Abstract
Due to its ornamental and medicinal value, pear flower has been historically loved and used in China. However, the current understanding of their odor-active compounds and aroma profiles is rather limited. This work aimed to evaluate and compare the overall aroma profile of pear flowers; the volatiles in flowers of seven pear cultivars (Anli, Bayuesu, Golden, Brown peel, KorlaXiangli, Lyubaoshi, Xizilü) were analyzed using solid-phase microextraction–gas chromatography-mass spectrometry (SPME-GC-MS). A total of 93 volatile compounds were identified and quantified within the amount of volatiles in the range of 62.7–691.8 μg kg−1 (FW) and showed high and significant variability in different cultivars. Anli and Brown peel flowers showed a relatively higher volatile abundance, while KorlaXiangli flowers were significantly lower than other cultivars. Although the composition of volatiles depended on the existence of different chemical classes, the odor activity values (OAVs) and odor descriptions showed some aldehydes were part of their main peculiarities and were considered as the basic active odorants that presented strong intensity of citrus and floral odor. Moreover, multivariate analysis showed the pear flower of different cultivars could be arranged in different clusters by the identified odorants. This study provides first-hand knowledge regarding pear flower aroma profiles, and that the cultivar differences were critical for the overall pattern.
1. Introduction
Pear (Pyrus L.) grows in moderate climate zones with many cultivars and is believed to have originated in the western mountainous area of China. It is widely distributed and cultivated in the north and south of China, with the largest production and richest germplasm resources in the world. Pear fruit has been used not only as a fruit but also as a traditional medicine with antitussive, anti-inflammatory, and diuretic effects for 1700 years [1].
The flower is an important part of plants. Except for the ornamental value, the aromatic substances released by flowers have high sensory and physiological values and even affect people’s appetite and mental state [2]. In China, people have used pear flowers to describe the beauty of artistic conception and they are regularly depicted in poems. The edible flower is often rich in nutrients, bringing food a good flavor or pleasant sensory effect. Therefore, Osmanthus and Dendranthema flowers have been famously used to make cakes by Chinese people since the Tang dynasty. Presently, in Yunnan province, a kind of pear flower is also used as an edible material favored by ethnic minorities. The medicinal effects of analgesic and diastolic of pear flower mean it is widely used in folk medicine treatments as a component of herbal mixtures [3]. According to the well-known traditional Chinese medical book Bencao Gangmu [4], pear flower could even eliminate black speckle, as a necessary material for women to whiten their skin in ancient days. Today, we know that the high content of arbutin is a representative active factor for whitening skin [1,5].
In the flowering process, the transition from vegetative to reproductive growth greatly affects fruit development and seed quality [6,7]. Previous studies have revealed that the most diverse and abundant volatiles are released predominantly from flowers as species-specific olfactory cues for attracting insect pollinators to ensure plant reproductive success [8,9]. Numerous insects possess olfactory receptors that respond specifically to plant volatiles and recognize the plant species [10]. The volatile compounds are the main constituents responsible for the aroma quality as a key factor attracting people and have a high correlation with the overall liking of the flower. Furthermore, many of the classes of volatile compounds isolated from plants show anti-microbial and anti-herbivore activity [8,11,12,13]. Based on their biosynthetic origin, volatiles can be divided into several classes, including terpenoids, fatty acid derivatives, and amino acid derivatives, involving complex enzymatic reactions [14]. The emission of volatiles is determined not only by genetics but can also be greatly influenced by environmental factors, such as light intensity, temperature, humidity, concentrations of CO2 and O3, and nutrient status [15,16,17]. The studies of flowers mainly focus on the morphology of ornamental aspects [18], however, aromatic properties are often neglected and some important odor-active compounds are gradually lost due to artificial selection and genetic erosion [19]. Previous studies on pear flowers have mainly concerned the structure and physiology aspects as well [3,20]; only Zhang et al. [21] has reported on the volatiles in KorlaXiangli flower to date.
To better understand the flavor of pear flowers, here we identify the volatile compounds and characterize the aroma profiles of seven pear cultivars. By combining the odor description, OAVs, aroma profiles, and multivariate analysis, the active odorants and aroma characteristics of pear flowers were evaluated.
2. Materials and Methods
2.1. Plant Material
Fresh flowers of seven pear cultivars (Anli, Bayuesu, Golden, Brown peel, KorlaXiangli, Lyubaoshi, Xizilü) were picked from the pear germplasm resources nursery at Hebei Normal University of Science and Technology (39.71° N, 119.185° E), China, in 2021. The entire nursery was managed with the same conditions of irrigation, pruning, and disease control. Forty flowers at the full-bloom stage from three mature trees of each cultivar were sampled and packed into polyethylene bags, sealed, labeled, and quickly transferred to the laboratory for analysis. The species of seven pear cultivars and sample collection date is shown in Table 1.

Table 1.
The species of seven pear cultivars and sample collection date.
2.2. Extraction of Volatile Compounds
The volatile components of the pear flower were absorbed by HP-SPME method. 2.0 g samples were weighed accurately and loaded into 20 mL headspace vials, then sealed. The 50/30 μm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) fiber (Supelco, Bellefonte, PA, USA) was used for SPME. The fiber was preconditioned and cleaned thermally at 270 °C for 1 h in GC injector according to prescriptions. Before extraction, the samples were conditioned at 50 °C for 30 min for equilibrium, then the fiber was exposed to a capped headspace vial for 30 min at 50 °C. All the extraction processes were performed by Agilent PAL 3 autosampler (Santa Clara, CA, USA). Three replicates of each cultivar were carried out.
