Plant Growth and Chemical Properties of Commercial Biochar- versus Peat-Based Growing Media
Abstract
:1. Introduction
2. Material and Methods
3. Results
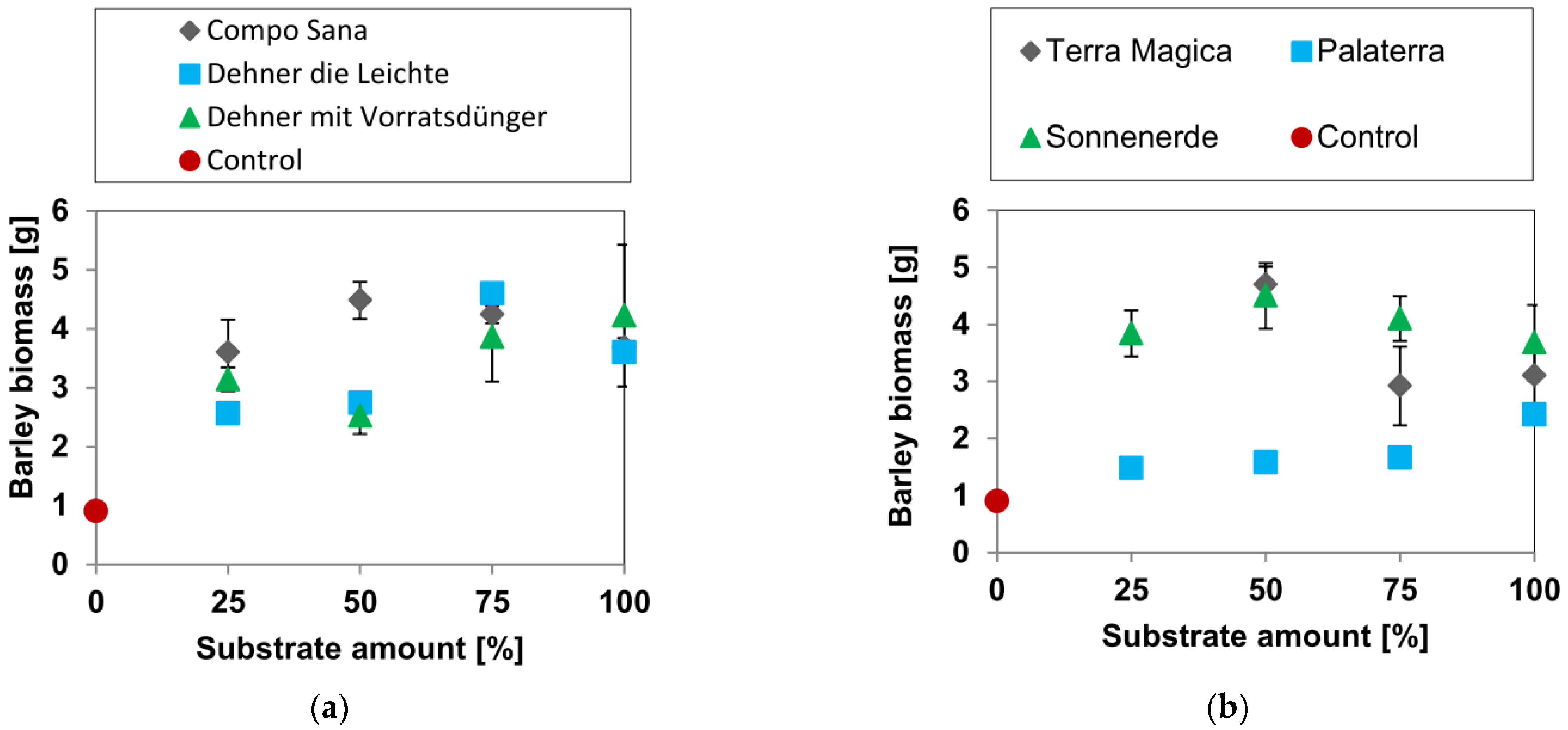
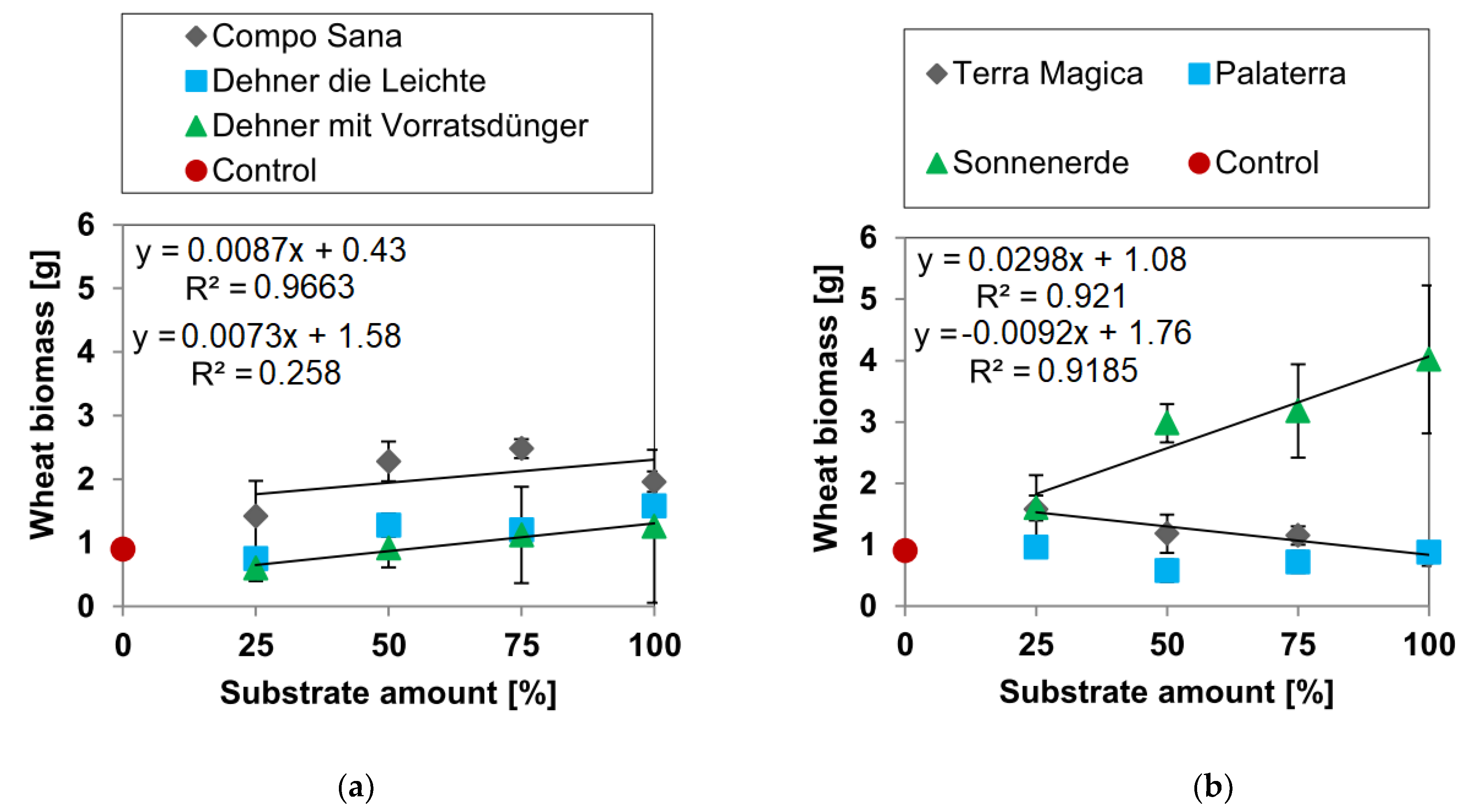
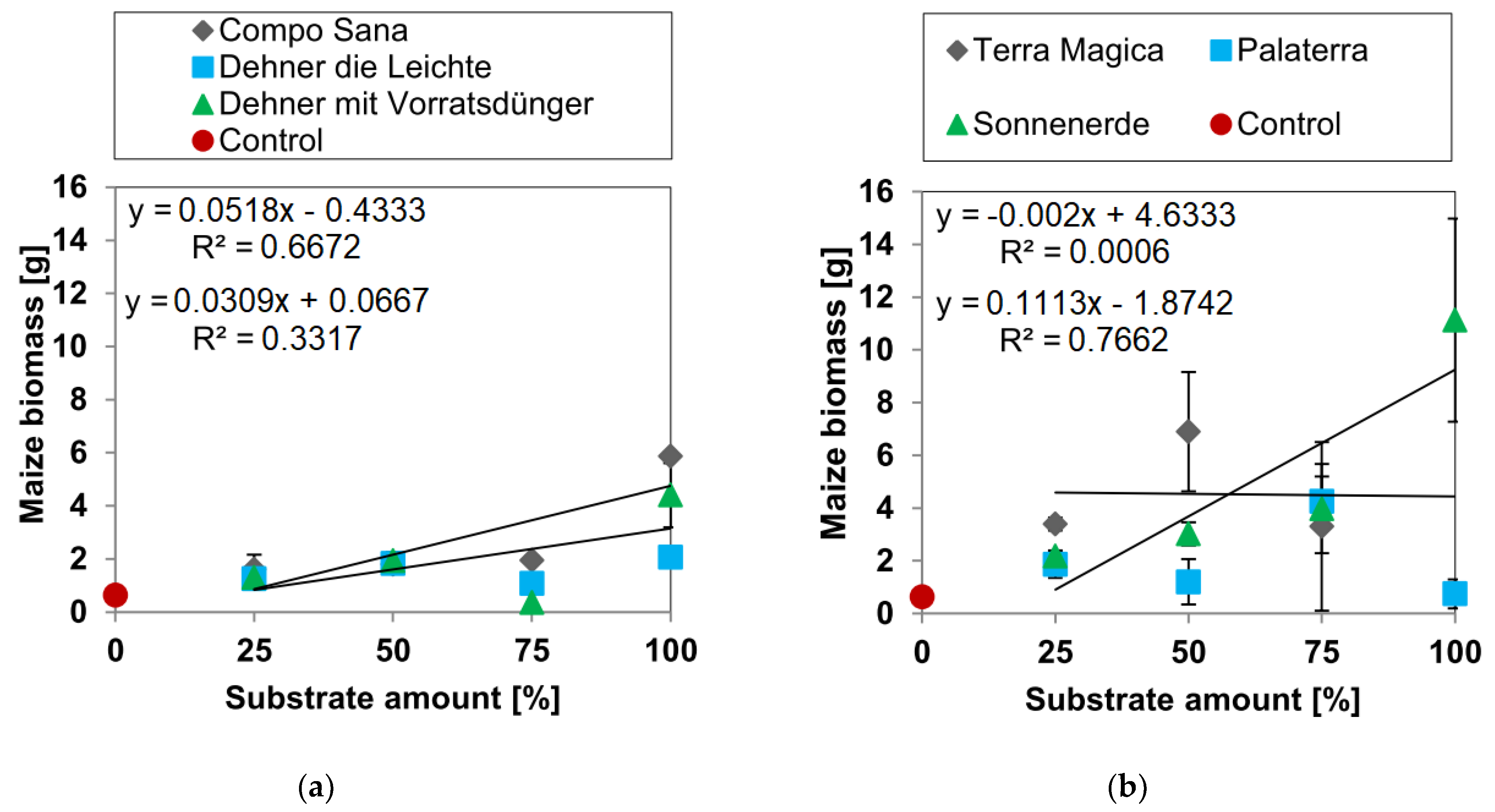
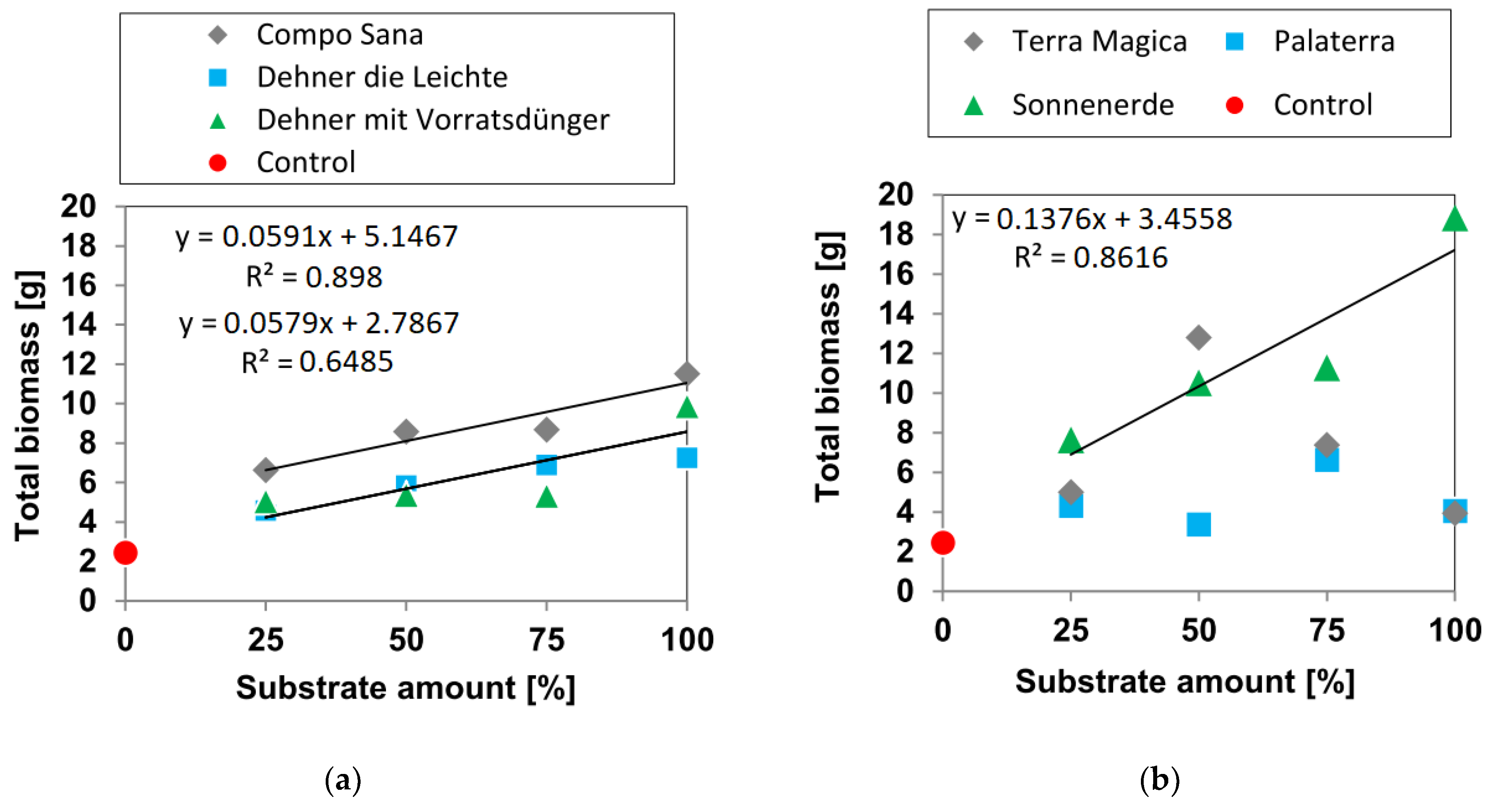
3.1. Biomass Yields
3.1.1. Barley
3.1.2. Wheat
3.1.3. Maize
3.1.4. Total Biomass Yield
3.2. Plant-Available Nutrient Stocks and Nutrient Retention
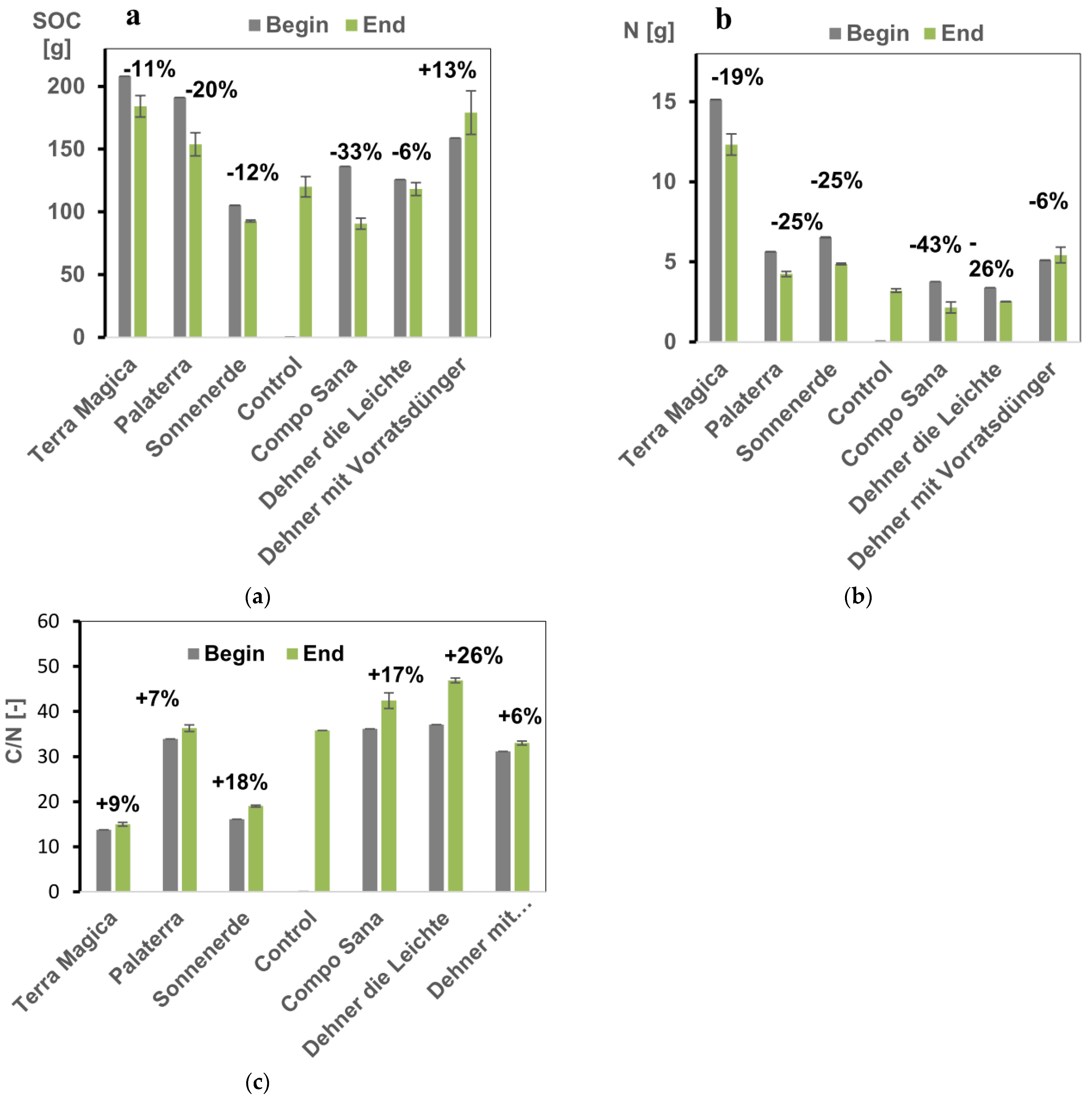
3.3. Soil Organic Matter Stability and Quality
3.4. Polycondensed Aromatic Carbon (Polycyclic Aromatic Hydrocarbons and Black Carbon)
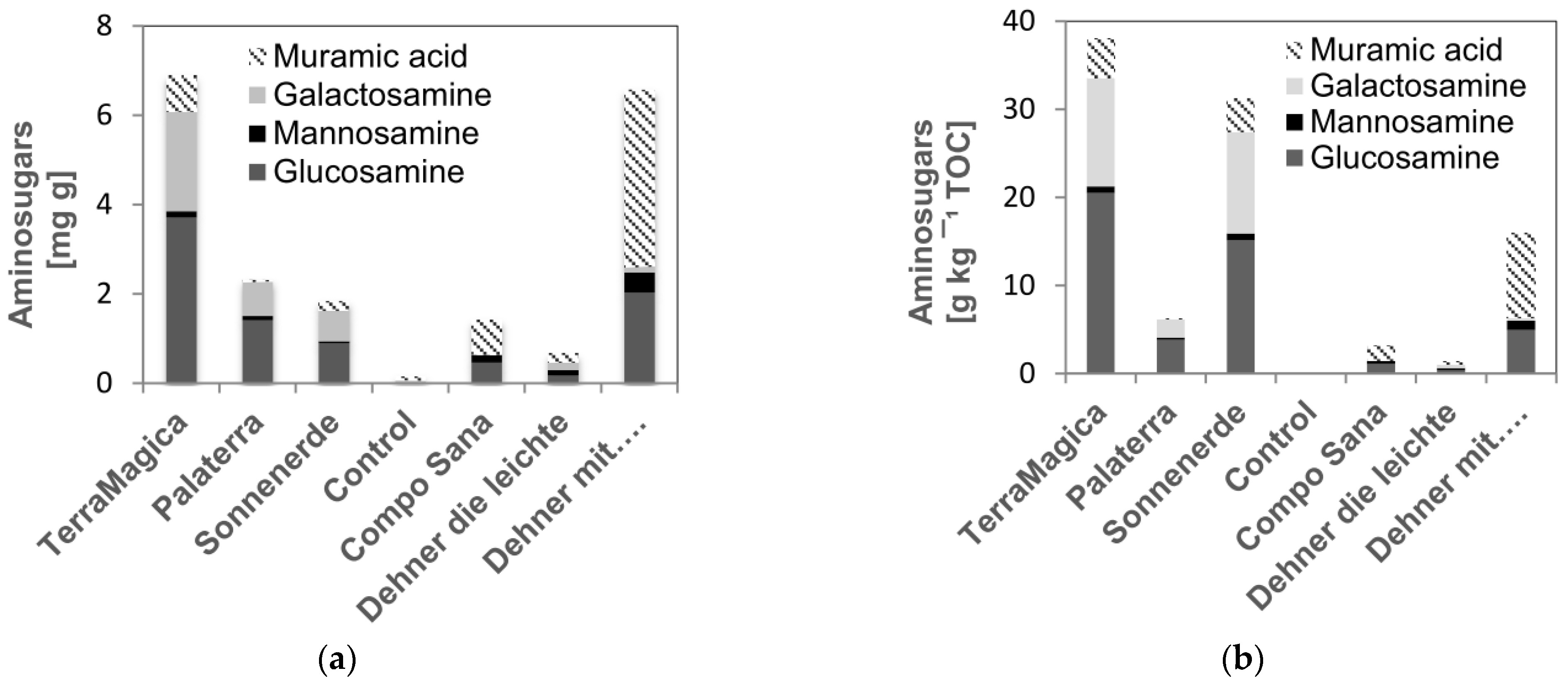
3.5. Microbial Residues (Amino Sugars)
4. Discussion
4.1. Biochar Effect
4.2. Bacterial and Fungal Residues/Activities
4.3. Dilution Effect and Long-Term Stability of Growing Media
5. Conclusions
- (i)
- After three growing cycles, the nutrient leaching is comparable in both biochar- and peat-based growing media. The only difference is that biochar-based growing media loose more calcium, while peat-based growing media loose more potassium.
- (ii)
- In Sonnenerde and all peat-based growing media, there was a clear amount yield relation when mixed with sand. All other growing media did not show such a trend when diluted with sand but their yield was not