci. 2011, 16, 568–578. [Google Scholar] [CrossRef]
- Yea, Q.; Zhu, W.; Li, L.; Zhang, S.; Yin, Y.; Ma, H.; Wang, X. Brassinosteroids control male fertility by regulating the expression of key genes involved in Arabidopsis anther and pollen development. Proc. Nat. Acad. Sci. USA 2010, 107, 6100–6105. [Google Scholar] [CrossRef]
- Chandra Sekhar, K.N.; Sawney, V.K. Role of ABA in stamen and pistil development in the normal and solanifolia mutant of tomato (Lycopersicom esculentum). Sex. Plant Reprod. 1991, 4, 279–283. [Google Scholar] [CrossRef]
- Peng, Y.B.; Zou, C.; Wang, D.H.; Gong, H.Q.; Xu, Z.H.; Bai, S.N. Preferential localization of abscisic acid in primordial and nursing cells of reproductive organs of Arabidopsis and cucumber. New Phytol. 2006, 170, 459–466. [Google Scholar] [CrossRef]
- Zhu, Y.; Dun, X.; Zhou, Z.; Xia, S.; Yi, B.; Wen, J.; Shen, J.; Ma, C.; Tu, J.; Fu, T. A separation defect of tapetum cells and microspore mother cells results in male, sterility in Brassica napus: The role of abscisic acid in early anther development. Plant Mol. Biol. 2010, 72, 111–123. [Google Scholar] [CrossRef]
- Takada, K.; Ishimaru, K.; Kamada, H.; Ezura, H. Anther-specific expression of mutated melon ethylene receptor gene Cm-ERS1/H70A affected tapetum degeneration and pollen grain production in transgenic tobacco plants. Plant Cell Rep. 2006, 25, 936–941. [Google Scholar] [CrossRef]
- De Storme, N.; Geelen, D. Cytokinesis in plant male meiosis. Plant Signal. Behav. 2013, 8, e23394. [Google Scholar] [CrossRef]
- Wang, F.; Cheng, Z.; Wang, J.; Zhang, F.; Zhang, B.; Luo, S.; Lei, C.; Pan, T.; Wang, Y.; Zhu, Y.; et al. Rice STOMATAL CYTOKINESIS DEFECTIVE2 regulates cell expansion by affecting vesicular trafficking in rice. Plant Physiol. 2022. [Google Scholar] [CrossRef]
- Kakimoto, T. Perception and signal transduction of cytokinins. Ann. Rev. Plant Biol. 2003, 54, 605–627. [Google Scholar] [CrossRef]
- Matsumoto-Kitano, M.; Kusumoto, T.; Tarkowski, P.; Kinoshita-Tsujimura, K.; Vaclavikova, K.; Miyawaki, K.; Kakimoto, T. Cytokinins are central regulators of cambial activity. Proc. Nat. Acad. Sci. USA 2008, 105, 20027–20031. [Google Scholar] [CrossRef]
- Mok, M.C. Cytokinins and plant development—An overview. In Cytokinins, 2nd ed.; Mok, D.W.S., Mok, M.C., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 155–166. [Google Scholar]
- Kieber, J.J.; Schaller, G.E. Cytokinin signaling in plant development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef]
- Hirose, N.; Takei, K.; Kuroha, T.; Kamada-Nobusada, T.; Hayashi, H.; Sakakibara, H. Regulation of cytokinin biosynthesis, compartmentalization and translocation. J. Exp. Bot. 2008, 59, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, Y.; Li, X.; Guo, S.; Huang, Y.; Xie, Q. Autophagy Dances with Phytohormones upon Multiple Stresses. Plants 2020, 9, 1038. [Google Scholar] [CrossRef]
- Sawhney, V.K.; Shukla, A. Male sterility in flowering plants: Are plant growth substances involved? Am. J. Bot. 1994, 81, 1640–1647. [Google Scholar] [CrossRef]
- Shukla, A.; Sawhney, V.K. Metabolism of dihydrozeatin in floral buds of wild-type and a genic male sterile line of rapeseed (Brassica napus L.). J. Exp. Bot. 1993, 44, 1497–1505. [Google Scholar] [CrossRef]