2.3. GC-MS Analysis
After adsorption, the fiber was withdrawn and introduced into the GC heated injector port for desorption at 230 °C for 5 min at splitless mode. Then volatile compounds were separated and identified on an Agilent 7890A-5975C gas chromatograph-mass spectrometer (Santa Clara, CA, USA), equipped with a HP-5MS capillary column (30 m × 320 μm × 0.25 μm). Helium (purity 99.999%) was employed as the carrier gas with a flow rate of 1.0 mL min−1. The temperature program started at 50 °C maintained for 2 min and was increased to 200 °C at a rate of 10 °C·min−1 maintained for 7 min. The mass spectrometry conditions included: ion source temperature was 230 °C; electronic impact mode (EI) at 70 eV; scanned in the m/z range of 50–550 amu with a speed of 2.9 scan s−1 in a full scan mode.
The qualitative identification of compounds was assigned by the retention indices (RI) determined through a C6-C30 n-alkane mixture (Sigma-Aldrich, St. Louis, MO, USA) and matching their recorded mass spectra using the NIST14 databases performed by automated mass spectral deconvolution and identification system (AMDIS). The semi-quantitative analysis of volatile compounds was performed was carried out as reported by Ye et al. [2] with some modifications. The compound 3-octanol (Sigma-Aldrich, St. Louis, MO, USA) was chosen as the internal standard equivalent by the GC peak area under the same conditions.
2.4. Statistical Analysis
Principal components analysis (PCA) were carried out by SIMCA 14.1, and hierarchical cluster analysis (HCA) were conducted using ClustVis, a web-based platform (www.biit.cs.ut.ee/clustvis/, accessed on 25 March 2022). Significant levels were obtained by Duncan’s new multiple range tests (p < 0.05) by DPS 7.05. The other data were compiled and analyzed using Microsoft Office 2016. All data represented the means of three experiments performed in triplicate.
3. Results
3.1. Total Ion Chromatograms
The GC chromatograms of the volatile compounds emitted by pear flowers are shown in Figure 1. The comparison of typical profiles of chromatograms demonstrated that the Anli flower had the largest abundance of several main volatiles, while most volatile peaks in KorlaXiangli were significantly smaller than other cultivars. Moreover, there was a visible difference between the spectral appearances that could be observed within different cultivars.
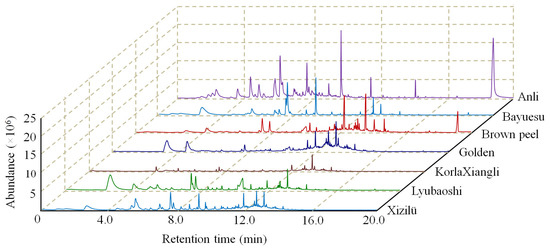
Figure 1.
Chromatographic profiles obtained by SPME-GC-MS of volatile compounds in pear flower of seven cultivars.
3.2. Identification of the Volatile Compounds
The volatile compounds identified in pear flowers are shown in Table 2. A total of ninety-three volatile compounds were identified and quantified in the flowers of seven pear cultivars, including alcohols (15), arenes (6), phenols (3), aldehydes (13), terpenoids (13), ketones (4), heterocycle (1), alkanes (10), and esters (28). Lyubaoshi flower contained the highest number of 52 volatiles, while Golden flower exhibited the least (39 compounds); the other flowers of Bayuesu, Xizilü, Anli, Brown peel, and KorlaXiangli contained 51, 47, 46, 42, and 42 volatile chemicals, respectively. The chemical classes of ester, alcohol, aldehyde, and terpene showed the largest numbers in most cultivars. Naphthalene, 1-decanal, 2-pentyl-furan, 2-ethyl hexanol, 1-nonanal, 1-nonanol, methyl 2-ethyl hexanoate, (E)-geranyl acetone, 6-methyl-5-hepten-2-one, (Z)-3-hexenyl acetate, methyl 2-hydroxy-3-methyl pentanoate, benzaldehyde, hydroquinone, 2-phenylethanol, 2-nitrophenol, 1-octanol, and all alkanes were the common volatiles existed in each cultivar.

Table 2.
The volatile compounds detected in the flower of seven pear cultivars and their semi-quantitative determination (μg kg−1 FW).
As shown in Figure 2, the total amount of volatiles in pear flowers was significantly different within the seven cultivars. Anli flower contained the highest amount (691.8 μg kg−1) close to Brown peel (667.2 μg kg−1), while KorlaXiangli showed the lowest amount of volatiles (62.7 μg kg−1), approximately the eleventh of Anli flower; the total amount of Golden flower (384.8 μg kg−1) was close to Xizilü (351.6 μg kg−1); cultivars Bayuesu and Lyubaoshi flower were 312.8 and 185.6 μg kg−1, respectively. Among these volatile chemicals, terpenoids such as geranyl linalool, linalool, and α-farnesene had relative high amounts in Anli, Bayuesu, and Golden flower; 3-methyltetradecane and hexadecane were the major volatiles that accounted for 29.0% and 31.9% in Brown peel flower; 2-phenylethanol, 1-decanal, pentadecane, 1-nonanal were the most abundant volatiles in Anli flower (15.5%), Bayuesu (26.0%), Golden (20.3%), and Lyubaoshi (20.1%), respectively. In addition, a range of esters (fatty acid-derived volatiles) was detected in each cultivar, while the levels were relatively lower compared to other chemicals. Even where volatiles in different pear flowers involved the same chemical classes, the amount varied greatly (Figure 2): alkanes existed in almost all cultivars as the background volatiles with a remarkable amounts level; alcohols mainly existed in the flowers of Brown peel, Anli, and Lyubaoshi; arenes were the dominant volatiles in Brown peel, Anli, and Golden flower; and the highest amount of aldehyde appeared in Bayuesu, Xizilü, and Lyubaoshi flower.