significantly higher than pure sand Palaterra had a negative amount yield relation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kern, J.; Tammeorg, P.; Shanskiy, M.; Sakrabani, R.; Knicker, H.; Kammann, C.; Tuhkanen, E.M.; Smidt, G.; Prasad, M.; Tiilikkala, K.; et al. Synergistic use of peat and charred material in growing media–an option to reduce the pressure on peatlands? J. Environ. Eng. Landsc. Manag. 2017, 25, 160–174. [Google Scholar] [CrossRef]
- Christoph, S.; Harttung, T. Biochar as a growing media additive and peat substitute. Solid Earth 2014, 5, 995–999. [Google Scholar]
- Olsson, L.; Barbosa, H.; Bhadwal, S.; Cowie, A.; Delusca, K.; Flores-Renteria, D.; Hermans, K.; Jobbagy, E.; Kurz, W.; Li, D.; et al. Climate Change and Land. Chapter 4: Land Degradation. 2019. Available online: https://www.ipcc.ch/site/assets/uploads/2019/08/2e.-Chapter-4_FINAL.pdf (accessed on 12 December 2021).
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Gross, A.; Bromm, T.; Glaser, B. Soil Organic Carbon Sequestration after Biochar Application: A Global Meta-Analysis. Agronomy 2021, 11, 2474. [Google Scholar] [CrossRef]
- Glaser, B.; Haumaier, L.; Guggenberger, G.; Zech, W. Black carbon in soils: The use of benzenecarboxylic acids as specific markers. Org. Geochem. 1998, 29, 811–819. [Google Scholar] [CrossRef]
