Abstract
The objective of this study was to determine the efficiency of balloon flower sprout’s saponin production in a plant factory with artificial lighting (PFAL). Balloon flower has been traditionally used as herbal medicine and now, it is used as a medicinal plant as well as a functional food. It is important to establish the cultivation conditions for the stable production of high-quality balloon flower. Therefore, this study aimed to investigate the effects of culture systems and temperature conditions on the growth and saponin accumulation of balloon flower sprouts in controlled environment systems. One-year balloon flower roots were cultivated in soil and soilless culture systems at different temperature conditions (20, 25, and 30 °C) for 17 days. The results showed that the shoot fresh weight and shoot dry weight of the balloon flower sprouts grown in the soilless culture system at 25 °C were significantly increased by about 1.29 and 1.58 times, respectively, as compared with those of the sprouts grown in a soil culture system. Sprouts grown in the soilless culture system at 25 °C also recorded the highest root fresh weight, whereas there was no significant difference in root dry weight among the treatments. The plant height results showed an increased trend similar to that of the shoot fresh weight and shoot dry weight of the balloon flower sprouts. The concentrations of platycodin D3 (Pd-D3), polygalcin D (Pc-D), and total saponin in the shoot parts were highest in the soilless culture system at 20 and 25 °C. The root parts of sprouts grown in the soilless culture system at 30 °C also had higher deapioplatycodin D (Dpd-D) and total saponin concentrations. Overall, these results suggest that a soilless culture system with temperature conditions at 20 and 25 °C is suitable for improving the growth and saponin concentration of balloon flower cultivated in PFALs. Ultimately, our research should be a valuable resource for future research on the production of medicinal plants such as sprouts and should provide basic information to establish methods for enhancing the growth and bioactive compounds in balloon flower.
1. Introduction
Balloon flower (Platycodon grandiflorum A. DC) is a perennial herb that belongs to the family Campanulaceae. P. grandiflorum A. DC is a rich source of bioactive compounds that are beneficial to health, such as flavonoids, phenolic acids, triterpenoid saponins, polyacetylene, and sterols [1]. Certain triterpenoid saponins (platycodin D; PD, polyglacin D; Pc-D, and platycodin D3; Pd-D3) are the major components of balloon flower and have various pharmacological activities and health benefits [2,3]. Balloon flower has been traditionally used as herbal medicine for treating various diseases, such as cough, excessive phlegm, sore throat, hyperlipidemia, hypertension, and diabetes [4]. Many studies have recently focused on investigating its biological activities, including antitumor, hepatoprotective, immunoregulatory, and antioxidant effects [5,6,7]. Further, the seedlings and roots of balloon flower have also been consumed as functional foods, and they have a great demand in broad markets in China, Japan, and Korea. With the recent increase in people’s awareness of the importance of nutritious and healthy foods for their health, balloon flower has been widely used as a medicinal health supplement and functional food, as well as for its dietary aspects and cosmetic components. The consumer demand as well as export of the balloon flower plant has sharply increased in many countries, and its price has significantly increased, resulting in high economic benefits, especially for farmers. Due to its huge development value and prospects for more applications in the coming years and the overexploitation of balloon flowers in the wild for industry applications, the natural resources of wild balloon flower are at risk of being gradually exhausted to meet society’s needs. Therefore, there is a need to manipulate modern cultivation methods in some controlled environment systems in order to ensure stable production and to improve the quality of balloon flower sprouts.
Plant factories with artificial lighting (PFALs), also called indoor farms or vertical farms, are one of the closed plant production systems that are attracting the interest of farmers and the food industry, as they can provide plant products with uniform quality year-round production and desired characteristics (taste, shape, and safety) [8]. The benefits of PFALs include high resource use efficiency, high annual productivity per unit land area, and production of high-quality plants without using pesticides [8]. By controlling the internal environment conditions, PFALs can be utilized for high-speed production of valuable plant products, which can be about two to four times faster than outdoor cultivation. Moreover, a multiple-shelf system can be used for cultivation in PFALs, facilitating the mass production of vegetables in a small space. Most PFALs have been applied to the commercial production of functional and leafy vegetables, such as lettuce, rocket salad, kale, and basil. However, the main disadvantage of PFALs is their high operation and management costs, which lead to increased production costs and low application of the output of low-value leafy vegetables. Therefore, the application of PFALs for the cultivation of functional vegetables and medicinal plants is one of the main strategies for ensuring the economic value of PFALs. PFALs are now used to grow herb and medicinal plants that provide materials in medicine, food, drink, cosmetics, health supplements, etc.