- Huang, S.; Cerny, R.E.; Qi, Y.; Bhat, D.; Aydt, C.M.; Hanson, D.D.; Malloy, K.P.; Ness, L.A. Transgenic studies on the involvement of cytokinin and gibberellin in male development. Plant Physiol. 2003, 131, 1270–1282. [Google Scholar] [CrossRef] [PubMed]
- Niemann, M.C.; Bartrina, I.; Ashikov, A.; Weber, H.; Novák, O.; Spíchal, L.; Strnad, M.; Strasser, R.; Bakker, H.; Schmülling, T.; et al. Arabidopsis ROCK1 transports UDP-GlcNAc/UDP-GalNAc and regulates ER protein quality control and cytokinin activity. Proc. Nat. Acad. Sci. USA 2015, 112, 291–296. [Google Scholar] [CrossRef]
- Kinoshita-Tsujimura, K.; Kakimoto, T. Cytokinin receptors in sporophytes are essential for male and female functions in Arabidopsis thaliana. Plant Signal. Behav. 2011, 6, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Firon, N.; Nepi, M.; Pacini, E. Water status and associated processes mark critical stages in pollen development and functioning. Ann. Bot. 2012, 109, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Wolters-Arts, M.; Lush, W.M.; Mariani, C. Lipids are required for directional pollen-tube growth. Nature 1998, 392, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, J.P. Molecular mechanisms of pollen tube growth and differentiation. Plant Cell 1993, 5, 1303. [Google Scholar] [CrossRef]
- Camacho, L.; Malhó, R. Endo/exocytosis in the pollen tube apex is differentially regulated by Ca2+ and GTPases. J. Exp. Bot. 2003, 54, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Feijó, J.A.; Costa, S.S.; Prado, A.M.; Becker, J.D.; Certal, A.C. Signalling by tips. Curr. Opin. Plant Biol. 2004, 7, 589–598. [Google Scholar] [CrossRef][Green Version]
- Romagnoli, S.; Cai, G.; Faleri, C.; Yokota, E.; Shimmen, T.; Cresti, M. Microtubule-and actin filament-dependent motors are distributed on pollen tube mitochondria and contribute differently to their movement. Plant Cell Physiol. 2007, 48, 345–361. [Google Scholar] [CrossRef]
- Iwano, M.; Entani, T.; Shiba, H.; Kakita, M.; Nagai, T.; Mizuno, H.; Miyawaki, A.; Shoji, T.; Kubo, K.; Isogai, A.; et al. Fine-tuning of the cytoplasmic Ca2+ concentration is essential for pollen tube growth. Plant Physiol. 2009, 150, 1322–1334. [Google Scholar] [CrossRef]
- Zhang, Y.; McCormick, S. The regulation of vesicle trafficking by small GTPases and phospholipids during pollen tube growth. Sex. Plant Reprod. 2010, 23, 87–93. [Google Scholar] [CrossRef]
- Guan, Y.; Guo, J.; Li, H.; Yang, Z. Signaling in pollen tube growth: Crosstalk, feedback, and missing links. Mol. Plant 2013, 6, 1053–1064. [Google Scholar] [CrossRef]
- Qin, T.; Liu, X.; Li, J.; Sun, J.; Song, L.; Mao, T. Arabidopsis microtubule-destabilizing protein 25 functions in pollen tube growth by severing actin filaments. Plant Cell 2014, 26, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Onelli, E.; Idilli, A.I.; Moscatelli, A. Emerging roles for microtubules in angiosperm pollen tube growth highlight new research cues. Front. Plant Sci. 2015, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Hafidh, S.; Potěšil, D.; Fíla, J.; Čapková, V.; Zdráhal, Z.; Honys, D. Quantitative proteomics of the tobacco pollen tube secretome identifies novel pollen tube guidance proteins important for fertilization. Genome Biol. 2016, 17, 81. [Google Scholar] [CrossRef] [PubMed]