- Brodowski, S.; Rodionov, A.; Haumaier, L.; Glaser, B.; Amelung, W. Revised black carbon assessment using benzene polycarboxylic acids. Org. Geochem. 2005, 36, 1299–1310. [Google Scholar] [CrossRef]
- Zhang, X.; Amelung, W. Gas chromatograph1c determination of muramic acid, glucosamine, mannosamine, and galactosamine in soils. Soil Biol. Biochem. 1996, 28, 1201–1206. [Google Scholar] [CrossRef]
- Glaser, B.; Birk, J.J. State of the scientific knowledge on properties and genesis of Anthropogenic Dark Earths in Central Amazonia (terra preta de índio). Geochim. Cosmochim. Acta 2012, 82, 39–51. [Google Scholar] [CrossRef]
- Glaser, B.; Haumaier, L.; Guggenberger, G.; Zech, W. The “Terra Preta” phenomenon: A model for sustainable agriculture in the humid tropics. Naturwissenschaften 2001, 88, 37–41. [Google Scholar] [CrossRef]
- Rodionov, A.; Amelung, W.; Peinemann, N.; Haumaier, L.; Zhang, X.; Kleber, M.; Glaser, B.; Urusevskaya, I.; Zech, W. Black carbon in grassland ecosystems of the world. Global Biogeochem. Cycles 2010, 24, 1–15. [Google Scholar] [CrossRef]
- Puglisi, E.; Zaccone, C.; Cappa, F.; Cocconcelli, P.S.; Shotyk, W.; Trevisan, M.; Miano, T.M. Changes in bacterial and archaeal community assemblages along an ombrotrophic peat bog profile. Biol. Fertil. Soils 2014, 50, 815–826. [Google Scholar] [CrossRef]
