Identification of Two Diamondback Moth Parasitoids, Diadegma fenestrale and Diadegma semiclausum, Using LAMP for Application in Biological Control
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and mt Genome Sequencing
2.2. Diagnostic LAMP and PCR
2.3. LAMP Amplification Using Biological Sample
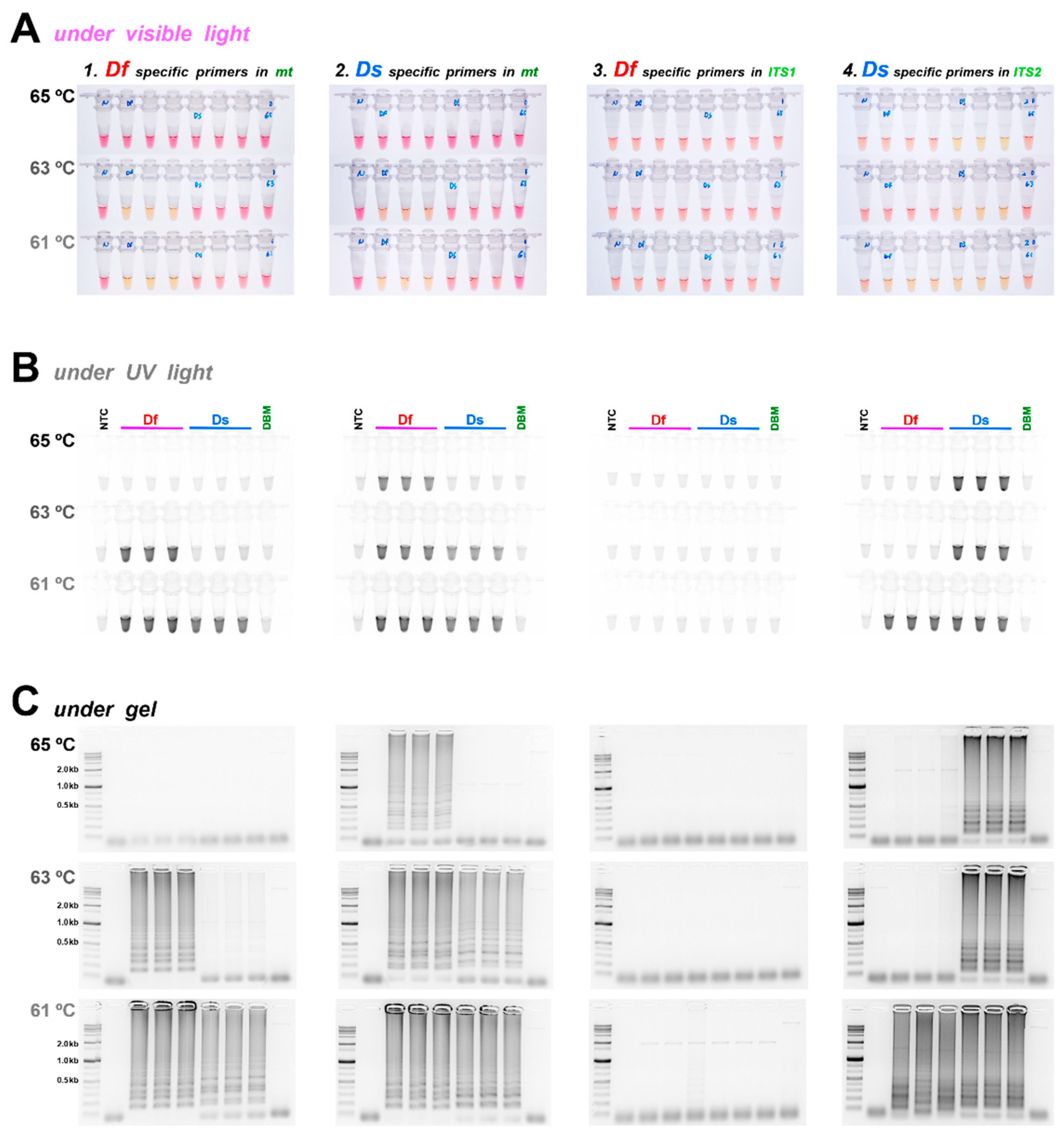
3. Results
3.1. mt Genome Sequencing, Primer Design, and Selection
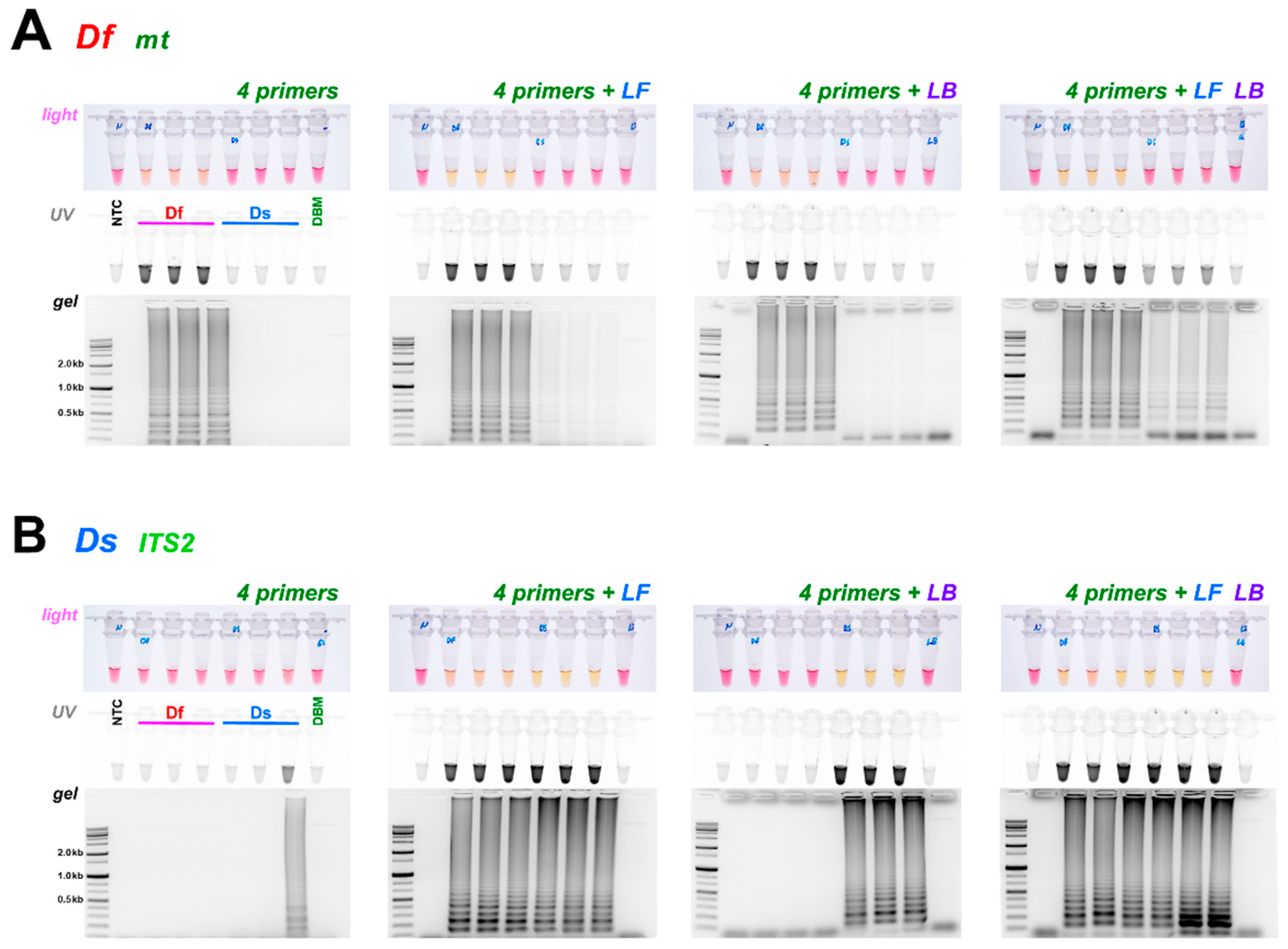
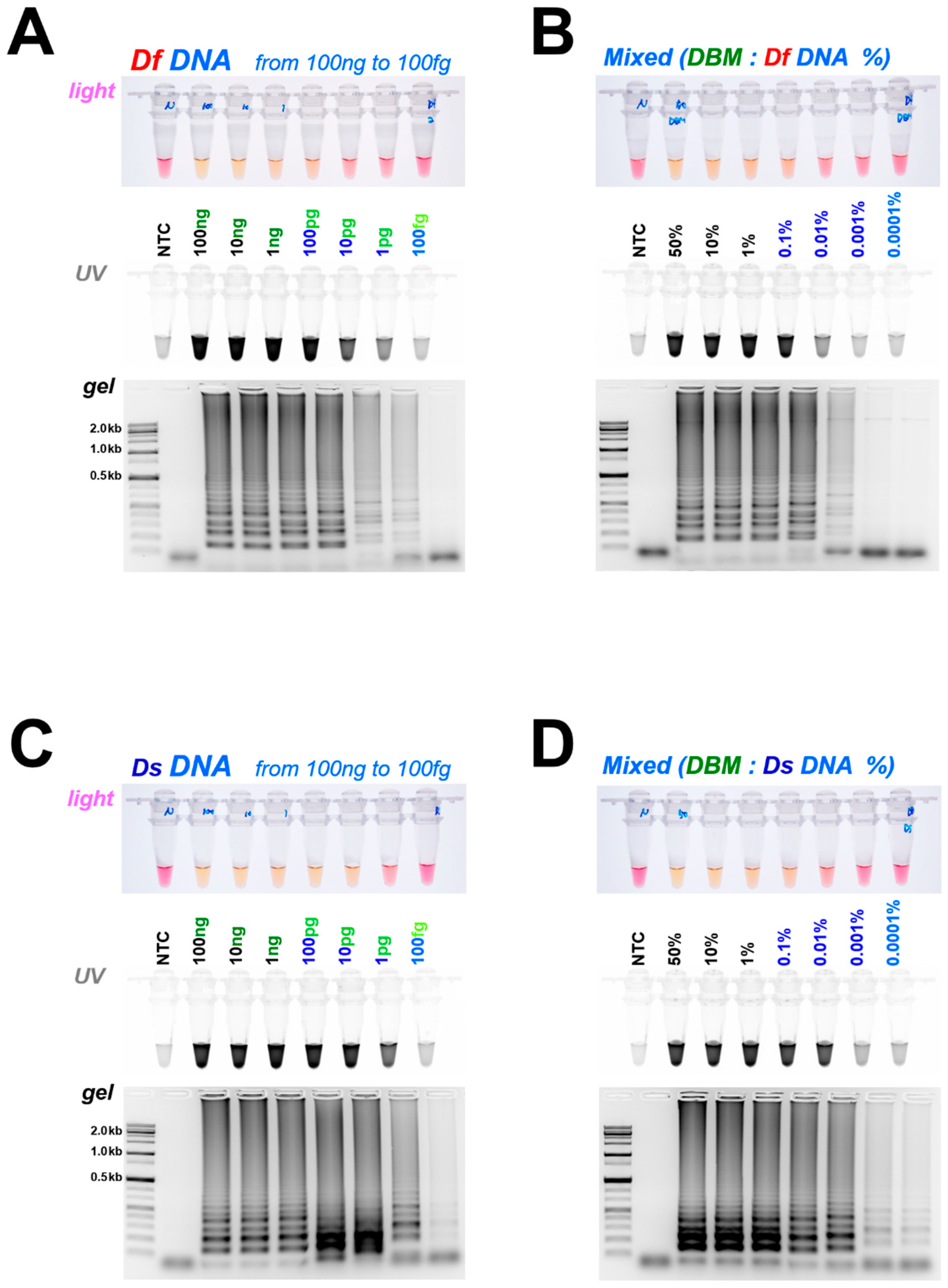
3.2. Diagnostic LAMP and PCR
3.3. Comparison of Loop Primer Effectiveness
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Talekar, N.; Shelton, A. Biology, ecology, and management of the diamondback moth. Annu. Rev. Entomol. 1993, 38, 275–301. [Google Scholar] [CrossRef]