- Winship, L.J.; Rounds, C.; Hepler, P.K. Perturbation analysis of calcium, alkalinity and secretion during growth of lily pollen tubes. Plants 2017, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; You, C.; Yang, S.; Zhang, Y.; Yang, F.; Li, X.; Chen, N.; Luo, Y.; Hu, X. The role of calcium/calcium-dependent protein kinases signal pathway in pollen tube growth. Front. Plant Sci. 2021, 12, 633293. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gong, P.; Guo, J.; Li, H.; Li, R.; Xing, W.; Yang, Z.; Guan, Y. Glycolysis regulates pollen tube polarity via Rho GTPase signaling. PLoS Genet. 2018, 14, e1007373. [Google Scholar] [CrossRef]
- Podolyan, A.; Luneva, O.; Klimenko, E.; Breygina, M. Oxygen radicals and cytoplasm zoning in growing lily pollen tubes. Plant Reprod. 2021, 34, 103–115. [Google Scholar] [CrossRef]
- Wu, J.; Qin, X.; Tao, S.; Jiang, X.; Liang, Y.-K.; Zhang, S. Long-chain base phosphates modulate pollen tube growth via channel-mediated influx of calcium. Plant J. 2014, 79, 507–516. [Google Scholar] [CrossRef]
- Dresselhaus, T.; Franklin-Tong, N. Male–female crosstalk during pollen germination, tube growth and guidance, and double fertilization. Mol. Plant 2013, 6, 1018–1036. [Google Scholar] [CrossRef]
- Higashiyama, T.; Takeuchi, H. The mechanism and key molecules involved in pollen tube guidance. Ann. Rev. Plant Biol. 2015, 66, 393–413. [Google Scholar] [CrossRef]
- Higashiyama, T.; Yang, W.C. Gametophytic pollen tube guidance: Attractant peptides, gametic controls, and receptors. Plant Physiol. 2017, 173, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Higashiyama, T. Tip-localized receptors control pollen tube growth and LURE sensing in Arabidopsis. Nature 2016, 531, 245–248. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.D.; Nadeau, J.A.; Zhang, X.S.; Bui, A.Q.; Halevy, A. Interorgan regulation of ethylene biosynthetic genes by pollination. Plant Cell 1993, 5, 419–432. [Google Scholar] [PubMed]
- Holden, M.J.; Marty, J.A.; Singh-Cundy, A. Pollination-induced ethylene promotes the early phase of pollen tube growth in Petunia inflata. J. Plant Physiol. 2003, 160, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Tsuchisaka, A.; Yu, G.; Jin, H.; Alonso, J.M.; Ecker, J.R.; Zhang, X.; Gao, S.; Theologis, A. A combinatorial interplay among the 1-aminocyclopropane-1-carboxylate isoforms regulates ethylene biosynthesis in Arabidopsis thaliana. Genetics 2009, 183, 979–1003. [Google Scholar] [CrossRef]
- Singh, A.; Evensen, K.B.; Kao, T.-H. Ethylene synthesis and floral senescence following compatible and incompatible pollination in Petunia inflata. Plant Physiol. 1992, 99, 38–45. [Google Scholar] [CrossRef]
- Kovaleva, L.V.; Timofeeva, G.V.; Rodionova, G.B.; Zakharova, E.V.; Rakitin, V.Y. Role of ethylene in the control of gametophyte-sporophyte interactions in the course of the progamic phase of fertilization. Russ. J. Dev. Biol. 2013, 44, 69–77. [Google Scholar] [CrossRef]
- Kovaleva, L.V.; Zakharova, E.V. Hormonal control of pollen-pistil interactions at the progamic phase of fertilization after compatible and incompatible pollination in petunia (Petunia hybrida L.). Sex. Plant Reprod. 2003, 16, 191–194. [Google Scholar] [CrossRef]