- Rein, G.; Hadden, R.; Zarccone, C. From organic matter to pyrogenic char to ash: The role of smouldering combustion in wildfires. EGU Gen. Assem. Conf. Abstr. 2012, 14, 12040. [Google Scholar]
- Hope, G.; Chokkalingam, U.; Anwar, S. The stratigraphy and fire history of the Kutai Peatlands, Kalimantan, Indonesia. Quat. Res. 2005, 64, 407–417. [Google Scholar] [CrossRef]
- Hartman, B.E.; Chen, H.; Hatcher, P.G. A non-thermogenic source of black carbon in peat and coal. Int. J. Coal Geol. 2015, 144–145, 15–22. [Google Scholar] [CrossRef]
- Glaser, B.; Knorr, K.-H.H. Isotopic evidence for condensed aromatics from non-pyrogenic sources in soils—Implications for current methods for quantifying soil black carbon. Rapid Commun. Mass Spectrom. 2008, 22, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Brodowski, S.; Amelung, W.; Haumaier, L.; Zech, W. Black carbon contribution to stable humus in German arable soils. Geoderma 2007, 139, 220–228. [Google Scholar] [CrossRef]
- Glaser, B.; Turrión, M.B.; Alef, K. Amino sugars and muramic acid—Biomarkers for soil microbial community structure analysis. Soil Biol. Biochem. 2004, 36, 399–407. [Google Scholar] [CrossRef]