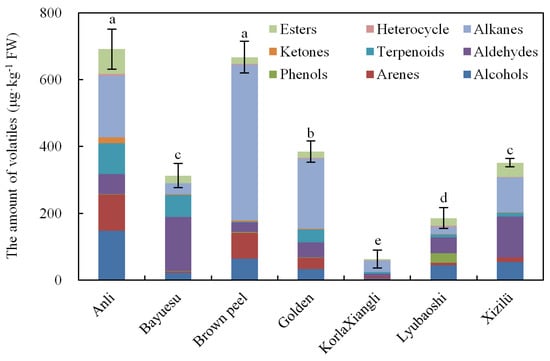
Figure 2.
The amount of different volatile chemical classes in flowers of seven pear cultivars. The error bars represent the standard deviation (n = 3). Different letters above the column indicate statistical differences between cultivars (p < 0.05), the same as below.
3.3. Odor Types, Description, and OAVs
The OAVs showed only a limited number of volatiles that could be detected at concentrations high enough to be perceived (OAV ≥ 1), which were considered as contributors responsible for the overall flower odor (Table 3). Due to the OAVs, 1-nonanal, 1-octanal, 1-decanal, phenethyl acetate, methyl 2-hydroxy-3-methyl pentanoate, and hexyl acetate were the key active odorants to the aroma of Anli flower; 1-octanal, 1-nonanal, 1-decanal, (E)-2-nonenal, 1-decanol were the most active odorants in Bayuesu and Xizilü flower; phenethyl acetate, 1-nonanal, 1-octanal, eugenol were the odor-active compounds to the aroma of Golden flower; 1-nonanal, phenethyl acetate, and phenyl acetaldehyde were the key contributors to Brown peel flower; 1-octanal, 1-nonanal, phenethyl acetate were the key contributors to KorlaXiangli flower; and 1-nonanal, phenethyl acetate, (E)-β-damascone were the active odorants in Lyubaoshi flower. To sum up, volatile aldehydes, particularly 1-octanal, 1-nonanal, 1decanal, and (E)-2-nonenal were present in higher amounts than their threshold and were considered the key factors to form the odor of pear flower. However, most esters and alcohols showed less importance due to their amounts being below the threshold.

Table 3.
Odor thresholds in water, odor descriptions, and OAVs of the studied aroma volatiles determined in pear flowers.
Based on odor description, eight main odor types were grouped (Table 3), described as fruity, aldehydic, green, floral, citrus, fatty, waxy, and pungent. Remarkably, several compounds had multiple flavors benefiting the flower’s fragrance. Total amounts of different odor types suggested, the floral, fruity, pungent, and green types showed most abundance in Anli, Golden, and Brown peel flowers; the aldehydic, waxy and floral types showed higher values in Bayuesu and Xizilü flowers; aldehydic type was present higher in Lyubaoshi, but all types were significantly lower in KorlaXiangli flowers (Figure 3). This finding suggested Bayuesu and Xizilü flowers had stronger aroma intensity of aldehydic and waxy, but the odor strength of KorlaXiangli was much weaker than the other cultivars.
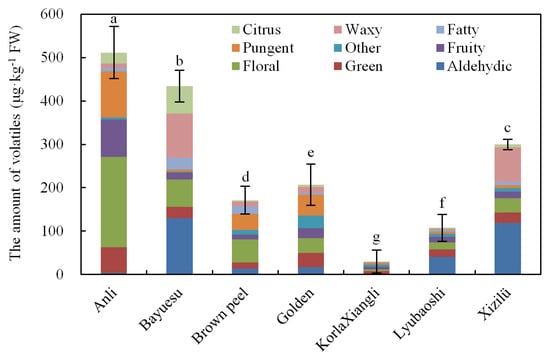
Figure 3.
The concentrations of different odor types in the flowers of seven pear cultivars.
3.4. Aroma Profiles Analysis
To visually and effectively explain the aroma features of pear flowers, the radar maps based on the accumulated OAVs were applied (Figure 4). Although most odor types were distributed in each cultivar, the radar maps indicated that the odor behaved significantly differently: Bayuesu flower was similar to Xizilü, which showed aldehydic, waxy, and fatty series were the predominant flavor characters, but the green odor was rich in Bayuesu; the profile of Brown peel flower was close to Golden flower and showed high intensity in aldehydic, waxy and fatty; and Lyubaoshi flower showed high abundance in aldehydic and fruity series.
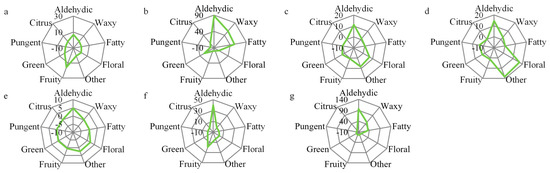
Figure 4.
Radar map of aroma series based on the accumulated OAVs. (a) Anli, (b) Bayuesu, (c) Brown peel, (d) Golden, (e) KorlaXiangli, (f) Lyubaoshi, and (g) Xizilü.