- Kovaleva, L.V.; Zakharova, E.V.; Timofeeva, G.V.; Andreev, I.M.; Golivanov, Y.Y.; Bogoutdinova, L.R.; Baranova, E.N.; Khaliluev, M.R. Aminooxyacetic acid (AOA), inhibitor of 1-aminocyclopropane-1-carboxilic acid (ACC) synthesis, suppresses self-incompatibility-induced programmed cell death in self-incompatible Petunia hybrida L. pollen tubes. Protoplasma 2020, 257, 213–227. [Google Scholar] [CrossRef]
- Zakharova, E.V.; Timofeeva, G.V.; Fateev, A.D.; Kovaleva, L.V. Caspase-like proteases and the phytohormone cytokinin as determinants of S-RNAse–based self-incompatibility–induced PCD in Petunia hybrida L. Protoplasma 2021, 258, 573–586. [Google Scholar] [CrossRef]
- Stepanova, A.N.; Yun, J.; Likhacheva, A.V.; Alonso, J.M. Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell 2007, 19, 2169–2185. [Google Scholar] [CrossRef] [PubMed]
- Völz, R.; Heydlauff, J.; Ripper, D.; von Lyncker, L.; Groß-Hardt, R. Ethylene signaling is required for synergid degeneration and the establishment of a pollen tube block. Dev. Cell 2013, 25, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Mou, W.; Kao, Y.T.; Michard, E.; Simon, A.A.; Li, D.; Wudick, M.M.; Lizzio, M.A.; Feijo, J.A.; Chang, C. Ethylene-independent signaling by the ethylene precursor ACC in Arabidopsis ovular pollen tube attraction. Nat. Comm. 2020, 11, 4082. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.-D.; Cho, Y.; Sheen, J. Emerging connections in the ethylene signaling network. Trends Plant Sci. 2009, 14, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Kovaleva, L.; Voronkov, A.; Zakharova, E.; Minkina, Y.; Timofeeva, G.; Andreev, I. Regulation of petunia pollen tube growth by phytohormones: Identification of their potential targets. J. Agric. Sci. Technol. A 2016, 6, 239–254. [Google Scholar] [CrossRef][Green Version]
- Dal Bosco, C.; Dovzhenko, A.; Palme, K. Intracellular auxin transport in pollen: PIN8, PIN5 and PILS5. Plant Signal. Behav. 2012, 7, 1504–1505. [Google Scholar] [CrossRef]
- Muday, G.K.; Rahman, A.; Binder, B.M. Auxin and ethylene: Collaborators or competitors? Trends Plant Sci. 2012, 17, 181–195. [Google Scholar] [CrossRef]
- Kovaleva, L.V.; Zakharova, E.V.; Voronkov, A.S.; Timofeeva, G.V. Auxin abolishes inhibitory effects of methylcyclopropen and amino oxyacetic acid on pollen grain germination, pollen tube growth, and the synthesis of ACC in petunia. Russ. J. Dev. Biol. 2017, 48, 122–129. [Google Scholar] [CrossRef]
- Kovaleva, L.V.; Zakharova, E.V.; Voronkov, A.S.; Timofeeva, G.V.; Andreev, I.M. Role of abscisic acid and ethylene in the control of water transport-driving forces in germinating petunia male gametophyte. Russ. J. Plant Physiol. 2017, 64, 782–793. [Google Scholar] [CrossRef]
- Cucinotta, M.; Manrique, S.; Guazzotti, A.; Quadrelli, N.E.; Mendes, M.A.; Benkova, E.; Colombo, L. Cytokinin response factors integrate auxin and cytokinin pathways for female reproductive organ development. Development 2016, 143, 4419–4424. [Google Scholar] [CrossRef]