- Amelung, W.; Miltner, A.; Zhang, X.; Zech, W. Fate of microbial residues during litter decomposition as affected by minerals. Soil Sci. 2001, 166, 598–606. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, J.; Da Silva, J.P.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Steiner, C.; Teixeira, W.G.; Lehmann, J.; Nehls, T.; De MacÊdo, J.L.V.; Blum, W.E.H.; Zech, W. Long term effects of manure, charcoal and mineral fertilization on crop production and fertility on a highly weathered Central Amazonian upland soil. Plant Soil 2007, 291, 275–290. [Google Scholar] [CrossRef] [Green Version]
- Borchard, N.; Siemens, J.; Ladd, B.; Möller, A.; Amelung, W. Application of biochars to sandy and silty soil failed to increase maize yield under common agricultural practice. Soil Tillage Res. 2014, 144, 184–194. [Google Scholar] [CrossRef]
- Jeffery, S.; van der Velde, M.; Bastos, A.C. Agronomic impact of biochar in soils: A meta-analysis Biochar and Crop Productivity. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Steiner, C.; Teixeira, W.G.; Lehmann, J.; Zech, W. Microbial Response to Charcoal Amendments of Highly Weathered Soils and Amazonian Dark Earths in Central Amazonia—Preliminary Results. Amaz. Dark Earths Explor. Sp. Time 2004, 195–212. [Google Scholar] [CrossRef]
- Birk, J.J.; Steiner, C.; Teixiera, W.C.; Zech, W.; Glaser, B. Microbial response to charcoal amendments and fertilization of a highly weathered tropical soil. In Amazonian Dark Earths: Wim Sombroek’s Vision; Springer: Berlin/Heidelberg, Germany, 2009; pp. 309–324. ISBN 9781402090301. [Google Scholar]