- Kok, L.T. Natural enemies of insect pests in the cruciferous crop ecosystem. Recent Res. Dev. Environ. Biol. 2004, 1, 31–42. [Google Scholar]
- Rowell, B.; Bunsong, N.; Satthaporn, K.; Phithamma, S.; Doungsa-Ard, C. Hymenopteran parasitoids of Diamondback moth (Lepidoptera: Ypeunomutidae) in northern Thailand. J. Econ. Entomol. 2005, 98, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J. Plutella maculipennis, Curt., its natural and biological Control in England. Bull. Entomol. Res. 1938, 29, 343–372. [Google Scholar] [CrossRef]
- Davies, A.P.; Takashino, K.; Watanabe, M.; Miura, K. Parental genetic traits in offspring from inter-specific crosses between introduced and indigenous Diadegma Foerster (Hymenoptera: Ichneumonidae): Possible effects on conservation genetics. Appl. Entomol. Zool. 2009, 44, 535–554. [Google Scholar] [CrossRef]
- Fitton, M.; Walker, A. Hymenopterous parasitoids associated with diamondback moth: Taxonomic dilemma. In Proceedings of the Second International Workshop, Tainan, Taiwan, 10–14 December 1990; Talekar, N.S., Ed.; Asian Vegetable Research and Development Center: Shanhua, Taiwan, 1992. AVRDC Publication No. 92–368. pp. 225–232. [Google Scholar]
- Thompson, W.R. A Catalogue of the Parasites and Predators of Insect Pests. Section 2, Host Parasite Catalogue. In Part 4, Hosts of Hymenoptera (Ichneumonidae); Commonwealth Institute of Biological Control: Ottawa, ON, Canada, 1957. [Google Scholar]
- Azidah, A.A.; Fitton, M.G.; Quicke, D.L.J. Identification of the Diadegma species (Hymenoptera: Ichneumonidae, Campopleginae) attacking the diamondback moth, Plutella xylostella, (Lepidoptera: Plutellidae). Bull. Entomol. Res. 2000, 90, 375–389. [Google Scholar] [CrossRef]
- Wagener, B.; Reineke, A.; Löhr, B.; Zebitz, C.P.W. Phylogenetic study of Diadegma species (Hymenoptera: Ichneumonidae) inferred from analysis of mitochondrial and nuclear DNA sequences. Biol. Control 2006, 37, 131–140. [Google Scholar] [CrossRef]
- Choi, J.; Kim, J.; Kwon, M.; Lee, J. Description of the Diadegma fenestrale (Hymenoptera:Ichneumonidae: Campopleginae) Attacking the Potato Tuber Moth, Phthorimaea operculella (Lep.: Gelechiidae) New to Korea. Anim. Syst. Evol. Divers. 2013, 29, 70–73. [Google Scholar] [CrossRef][Green Version]
- CABI Invasive Species Compendium. Available online: https://www.cabi.org/isc/search/index (accessed on 26 February 2022).