- Chilley, P.M.; Casson, S.A.; Tarkowski, P.; Hawkins, N.; Wang, K.L.; Hussey, P.J.; Beale, M.; Ecker, J.R.; Sandberg, G.K.; Lindsey, K. The POLARIS peptide of Arabidopsis regulates auxin transport and root growth via effects on ethylene signaling. Plant Cell 2006, 18, 3058–3072. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Chae, H.S.; Kieber, J.J. Regulation of ACS protein stability by cytokinin and brassinosteroid. Plant J. 2008, 57, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ma, N.; Jia, Y.; Zhang, Y.; Feng, M.; Jiang, C.Z.; Ma, C.; Gao, J. An ethylene-induced regulatory module delays flower senescence by regulating cytokinin content. Plant Physiol. 2017, 173, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene role in plant growth, development and senescence, Interaction with other phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- Vogler, F.; Schmalzl, C.; Englhart, M.; Bircheneder, M.; Sprunck, S. Brassinosteroids promote Arabidopsis pollen germination and growth. Plant Reprod. 2014, 27, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Takayama, S.; Isogai, A. Self-incompatibility in plants. Ann. Rev. Plant Biol. 2005, 56, 467–489. [Google Scholar] [CrossRef]
- Bedinger, P.A.; Broz, A.K.; Tovar-Mendez, A.; McClure, B. Pollen-pistil interactions and their role in mate selection. Plant Physiol. 2017, 173, 79–90. [Google Scholar] [CrossRef]
- Bosch, M.; Franklin-Tong, V.E. Self-incompatibility in Papaver: Signalling to trigger PCD in incompatible pollen. J. Exp. Bot. 2008, 59, 481–490. [Google Scholar] [CrossRef]
- Bosch, M.; Poulter, N.S.; Vatovec, S.; Franklin-Tong, V.E. Initiation of programmed cell death in self-incompatibility: Role for cytoskeleton modifications and several caspase-like activities. Mol. Plant. 2008, 1, 879–887. [Google Scholar] [CrossRef]
- Wilkins, K.A.; Bosch, M.; Haque, T.; Teng, N.; Poulter, N.S.; Franklin-Tong, V.E. Self-incompatibility-induced programmed cell death in field poppy pollen involves dramatic acidification of the incompatible pollen tube cytosol. Plant Physiol. 2015, 167, 766–779. [Google Scholar] [CrossRef]
- Kubo, K.; Entani, T.; Takara, A.; Wang, N.; Fields, A.M.; Hua, Z.; Toyoda, M.; Kawashima, S.; Ando, T.; Isogai, A.; et al. Collaborative non-self recognition system in S-RNase-based self-incompatibility. Science 2010, 330, 796–799. [Google Scholar] [CrossRef]
- Kubo, K.I.; Tsukahara, M.; Fujii, S.; Murase, K.; Wada, Y.; Entani, T.; Iwano, M.; Takayama, S. Cullin1-P is an essential component of non-self recognition system in self-incompatibility in Petunia. Plant Cell Physiol. 2016, 57, 2403–2416. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Zhang, S.L. A cascade signal pathway occurs in self-incompatibility of Pyrus pyrifolia. Plant Signal. Behav. 2011, 6, 420–421. [Google Scholar] [CrossRef] [PubMed][Green Version]
- García-Valencia, L.E.; Bravo-Alberto, C.E.; Wu, H.M.; Rodríguez-Sotres, R.; Cheung, A.Y.; Cruz-García, F. SIPP, a novel mitochondrial phosphate carrier, mediates in self-incompatibility. Plant Physiol. 2017, 175, 1105–1120. [Google Scholar] [CrossRef] [PubMed]
- Roldán, J.A.; Rojas, H.J.; Goldraij, A. Disorganization of F-actin cytoskeleton precedes vacuolar disruption in pollen tubes during the in vivo self-incompatibility response in Nicotiana alata. Ann. Bot. 2012, 110, 787–795. [Google Scholar] [CrossRef]
- Bleecker, A.B.; Kende, H. Ethylene: A gaseous signal molecule in plants. Ann. Rev. Cell Dev. Biol. 2000, 16, 1–18. [Google Scholar] [CrossRef]
- Rogers, H.J. From models to ornamentals: How is flower senescence regulated? Plant Mol. Biol. 2013, 82, 563–574. [Google Scholar] [CrossRef]