Hydroponic systems (or soilless culture. systems) are commonly applied in PFALs for growing crops in a nutrient solution with or without a growing media, such as sponge, vermiculite, rockwool, peat moss, saw dust, and coir dust. Most hydroponic systems automatically operate to control the amount of nutrients suitable to the demands of different species. The deep flow technique (DFT), ebb-and-flow, the nutrient film technique (NFT), and aeroponic systems are the most widespread systems adopted in PFALs to enhance water and nutrient use efficiencies of a system and to eliminate waste [9]. These systems allow for the optimization of irrigation and fertilization management, which saves labor and improves product quality [9]. Furthermore, various environmental factors in controlled environment systems, such as the relative humidity, light, and air temperature, can affect plant growth. Air temperature plays an important role in plant growth, and provides the optimal range of air temperature for maximum productivity [10]. Meanwhile, exposing plants to excessively low air temperatures can damage the photosynthetic apparatus, inhibit the synthesis or degradation of proteins, damage the thylakoid membrane, and reduce the electron transfer capacity of the plant [11,12]. Increased air temperatures above critical levels have lead to damage of the thylakoid membranes, reduction in photosynthesis and respiration, and membrane disruption [13,14]. In PFALs, defining a suitable culture system and optimal environmental conditions are important steps for successfully establishing the whole cultivation processes of vegetables and medicinal plants with year-round production and high quality. The results in this study confirm our hypothesis that cultivation type and temperature can affect the growth and change the accumulation of secondary metabolites in each part (shoot and root) of P. grandiflorum A. DC. Therefore, the aim of this study is to investigate the effects of culture systems and temperature conditions on the growth and saponin accumulation of balloon flower sprouts cultivated in PFALs.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
One-year-old P. grandiflorum A. DC seedlings (the average length of the seedlings was 7 cm) were used as experimental material. Soil cultivation involved transplanting seedlings into a plastic container (31.5 × 22 × 6.5 cm, L × W × H, respectively). Soilless cultivation involved transplanting seedlings into a deep flow technique (DFT) system with a plastic container (31.5 × 22 × 6.5 cm, L × W × H, respectively) filled with 40 sponges (3.5 × 3.5 × 3 cm, L × W × H, respectively). Growing took place in a growth chamber (VS-91G09M-4R, Vision Scientific Co., Ltd., Daejeon, Korea) with different temperatures (20, 25, and 30 °C). The growing conditions were maintained at 70 ± 10% relative humidity, 100 ± 1.01 μmol·m−2·s−1 photosynthetic photon flux density (PPFD), and 12 h photoperiod for 17 days. Distilled water was used for growing P. grandiflorum A. DC. Samples for growth characteristics and saponin analysis were harvested 17 days after transplanting, and 30 sprouts (divided shoot and root part) were randomly taken from each treatment (three repetitions per treatment).
2.2. Growth Characteristics
The sprouts were harvested 17 days after transplanting, for measuring growth characteristics such as shoot fresh weight, shoot dry weight, and plant height. The fresh weights of shoots and roots were measured using an electronic scale (PAG214C, Ohaus Corp, Parsippany, NJ, USA) and then, were dried in an oven (WOF-155, Daihan, Wonju, Korea) at 70 °C for 3 days to weigh dry mass. Plant heights were measured manually using a ruler.