3.5. Multivariate Analysis
In this study, PCA was carried out using OAVs and samples as the variables, to obtain an overview of relationships among different cultivars in terms of their active odorants. As can be seen from Figure 5, the first principal component (PC1) and second principal component (PC2) were extracted, it was noted that the linear combination of PC1 and PC2 explained 62.1% of the total variance of pear flower samples, indicated that these two factors were considered sufficient for further discussion. The information on relationships between the active odorants and the PCs was provided according to the location of plotted data. As shown, the seven cultivars can be classified into four clusters: cultivars Golden and Brown peel flowers were group together in the first quadrant, the relevant odorants were naphthalene, β-ocimene, phenethyl acetate, α-farnesene, eugenol, 2-methyl naphthalene, and phenyl acetaldehyde; the second group contained Bayuesu and Xizilü flowers located in the left of the third quadrant, with high amounts of the chemicals 1-decanol, 1-heptanol, 1-undecanal, 1-octanal, 1-decanal, 1-nonanal, hexyl 2-methylbutyrate, (E)-citral, 1-octanol, 1-nonanol, (E)-2-nonenal, and linaloolhe as the relevant odorants, which meant that these cultivars were positive related with the aldehydic, waxy, and fatty series; KorlaXiangli and Lyubaoshi flower were clustered as the third group distributed mostly near the origin, revealed that the flowers were slightly in floral, pungent and green series; cultivars Anli flower situated individual in the lower right corner of the fourth quadrant clustered as the forth group, related to the typical chemicals of methyl 2-hydroxy-3-methyl pentanoate, (E)-geranyl acetone, 1-hexanol, 6-methyl-5-hepten-2-one, (Z)-3-hexenyl acetate, acetophenone, 2-phenylethanol, benzaldehyde, 2-pentyl-furan, and 2-phenylethyl formate, indicated this cultivar was rich in floral, fruity, and green odor.
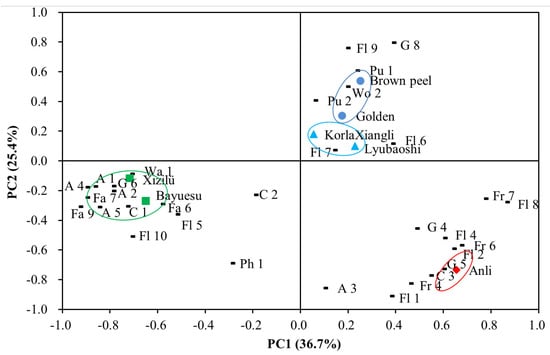
Figure 5.
PCA scores plots result using seven pear flower samples as score values and OAVs of selected odorants as loading values. The odorant names correspond to the codes of compounds listed in Table 3.
Application of HCA can interpret the results in an intuitive graphical way; cultivars in the same cluster displayed similar characters, whereas the samples in different clusters were relatively different. In this study, the OAV data were also subjected to HCA and used to explore the heterogeneity between cultivars (Figure 6). Results showed the aroma profiles of different pear flowers were appropriately divided into two main branches, then were again subdivided into three groups. According to the dendrogram and heatmap obtained, Bayuesu had the highest odor-active compound similarity to the Xizilü flower and these were grouped in one cluster, with Golden and Brown peel flowers grouped in a second cluster, and Lyubaoshi and KorlaXiangli flowers grouped in the third cluster. The observed clusters can be explained by the similar odor type of the identified odorants. HCA also showed the highest diversity among the Bayuesu and KorlaXiangli flowers due to the significant differences in the kinds and amounts of the identified volatiles. Futhermore, the results suggested that the aroma of Anli flower was distinct from the other six cultivars. These results were consistent with the PCA analysis, indicating HCA was a powerful tool to validate the rationality of the clustering and reveal the characteristics of each group [38].
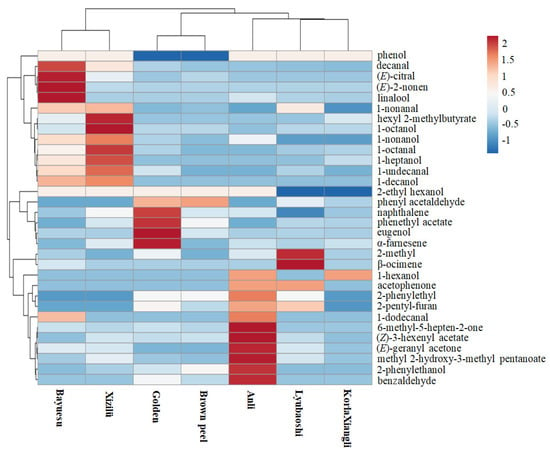
Figure 6.
Hierarchical clustering heatmap of selected odorants in flowers of seven pear cultivars. Each colored cell on the map corresponds to OAVs of the odorants, with the samples in the rows and compounds in the columns.
4. Discussion
In this study, ninety-three volatile compounds were identified from the flower of different pear cultivars. Of those ninety-three chemicals, esters, alcohols, aldehydes, alkanes, and terpenoids were the dominant volatiles but showed noticeable differences in their amounts among different cultivars. Overall, volatiles in all cultivars were not distributed uniformly. This phenomenon was consistent with the previous studies on citrus flowers [39], Watermelon Rind [40], Polianthes tuberosa L. flower [41], and peach [42].
The aroma characteristics showed pear flower odor was complicated, involving a series of odor types. In this study, several aldehydes, alcohols, and terpenoids were commonly found in each cultivar and presented an intense citrus odor like orange or lemon, and floral fragrance like rose or hyacinth [42]. Moreover, a certain amount of fatty and waxy odorants with oil-like or balsam-like were found in Bayuesu and Xizilü flowers and several C6 aldehydes, alcohols, and related esters were detected in Anli flowers known as green leaf volatiles (GLVs) [11]. However, not all volatile compounds observed in pear flowers actually affect the aroma [17,43]; alkanes, as the major components of plant wax, were ubiquitous in all pear cultivars, always had less contribution to aroma but more for the fragrance lasting [42]. Somewhat unexpectedy, α-pinene, γ-terpinene, d-camphor, and geraniol generally exist in the flowers of plants but were hardly recognized in this experiment [44,45].