- Trobacher, C.P. Ethylene and programmed cell death in plants. Botany 2009, 87, 757–769. [Google Scholar] [CrossRef]
- Müller, M.; Munné-Bosch, S. Ethylene response factors: A key regulatory hub in hormone and stress signaling. Plant Physiol. 2015, 169, 32–41. [Google Scholar] [CrossRef]
- Rantong, G.; Evans, R.; Gunawardena, A.H. Lace plant ethylene receptors, AmERS1a and AmERS1c, regulate ethylene-induced programmed cell death during leaf morphogenesis. Plant Mol. Biol. 2015, 89, 215–227. [Google Scholar] [CrossRef]
- Shibuya, K.; Niki, T.; Ichimura, K. Pollination induces autophagy in petunia petals via ethylene. J. Exp. Bot. 2013, 64, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Young, T.E.; Gallie, D.R. Regulation of programmed cell death in maize endosperm by abscisic acid. Plant Mol. Biol. 2000, 42, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Trivellini, A.; Ferrante, A.; Vernieri, P.; Serra, G. Effects of abscisic acid on ethylene biosynthesis and perception in Hibiscus rosa-sinensis L. flower development. J. Exp. Bot. 2011, 62, 5437–5452. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hou, L.; Meng, J.; You, H.; Li, Z.; Gong, Z.; Yang, S.; Shi, Y. The antagonistic action of abscisic acid and cytokinin signaling mediates drought stress response in Arabidopsis. Mol. Plant 2018, 11, 970–982. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Su, S.; Dai, H.; Liu, C.; Wei, X.; Zhao, Y.; Wang, Z.; Zhang, X.; Yuan, Y.; Yu, X.; et al. Programmed cell death in stigmatic papilla cells is associated with senescence-induced self-incompatibility breakdown in Chinese cabbage and radish. Front. Plant Sci. 2020, 11, 1847. [Google Scholar] [CrossRef]
- Su, S.; Dai, H.; Wang, X.; Wang, C.; Zeng, W.; Huang, J.; Duan, Q. Ethylene negatively mediates self-incompatibility response in Brassica rapa. Biochem. Biophys. Res. Comm. 2020, 525, 600–606. [Google Scholar] [CrossRef]
- Kunikowska, A.; Byczkowska, A.; Doniak, M.; Kaźmierczak, A. Cytokinins résumé: Their signaling and role in programmed cell death in plants. Plant Cell Rep. 2013, 32, 771–780. [Google Scholar] [CrossRef]
- Thomas, S.G.; Huang, S.; Li, S.; Staiger, C.J.; Franklin-Tong, V.E. Actin depolymerization is sufficient to induce programmed cell death in self-incompatible pollen. J. Cell Biol. 2006, 174, 221–229. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakharova, E.V.; Khaliluev, M.R.; Kovaleva, L.V. Hormonal Signaling in the Progamic Phase of Fertilization in Plants. Horticulturae 2022, 8, 365. https://doi.org/10.3390/horticulturae8050365
Zakharova EV, Khaliluev MR, Kovaleva LV. Hormonal Signaling in the Progamic Phase of Fertilization in Plants. Horticulturae. 2022; 8(5):365. https://doi.org/10.3390/horticulturae8050365
Chicago/Turabian StyleZakharova, Ekaterina V., Marat R. Khaliluev, and Lidia V. Kovaleva. 2022. "Hormonal Signaling in the Progamic Phase of Fertilization in Plants" Horticulturae 8, no. 5: 365. https://doi.org/10.3390/horticulturae8050365
APA StyleZakharova, E. V., Khaliluev, M. R., & Kovaleva, L. V. (2022). Hormonal Signaling in the Progamic Phase of Fertilization in Plants. Horticulturae, 8(5), 365. https://doi.org/10.3390/horticulturae8050365