- Kwon, M.; Kim, J.; Hong, E.; Lee, Y. Ecological characteristics of Cotesia glomerata L. (Hymenoptera: Braconidae) and its parasitism rates for diamondback moth (Plutella xylostella L.) in a kimchi cabbage field in the Korean highland area. Korean J. Appl. Entomol. 2019, 58, 355–362. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Juric, I.; Salzburger, W.; Balmer, O. Spread and global population structure of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae), and its larval parasitoids, Diadegma semiclausum and Diadegma fenestrale (Hymenoptera: Ichneumonidae), based on mtDNA. Bull. Enotomol. Res. 2017, 107, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Yara, K. Identification of Torymus sinensis and T. beneficus (Hymenoptera: Torymidae), introduced and indigenous parasitoids of the chestnut gall wasp Dryocosmus kuriphilus (Hymenoptera: Cynipidae), using the ribosomal ITS2 region. Biol. Control 2006, 36, 15–21. [Google Scholar] [CrossRef]
- Wacławik, B.; Nugnes, F.; Bernardo, U.; Gebiola, M.; Przybycień, M.; Lachowska-Cierlik, D. An integrative revision of the subgenus Liophloeodes (Coleoptera: Curculionidae: Entiminae: Polydrusini): Taxonomic, systematic, biogeographic and evolutionary insights. Arthropod Syst. Phylogeny 2021, 79, 419–441. [Google Scholar]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Park, K.; Kwon, H. Developmental characteristics of Diadegma semiclausum Hellen (Hymenoptera: Ichneumonidae), a larval parasitoid of Plutella xylostella L. (Lepidoptera: Yponomeutidae). J. Asia Pac. Entomol. 2003, 6, 105–110. [Google Scholar] [CrossRef]
- Schwartz, S.; Kent, W.J.; Smit, A.; Zhang, Z.; Baertsch, R.; Hardison, R.C.; Haussler, D.; Miller, W. Human-mouse alignments with BLASTZ. Genome Res. 2003, 13, 103–107. [Google Scholar] [CrossRef]
- Tillich, M.; Lehwark, P.; Pellizzer, T.; Ulbricht-Jones, E.S.; Fischer, A.; Bock, R.; Greiner, S. GeSeq-versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 2017, 45, W6–W11. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Frazer, K.A.; Pachter, L.; Poliakov, A.; Rubin, E.M.; Dubchak, I. VISTA: Computational tools for comparative genomics. Nucleic Acids Res. 2004, 32, W273–W279. [Google Scholar] [CrossRef]
- Mayor, C.; Brudno, M.; Schwartz, J.R.; Poliakov, A.; Rubin, E.M.; Frazer, K.A.; Patcher, L.S.; Dubchak, I. VISTA: Visualizing global DNA sequence alignments of arbitrary length. Bioinformatics 2000, 16, 1046–1047. [Google Scholar] [CrossRef]
- Kim, J.I.; Kwon, M.; Lee, S.H.; Kim, Y. Parasitism and survival rate of Diadegma fenestrale (Hymenoptera: Ichneumonidae) and DfIV gene expression patterns in two lepidopteran hosts. Biochem. Biophys. Res. Commun. 2015, 459, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Nam, H.Y.; Kwon, M.; Kim, H.J.; Yi, H.J.; Haenniger, S.; Unbehend, M.; Heckel, D.G. Development of a simple and accurate molecular tool for Spodoptera frugiperda species identification using LAMP. Pest Manag. Sci. 2021, 77, 3145–3153. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Nam, H.Y.; Kwon, M.; Choi, J.H.; Cho, S.R.; Kim, G.H. Novel diamide resistance-linked mutation in Korean Spodop-tera exigua and a LAMP assay based on a mutation-associated intronic InDel. J. Pest Sci. 2021, 94, 1017–1029. [Google Scholar] [CrossRef]