- Fischer, D.; Erben, G.; Dunst, G.; Glaser, B. Dynamics of labile and stable carbon and priming effects during composting of sludge and lop mixtures amended with low and high amounts of biochar. Waste Manag. 2018, 78, 880–893. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Bogomolova, I.; Glaser, B. Biochar stability in soil: Decomposition during eight years and transformation as assessed by compound-specific 14C analysis. Soil Biol. Biochem. 2014, 70, 229–236. [Google Scholar] [CrossRef]
- Amlinger, F.; Peyr, S.; Geszti, J.; Dreher, P.; Karlheinz, W.; Nortcliff, S. Beneficial Effects of Compost Application on Fertility and Productivity of Soils; Federal Ministry for Agriculture and Forestry, Environment and Water Management: Vienna, Austria, 2007; p. 225. [Google Scholar]
- Parvage, M.M.; Ulén, B.; Eriksson, J.; Strock, J.; Kirchmann, H. Phosphorus availability in soils amended with wheat residue char. Biol. Fertil. Soils 2013, 49, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Borchard, N.; Schirrmann, M.; Cayuela, M.L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizábal, T.; Sigua, G.; Spokas, K.; Ippolito, J.A.; et al. Biochar, soil and land-use interactions that reduce nitrate leaching and N2O emissions: A meta-analysis. Sci. Total Environ. 