OAV was the ratio of concentration to the sensory threshold for a particular volatile compound [42]. The specific intensity and tendency of active odorants, which could be obtained by combining OAV and odor descriptor [43], provided further information on their important role in flavor [46]. In this study, OAVs of related odorants were further calculated to evaluate the aroma attributes of pear flowers. The lower threshold values of phenethyl acetate, 1-octanal, 1-nonanal, methyl 2-hydroxy-3-methyl pentanoate, 1-decanal, phenyl acetaldehyde, 2-pentyl-furan, and eugenol, typically showed higher OAVs and can be considered as the primary contributors of pear flower, presenting strong citrus, floral, and waxy notes [32]. Furthermore, some other volatiles with high OAVs were also observed in special cultivars, such as hexyl acetate, (Z)-3-hexenyl acetate correlated with the fruity and green odor detected in Anli flower; (E)-2-nonenal, 1-decanol, linalool correlated with fatty and floral odor [47] in Bayuesu and Xizilü flower; phenyl acetaldehyde and eugenol correlated with hyacinth and clove flavor [23] in Golden and Brown peel flower; and (E)-β-damascone correlated with floral and fruity notes [47,48] was just observed in Lyubaoshi flower. To sum up, the pear flower samples were characterized mainly by fruity, aldehydic, green, floral, citrus, fatty, waxy, and pungent notes. As reported previously [45,49,50], the relationship between aroma components and people’s perception have always been a difficult and inadequately settled problem. For pear flowers, there is a lack of reference for evaluating the aromatic series and indicators for understanding the aroma characters. Still, in this study, there were obvious differences observed based on the aroma profiles conducted to compare the aroma attributes of different pear flowers. The aroma profiles indicated the major aroma characteristic between Bayuesu and Xizilü flower were much similar, with higher aldehydic, waxy, and fatty odor; the profile of Brown peel flowers were close to Golden pear, showing a high abundance in the series of aldehydic and floral flavor; and Lyubaoshi flower showed a high abundance of aldehydic and fruity series. These results further demonstrated that pear flowers mainly presented a sweet-orange or citrus-like flavor; these aroma descriptions were also consistent with the actual sensory characteristics. To the best of our knowledge, this was the first paper focused on preferred aromatic series and their components.
PCA is a multivariate technique used to analyze data in which observations were described by several inter-correlated quantitative dependent variables and samples [41]. HCA is suitable for classifying samples into different classes or clusters. Due to the significant similarities or differences in the quantities of the identified volatiles, Anli flower was discriminated from the others and existed as an independent category, indicating that the green leaf flavor was the preferred aromatic property; Bayuesu was similar to Xizilü flowers, discriminated from the others indicated that the citrus flavor was the preferred aroma correlated with the typical odorants of 1-octanal, 1-nonanal, 1-decanal with aldehydic odor [24,51]; in contrast, Bayuesu showed the highest diversity to KorlaXiangli flower because of the significant differences in the identified volatiles. It can be concluded that cluster analysis based on aroma components performed well in the discrimination of the flowers of different pear cultivars. To some extent, we also found the cluster results were consistent with the species difference (Table 1) and genetic relationship between cultivars. According to the obtained results, the PCA and HCA of volatile compounds can be successfully applied for the aroma evaluation of pear flowers, and the cultivars’ differences were critical for the overall pattern.
As reported, several key volatile chemical compositions had previously been found to be bio-activator to plant species [52,53]. Here, we showed that the pear flower contained phenolic compounds such as hydroquinone, and its derivatives were investigated for their most important antioxidant-active and skin depigment [3]; linalool can repel agricultural pests such as aphid Myzus persicae; the C6 and C9 aldehydes and alcohols such as 3-hexenol, 2-hexenal, 1-hexanol were produced by plants in response to the physical injury and played an important role in plants defense strategies and pest resistance;11 furthermore, eugenol exhibits an excellent bactericidal activity against a wide range of organisms [54]. In addition, volatiles of 1-octanal, 1-nonanal, 1-dodecanal, 1-undecanal, 1-decanal, (E)-β-damascone, α-farnesene, benzaldehyde, 6-methyl-5-hepten-2-one, 2-hexenal were commonly found in diverse plants, and it had been recognized that the biological behavior responded by the blend of volatiles rather than individual compound [55].
5. Conclusions
This study determined the volatile compounds in the flowers of seven pear cultivars and demonstrated evidence of differences were statistical significance between cultivars. Considering the lower threshold perception and higher OAVs, volatile aldehydes were identified as key compounds for the pear flower in most cultivars. Being the basic active odorants, aldehydes displayed an intensely citrus and floral note. For the overall aroma profiles, pear flowers were characterized mainly by aldehydic, waxy, floral, and pungent series, moreover, it was essential for the attraction of insect pollinators to ensure plant reproductive success. Combined the aroma profiles and multivariate analysis, a clear classification of pear flowers was carried out. In conclusion, Bayuesu flower was very similar to Xizilü and grouped together; Golden flower was close to Brown peel and grouped together; Bayuesu showed the highest diversity to KorlaXiangli flower; and Anli flower showed many special elements to the other cultivars. Here, a comprehensive evaluation was performed to indicate the odor characteristics and relationship among the flower samples. Overall, these results support an important step in determining the role of aroma components and evaluated the characteristic aroma of pear flower, which could contribute to the cultivars distinction and further utilization of pear flower.