2.3. Saponin Analysis (Extraction of Samples Three Types of Saponins (Pd-D3, Dpd-D, and PC-D) by High-Performance Liquid Chromatography (HPLC))
The P. grandiflorum A. DC sprouts (n = 30) were dried in a dry oven at 70 °C for 3 days, then, pulverized using a blender (GMFC-670, HANIL ELECTRIC, Korea) and kept frozen at −4 °C until analysis. A total of 20 mL of 70% HPLC-grade methanol was added to 1 g of the powdered sample, and the samples were extracted in a constant temperature water bath at 70 °C for 1 h and centrifuged at 3000 rpm for 10 min (1730R, GYROZEN Co., Ltd., Gimpo, Korea). The extracted solution was filtered through a 0.45 μm pore size membrane filter (Dismic-25CS, Toyoroshikaisha Ltd., Tokyo, Japan). This process was performed twice to obtain 40 mL of extract solution. Extracted samples were transferred to the 40 mL vial, and then, preconcentrated on a hot-plate stirrer at 140 °C. The solution was also concentrated by evaporation using a vacuum evaporator at 60 °C, and residue was dissolved in 2 mL of HPLC-grade water. The dissolved solution was filtered through a 0.45 μm pore size membrane filter and used for HPLC analysis. The analysis of saponin was performed using HPLC (Agilent 1260 system, Agilent Technologies Inc., Waldbronn, Germany) with a diode array detector, and all samples were analyzed using a C18 (250 × 4.6 mm, 5 μm, Shiseido, Japan) with an injection volume of 10 μL. The column temperature was maintained at 35 °C. Chromatographic separation of saponins was performed using a gradient consisting of solvent A (water) and solvent B (100% acetonitrile). The gradient program was as follows: 0–22 min 18%, 22–32 min 18–30%, and 32–60 min 30–50%. The absorption wavelength and flow rate were 203 nm and 1.0 mL/min, respectively [15]. The three saponin standards (platycodin D3 (Pd-D3), deapioplatycodin D (Dpd-D), and polygalcin D (Pc-D) used in the present work were purchased from Sigma-Aldrich (Darmstadt, Germany). All the solvents used for extraction were purchased from Fisher Scientific (Fair Lawn, NJ, USA).
2.4. Statistical Analysis
The growth characteristics and saponin analysis were measured in 30 replicates per treatment. For statistical analysis, data were analyzed by the SAS 9.2 program (SAS Institute Inc., Cary, NC, USA) with variance analysis. Duncan’s multiple range test was used to verify the significant differences in all treatments at p < 0.05. All graphs were created using the SigmaPlot 12.0 (Systat Software Inc., San Jose, CA, USA).
3. Results
3.1. Growth Characteristics
Different culture systems and temperatures significantly affected the growth of balloon flower. The soilless culture system tended to increase plant growth, and temperatures of 20, 25, and 30 °C showed similar effects on growth. In particular, the shoot fresh weight and shoot dry weight of balloon flower sprouts grown in the soilless culture system at 25 °C were significantly increased by about 1.71 and 1.58 times, respectively, as compared with those of the plants grown in a soil culture system at 25 °C (Figure 1A,B) The plant height results showed an increased trend similar to that of the shoot fresh weight and shoot dry weight. Sprouts grown in the soilless culture system at 25 °C also had the highest plant heights among the treatments (Figure 1C). The lowest shoot fresh weight, shoot dry weight and plant heights were observed in the soil and the soilless cultures at 15 °C. The root fresh weight and root dry weight tended to decrease as the temperature increased. These values also differed depending on the cultivation system (Figure 2A,B). The root growth was high in the soilless culture system in all the temperature treatments, except for the 20 °C treatment. In particular, in the soilless culture system, the root dry weights of sprouts grown at 15 °C were significantly higher (i.e., 1.69 times) than those of sprouts grown at 30 °C (Figure 2B).
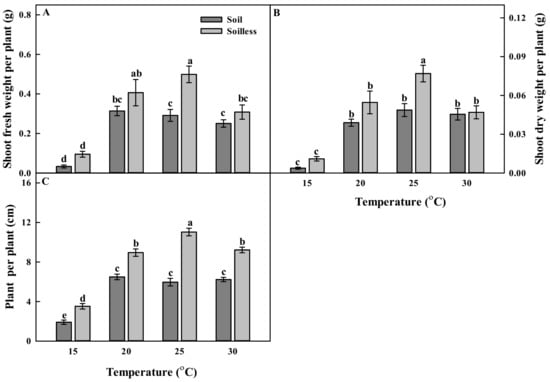
Figure 1.