2019, 651, 2354–2364. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Liu, B.; Amonette, J.E.; Lin, Z.; Liu, G.; Ambus, P.; Xie, Z. How does biochar influence soil N cycle? A meta-analysis. Plant Soil 2018, 426, 211–225. [Google Scholar] [CrossRef]

| Commercial Growing Media | Ingredients | Production Process |
|---|---|---|
| Peat-Based | ||
| Compo Sana | 95% Bog peat (H2 - H7) 1, NPK fertilizer, wetting agent, perlite, lime, phosphate with silica (Agrosil) | Only mixing |
| Dehner die Leichte | 98% Bog peat (H2 - H7) 1, NPK fertilizer | Only mixing |
| Dehner mit Vorratsdünger | 92% Bog peat (H3 - H7) 1, NPK fertilizer, perlite, lime seabird guano (0.07%) | Only mixing |
| Biochar-Based | ||
| Palaterra | Green waste compost, wood fibre, bark humus, biochar, bentonite, basalt, bugle fertilizer, microorganism, fungi | Aerobic rotting, fermentation 1, fermentation 2 |
| Sonnenerde | Green waste compost, fruit waste, rock flour, biochar, mycorrhiza, N-binding bacteria | Aerobic composting |
| Terra Magica | Biomass, biochar, microorganisms rock flour, | Aerobic composting |
| Commercial Growing Media | PAH 1 [mg kg−1] | Black Carbon 2 [g kg−1] | Black Carbon 3 [g kg−1 TOC] | B6CA/BPCA 4 [%] |
|---|---|---|---|---|
| Peat-Based | ||||
| Compo Sana | 0.07 | 20.1 | 46 | 38 |
| Dehner die Leichte | 0.23 | 27.9 | 60 | 35 |