- Nagamine, K.; Hase, T.; Notomi, T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol. Cell. Probe 2002, 16, 223–229. [Google Scholar] [CrossRef] [PubMed]



| Primers | Sequence (5′->3′) |
|---|---|
| For identifying D. fenestrale by using primers for the mitochondrial region | |
| Df-F3 | GGGAATATTTCCATATATTTTTACTTCT |
| Df-B3 | TATATGAAAGGGGTGATTATGTAAAAATT |
| Dfs-FIP | GTGCAAATGCTAATAAATAATTTTTTGATCATTTTTTCTTTAAGATTTTCTTTACCA |
| Dfs-BIP | AATTCCTCAAGGAACACCAAATATTTTGATAAAGTTAATGGACGAATT |
| Df-LF | AATCATTAATCTAAATCATAATGGT |
| Df-LB | TATAGTTTTAATTGAAACAATTAGAA |
| For identifying D. semiclausum by using primers for the mitochondrial region | |
| Ds-F3 | GAATTATTCCCATATATTTTTACTGCC |
| Ds-B3 | GCTATATGAAAAGGATGATTATGTAAATT |
| For identifying D. fenestrale by using primers for the internal transcribed spacer (ITS) 1 region | |
| Df_ITS1-F3 | GTCTGTGTTCCTCTCTTTGAGT |
| Df_ITS1-B3 | GATTGCAGGAGAGCAACACG |
| Df_ITS1-FIP | AGAGACGAGCGTAACCGGGCTAAAACAACTCGAGATATCCGACAG |
| Df_ITS1-BIP | CGCGTGCGGCATCGATGAAAATCTTCGCTCCGCGATTC |
| For identifying D. semiclausum by using primers for the internal transcribed spacer (ITS) 2 region | |
| Ds_ITS2-F3 | GAGCCGAGCCACAAAGTTTGA |
| Ds_ITS2-B3 | TCATTATTGGAAGAGGCCGAGAT |
| Ds_ITS2-FIP | TCGGACCGTTCACTTGTCAATGTACGCTCGTCGTTCGTAGG |
| Ds_ITS2-BIP | CGCTAAACGGCCGGTCGATCCCAACGTACACGGGTCGTA |
| Ds_ITS2-LF | GTATTCTCTCTTACGCGAGATT |
| Ds_ITS2-LB | GGGGAGCTATATTCATAGTTC |
| For PCR | |
| LCO1490 | GGTCAACAAATCATAAAGATATTGG |
| HCO2198 | TAAACTTCAGGCTGACCAAAAAATCA |
| 18S_rRNA-F | GGCAAGTCTGGTGCCAGCA |
| 18S_rRNA-R | GGTGTGTACAAAGGGCAGGGAC |
| 18S_rRNA-R-F | GTCCCTGCCCTTTGTACACAC |
| 28S_rRNA-R | CCACCCACTTAGAGCTGCACT |
| Wasp_ITS2R | ATATGCTTAAATTCAGCGGG |
| Wasp_ITS2R2 | CGCCTGCTCTGAGGTCGTT |
| Primer Set | Incubation Condition | ||
|---|---|---|---|
| 65 °C, 40 min | 63 °C, 40 min | 61 °C, 40 min | |
| Df (mt) | Not amplified | Good | False positive |
| Ds (mt) | Good | False positive | False positive |
| Df (ITS1) | Not amplified | Not amplified | Not amplified |
| Ds (ITS2) | Good | Good | False positive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, H.; Kwon, M.; Ramasamy, S.; Kim, J. Identification of Two Diamondback Moth Parasitoids, Diadegma fenestrale and Diadegma semiclausum, Using LAMP for Application in Biological Control. Horticulturae 2022, 8, 366. https://doi.org/10.3390/horticulturae8050366
Nam H, Kwon M, Ramasamy S, Kim J. Identification of Two Diamondback Moth Parasitoids, Diadegma fenestrale and Diadegma semiclausum, Using LAMP for Application in Biological Control. Horticulturae. 2022; 8(5):366. https://doi.org/10.3390/horticulturae8050366
Chicago/Turabian StyleNam, Hwayeun, Min Kwon, Srinivasan Ramasamy, and Juil Kim. 2022. "Identification of Two Diamondback Moth Parasitoids, Diadegma fenestrale and Diadegma semiclausum, Using LAMP for Application in Biological Control" Horticulturae 8, no. 5: 366. https://doi.org/10.3390/horticulturae8050366
APA StyleNam, H., Kwon, M., Ramasamy, S., & Kim, J. (2022). Identification of Two Diamondback Moth Parasitoids, Diadegma fenestrale and Diadegma semiclausum, Using LAMP for Application in Biological Control. Horticulturae, 8(5), 366. https://doi.org/10.3390/horticulturae8050366