Author Contributions
Conceptualization, X.L., J.W., H.W., K.Z. and F.S.; data curation, X.L., J.W. and F.S.; formal analysis, X.L. and J.W.; funding acquisition, J.W. and F.S.; investigation, X.L., J.W., K.Z. and F.S.; methodology, X.L., J.W. and F.S.; project administration, X.L., J.W. and F.S.; resources, X.L., J.W. and H.W.; software, X.L.; supervision, X.L., J.W. and F.S.; validation, X.L.; visualization, X.L., J.W. and H.W.; writing—original draft, X.L.; writing-review and editing, X.L., J.W., H.W., K.Z. and F.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Foundation for Advanced Talents of Beijing Technology and Business University, Grant Number 19008021176 and the Key Research and Development Program of Hebei Province, Grant Number 16226313D.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All results are included within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cui, T.; Nakamura, K.; Ma, L.; Li, J.Z.; Kayahara, H. Analyses of Arbutin and Chlorogenic Acid, the Major Phenolic Constituents in Oriental Pear. J. Agric. Food Chem. 2005, 53, 3882–3887. [Google Scholar] [CrossRef] [PubMed]
- Dimita, R.; Min Allah, S.; Luvisi, A.; Greco, D.; De Bellis, L.; Accogli, R.; Mininni, C.; Negro, C. Volatile Compounds and Total Phenolic Content of Perilla Frutescens at Microgreens and Mature Stages. Horticulturae 2022, 8, 71. [Google Scholar] [CrossRef]
- Rychlińska, I.; Gudej, J. Qualitative and quantitative chromatographic investigation of flavonoids in Pyrus communis L. flowers. Acta Pol. Pharm. 2003, 60, 81–85. [Google Scholar] [PubMed]
- Li, S. Bencao Gangmu; Renmin Weisheng Press: Beijing, China, 1982; p. 1756. [Google Scholar]
- Aires, C.P.; Koo, H.; Sassaki, G.L.; Iacomini, M.; Cury, J.A. A Procedure for Characterizing Glucans Synthesized by Purified Enzymes of Cariogenic Streptococcus mutans. Int. J. Biol. Macromol. 2010, 46, 551–554. [Google Scholar] [CrossRef]
- Pellegrino, G.; Bellusci, F.; Musacchio, A. The Effects of Inflorescence Size and Flower Position on Female Reproductive Success in Three Deceptive Orchids. Bot. Stud. 2010, 51, 351–356. [Google Scholar]
- Wetzstein, H.Y.; Yi, W.; Porter, J.A.; Ravid, N. Flower Position and Size Impact Ovule Number Per Flower, Fruitset, and Fruit Size in Pomegranate. J. Am. Soc. Hortic. Sci. 2013, 138, 159–166. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, Function and Metabolic Engineering of Plant Volatile Organic Compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Ceuppens, B.; Ameye, M.; Van Langenhove, H.; Roldan-Ruiz, I.; Smagghe, G. Characterization of Volatiles in Strawberry Varieties ‘Elsanta’and ‘Sonata’and Their Effect on Bumblebee Flower Visiting. Arthropod-Plant Interact. 2015, 9, 281–287. [Google Scholar] [CrossRef]
- Webster, B.; Gezan, S.; Bruce, T.; Hardie, J.; Pickett, J. Between Plant and Diurnal Variation in Quantities and Ratios of Volatile Compounds Emitted by Vicia Faba Plants. Phytochemistry 2010, 71, 81–89. [Google Scholar] [CrossRef]
- Xu, L.; Liu, H.; Ma, Y.; Wu, C.; Li, R.; Chao, Z. Comparative Study of Volatile Components from Male and Female Flower Buds of Populus × Tomentosa by HS-SPME-GC-MS. Nat. Prod. Res. 2019, 33, 2105–2108. [Google Scholar] [CrossRef]
- Junker, R.R.; Gershenzon, J.; Unsicker, S.B. Floral Odor Bouquet Loses Its Ant Repellent Properties after Inhibition of Terpene Biosynthesis. J. Chem. Ecol. 2011, 37, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Hammerbacher, A.; Coutinho, T.A.; Gershenzon, J. Roles of Plant Volatiles in Defence against Microbial Pathogens and Microbial Exploitation of Volatiles. Plant Cell Environ. 2019, 42, 2827–2843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Wang, W.; Zhao, L.; Zheng, C.; Ma, F. Changes in Volatile Organic Compounds and Differential Expression of Aroma-Related Genes During Flowering of Rosa Rugosa ‘Shanxian’. Hortic. Environ. Biotechnol. 2019, 60, 741–751. [Google Scholar] [CrossRef]
- Xu, C.; Ma, Y.; Tian, Z.; Luo, Q.; Zheng, T.; Wang, B.; Zuo, Z. Monoterpene Emissions and Their Protection Effects on Adult Cinnamomum Camphora against High Temperature. Trees 2022, 36, 711–721. [Google Scholar] [CrossRef]
- Mochizuki, T.; Watanabe, M.; Koike, T.; Tani, A. Monoterpene Emissions from Needles of Hybrid Larch F1 (Larix Gmelinii Var. Japonica × larix Kaempferi) Grown under Elevated Carbon Dioxide and Ozone. Atmos. Environ. 2017, 148, 197–202. [Google Scholar] [CrossRef]