| Dehner mit Vorratsdünger | BDL 5 | 27.2 | 66 | 35 |
| Biochar-Based | ||||
| Palaterra | 0.08 | 46.6 | 126 | 45 |
| Sonnenerde | 0.24 | 7.3 | 123 | 37 |
| Terra Magica | 0.44 | 19.6 | 108 | 43 |
| Growing Media | GlcN/MurAc 1 | GalN/MurAc 2 |
|---|---|---|
| Compo Sana | 0.58 | 0.01 |
| Dehner die Leichte | 0.79 | 0.72 |
| Dehner mit Vorratsdünger | 0.51 | 0.03 |
| Palaterra | 24.30 | 13.01 |
| Sonnenerde | 3.95 | 3.01 |
| Terra Magica | 4.58 | 2.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glaser, B.; Asomah, A.A.A. Plant Growth and Chemical Properties of Commercial Biochar- versus Peat-Based Growing Media. Horticulturae 2022, 8, 339. https://doi.org/10.3390/horticulturae8040339
Glaser B, Asomah AAA. Plant Growth and Chemical Properties of Commercial Biochar- versus Peat-Based Growing Media. Horticulturae. 2022; 8(4):339. https://doi.org/10.3390/horticulturae8040339
Chicago/Turabian StyleGlaser, Bruno, and Angela Amma Asieduaa Asomah. 2022. "Plant Growth and Chemical Properties of Commercial Biochar- versus Peat-Based Growing Media" Horticulturae 8, no. 4: 339. https://doi.org/10.3390/horticulturae8040339
APA StyleGlaser, B., & Asomah, A. A. A. (2022). Plant Growth and Chemical Properties of Commercial Biochar- versus Peat-Based Growing Media. Horticulturae, 8(4), 339. https://doi.org/10.3390/horticulturae8040339