- Yan, J.W.; Ban, Z.J.; Lu, H.Y.; Li, D.; Poverenov, E.; Luo, Z.S.; Li, L. The Aroma Volatile Repertoire in Strawberry Fruit: A Review. J. Sci. Food Agric. 2018, 98, 4395–4402. [Google Scholar] [CrossRef]
- Mus, A.A.; Gansau, J.A.; Kumar, V.S.; Rusdi, N.A. The Variation of Volatile Compounds Emitted from Aromatic Orchid (Phalaenopsis Bellina) at Different Timing and Flowering Stages. Plant Omics 2020, 13, 78–85. [Google Scholar] [CrossRef]
- Du, F.; Wang, T.; Fan, J.M.; Liu, Z.Z.; Zong, J.X.; Fan, W.X.; Han, Y.H.; Grierson, D. Volatile Composition and Classification of Lilium Flower Aroma Types and Identification, Polymorphisms, and Alternative Splicing of Their Monoterpene Synthase Genes. Hortic. Res. 2019, 6, 110. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Xu, Y.; Niu, Q.; He, L.; Teng, Y.; Bai, S. Abscisic Acid (ABA) Promotes the Induction and Maintenance of Pear (Pyrus Pyrifolia White Pear Group) Flower Bud Endodormancy. Int. J. Mol. Sci. 2018, 19, 310. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, J.; Fan, Y.; Zexin, M.A.; Jiang, L.I. Analysis of Aroma Components of Pyrus Sinkiangensis Yü. Cv. ‘Korla Xiangli’ Inflorescence by Headspace Solid Phase Micro-Extraction Coupled to Gas Chromatography-Mass Spectrometry. Food Sci. 2016, 37, 115–120. [Google Scholar] [CrossRef]
- Lin, X. Perfumery, 2nd ed.; Chemical Industry Press: Beijing, China, 2011. [Google Scholar]
- Pino, J.A.; Mesa, J. Contribution of Volatile Compounds to Mango (Mangifera Indica, L.) Aroma. Flavour. Frag. J. 2006, 21, 207–213. [Google Scholar] [CrossRef]
- Averbeck, M.; Schieberle, P. Influence of Different Storage Conditions on Changes in the Key Aroma Compounds of Orange Juice Reconstituted from Concentrate. Eur. Food Res. Technol. 2011, 232, 129–142. [Google Scholar] [CrossRef]
- Muhlemann, J.K.; Hiroshi, M.; Chang, C.Y.; Phillip, S.M.; Ivan, B.; Bruce, C.; Ann, P.M.; Nikolau, B.J.; Olga, V.; Morgan, J.A. Developmental Changes in the Metabolic Network of Snapdragon Flowers. PLoS ONE 2012, 7, e40381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boonbumrung, S.; Tamura, H.; Mookdasanit, J.; Nakamoto, H.; Ishihara, S.; Yoshizawa, T.; Varanyanond, W. Characteristic Aroma Components of the Volatile Oil of Yellow Keaw Mango Fruits Determined by Limited Odor Unit Method. Food Sci. Technol. Res. 2001, 7, 200–206. [Google Scholar] [CrossRef] [Green Version]
- Limpawatanna, M. An Integrated Approach to Sensory Analysis of Rice Flavor; University of Georgia: Athens, GA, USA, 2007. [Google Scholar]
- Jin, Q.L.; Jiang, Z.D.; Ni, H.; Chen, F.; Huang, G.L.; Yang, Y.F. Volatile Components of Instant Oolong Tea Powder. Mod. Food Sci. Technol. 2015, 31, 372–379. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Buttery, R.G.; Turnbaugh, J.G.; Benson, M. Odor Thresholds of Various Branched Esters. LWT-Food Sci. Technol. 1995, 28, 153–156. [Google Scholar] [CrossRef]
- Pyysalo, T.; Honkanen, E.; Hirvi, T. Volatiles of Wild Strawberries, Fragaria Vesca, L., Compared to Those of Cultivated Berries, Fragaria × Ananassa Cv. Senga Sengana. J. Agric. Food Chem. 1979, 27, 19–22. [Google Scholar] [CrossRef]
- Aaby, K.; Haffner, K.; Skrede, G. Aroma Quality of Gravenstein Apples Influenced by Regular and Controlled Atmosphere Storage. LWT Food Sci. Technol. 2002, 35, 254–259. [Google Scholar] [CrossRef]
- Du, X.; Finn, C.E.; Qian, M.C. Volatile Composition and Odour-Activity Value of Thornless ‘Black Diamond’ and ‘Marion’ Blackberries. Food Chem. 2010, 119, 1127–1134. [Google Scholar] [CrossRef]
- Sinuco, D.C.; Steinhaus, M.; Schieberle, P.; Osorio, C. Changes in Odour-Active Compounds of Two Varieties of Colombian Guava (Psidium guajava, L.) During Ripening. Eur. Food Res. Technol. 2010, 230, 859–864. [Google Scholar] [CrossRef]
- Feng, Y.; Su, G.; Zhao, H.; Cai, Y.; Cui, C.; Sun-Waterhouse, D.; Zhao, M. Characterisation of Aroma Profiles of Commercial Soy Sauce by Odour Activity Value and Omission Test. Food Chem. 2015, 167, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Dietz, F.; Traud, J. Smell and Taste Threshold Concentrations of Phenolic Substances. Its Relation among Each Other and in Comparison with the Instrumental Analysis as Well as with the Acute Fish Toxicity. Gas. Wasser Abwasser 1978, 119, 318–325. [Google Scholar]
- Aznar, M.; López, R.; Cacho, J.; Ferreira, V. Prediction of Aged Red Wine Aroma Properties from Aroma Chemical Composition. Partial Least Squares Regression Models. J. Agric. Food Chem. 2003, 51, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Padrayuttawat, A.; Tokunaga, T. Seasonal Change of Volatile Compounds of Citrus Sudachi During Maturation. Food Sci. Technol. Res. 2006, 5, 156–160. [Google Scholar] [CrossRef] [Green Version]
- Khalil, M.N.; Fekry, M.I.; Farag, M.A. Metabolome Based Volatiles Profiling in 13 Date Palm Fruit Varieties from Egypt via Spme GC–MS and Chemometrics. Food Chem. 2017, 217, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.; Song, M.; Fan, F.; Zhang, B.; Xu, Y.; Xu, C.; Chen, K. Comparative Analysis of Flower Volatiles from Nine Citrus at Three Blooming Stages. Int. J. Mol. Sci. 2013, 14, 22346–22367. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Ramirez, J. Watermelon Rind and Flesh Volatile Profiles and Cultivar Difference. Horticulturae 2022, 8, 99. [Google Scholar] [CrossRef]
- Kutty, N.N.; Mitra, A. Profiling of Volatile and Non-Volatile Metabolites in Polianthes Tuberosa, L. Flowers Reveals Intraspecific Variation among Cultivars. Phytochemistry 2019, 162, 10–20. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Ma, R.; Yu, M. Comparison of Aroma Trait of the White-Fleshed Peach ‘Hu Jing Mi Lu’ and the Yellow-Fleshed Peach ‘Jin Yuan’ Based on Odor Activity Value and Odor Characteristics. Horticulturae 2022, 8, 245. [Google Scholar] [CrossRef]
- Wu, Y.; Duan, S.; Zhao, L.; Gao, Z.; Luo, M.; Song, S.; Xu, W.; Zhang, C.; Ma, C.; Wang, S. Aroma Characterization Based on Aromatic Series Analysis in Table Grapes. Sci. Rep. 2016, 6, 31116. [Google Scholar] [CrossRef] [Green Version]
- Xin, H.P.; Wu, B.H.; Zhang, H.H.; Wang, C.Y.; Li, J.T.; Yang, B.; Li, S.H. Characterization of Volatile Compounds in Flowers from Four Groups of Sweet Osmanthus (Osmanthus Fragrans) Cultivars. Can. J. Plant. Sci. 2013, 93, 923–931. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.C.; Yin, Z.H.; Kang, W.Y. Study of Volatiles in Lysimachia Parvifolia Flower Using HS-SPME-GC-MS. Chem. Nat. Compd. 2014, 50, 1130–1131. [Google Scholar] [CrossRef]
- Giri, A.; Osako, K.; Ohshima, T. Identification and Characterisation of Headspace Volatiles of Fish Miso, a Japanese Fish Meat Based Fermented Paste, with Special Emphasis on Effect of Fish Species and Meat Washing. Food Chem. 2010, 120, 621–631. [Google Scholar] [CrossRef]
- Yao, H.; Jin, X.; Feng, M.; Xu, G.; Zhang, P.; Fang, Y.; Xu, T.; Meng, J. Evolution of Volatile Profile and Aroma Potential of Table Grape Hutai-8 During Berry Ripening. Food Res. Int. 2021, 143, 110330. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Tong, G.; Yang, Q.; Huang, M.; Ye, H.; Liu, Y.; Wu, J.; Zhang, J.; Sun, X.; Zhao, D. Characterization of Key Aroma Compounds in Tartary Buckwheat (Fagopyrum Tataricum Gaertn.) by Means of Sensory-Directed Flavor Analysis. J. Agric. Food Chem. 2021, 69, 11361–11371. [Google Scholar] [CrossRef]
- Ni, R.; Michalski, M.H.; Brown, E.; Doan, N.; Zinter, J.; Ouellette, N.T.; Shepherd, G.M. Optimal Directional Volatile Transport in Retronasal Olfaction. Proc. Natl. Acad. Sci. USA 2015, 112, 14700–14704. [Google Scholar] [CrossRef] [Green Version]
- Tieman, D.; Bliss, P.; McIntyre, L.M.; Blandon-Ubeda, A.; Bies, D.; Odabasi, A.Z.; Rodríguez, G.R.; van der Knaap, E.; Taylor, M.G.; Goulet, C.; et al. The Chemical Interactions Underlying Tomato Flavor Preferences. Curr. Biol. 2012, 22, 1035–1039. [Google Scholar] [CrossRef] [Green Version]
- Shen, D.Y.; Li, M.K.; Song, H.L.; Zou, T.T.; Zhang, L.; Xiong, J. Characterization of Aroma in Response Surface Optimized No-Salt Bovine Bone Protein Extract by Switchable GC/GC×GC-Olfactometry-Mass Spectrometry, Electronic Nose, and Sensory Evaluation. LWT 2021, 147, 111559. [Google Scholar] [CrossRef]
- Pérez-Hedo, M.; Rambla, J.L.; Granell, A.; Urbaneja, A. Biological Activity and Specificity of Miridae-Induced Plant Volatiles. BioControl 2018, 63, 203–213. [Google Scholar] [CrossRef]
- Hori, M.; Namatame, M. Host Plant Volatiles Responsible for the Invasion of S Tenotus Rubrovittatus (H Eteroptera: M Iridae) into Paddy Fields. J. Appl. Entomol. 2013, 137, 340–346. [Google Scholar] [CrossRef]
- Hu, Q.; Zhou, M.; Wei, S. Progress on the Antimicrobial Activity Research of Clove Oil and Eugenol in the Food Antisepsis Field. J. Food Sci. 2018, 83, 1476–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šimpraga, M.; Takabayashi, J.; Holopainen, J.K. Language of Plants: Where Is the Word? J. Integr. Plant Biol. 2016, 58, 343–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).