Shoot fresh weights (A); shoot dry weights (B); and plant heights (C) of balloon flower sprouts grown under soil culture and soilless culture systems with different temperatures at 17 days after transplanting. Different letters indicate significant difference at p < 0.05 (n = 30).
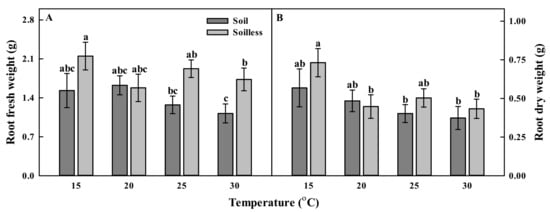
Figure 2.
Root fresh weights (A); root dry weights (B) of balloon flower sprouts grown under soil culture and soilless culture systems with different temperatures at 17 days after transplanting. Different letters indicate significant difference at p < 0.05 (n = 30).
3.2. Saponin Concentrations
The total and specific saponin concentrations in both shoot and root parts were measured to evaluate the effect of culture systems and temperature (Table 1 and Table 2). The saponin concentrations of the balloon flower shoots showed different trends with regards to the soil and the soilless culture systems. All individual (Pd-D3, Dpd-D, and Pc-D) and total saponin concentrations were significantly higher in the sprouts grown under a soilless culture system as compared with those grown under a soil culture system. In particular, the total saponin concentrations of the sprouts grown in the soilless culture system at 20, 25, and 30 °C were markedly increased by factors of 3.64, 3.34, and 2.78, respectively, as compared with those in the soil culture system at corresponding temperatures, and the highest value was obtained in the soilless culture system at 20 °C (Table 1). The results for specific saponin concentrations, such as Pd-D3, Dpd-D, and Pc-D, exhibited a similar trend to those for total saponin concentration. Additionally, the concentrations of individual saponins showed different trends depending on the culture system (soil and soilless). In the soil culture system, Pc-D accounted for the highest value of specific saponin, followed by Pd-D3, and then Dpd-D. In the soilless culture system, Pd-D3 accounted for the highest concentration of specific saponin, followed by Pc-D, and then Dpd-D. The Pd-D3 and Pc-D concentrations of the sprouts grown in the soilless culture system at 20 °C were the highest among the treatments, and the Dpd-D concentrations were higher in soilless cultures at 20 and 25 °C than in the other treatments. The Pc-D concentration of the sprouts grown in the soilless culture system at 20 °C was highest among the other treatments (Table 1).

Table 1.
Saponin concentrations in shoots of balloon flower sprouts grown under the soil and soilless culture systems with different temperatures at 17 days after transplanting.

Table 2.
Saponin concentrations in roots of balloon flower sprouts grown under soil and soilless culture systems with different temperatures at 17 days after transplanting.
Meanwhile, the total and specific saponin concentrations of the root part were slightly different from those of the shoot part (Table 2). Similar to the results for the shoot part, the saponin concentration was significantly higher in the soilless culture system than in the soil culture system (Table 1 and Table 2). In the case of saponin concentration in the root part, different increasing patterns were exhibited at the three temperatures depending on the cultivation system. In the soil culture system, the total saponin concentration was highest at 20 °C, and a tendency for it to decrease was observed as the temperature increased. However, in the soilless culture system, the total saponin concentration increased as the temperature increased. The total saponin concentration of the sprouts grown in the soilless culture system at 30 °C was the highest among the treatments, and was 3.1 times higher than that of the sprouts grown in the soil culture system at 30 °C. The Ddp-D concentration was markedly increased in the soilless culture system at 30 °C, which was about 10~14 times higher than that in the soil culture system and was about 3.3 and 2.4 times higher than that in the soilless culture system at 20 and 25 °C, respectively. Higher Pd-D3 concentrations were observed in the soilless culture system at 20 and 25 °C as compared with the other treatments, while the Pc-D concentrations were slightly increased in the soil culture system at 20 and 25 °C.
The saponin contents of the whole shoot part and root part (fresh weight) were significantly higher in the soilless culture system than in the soil culture system (Figure 3). In the shoot part, as the temperature decreased, the total saponin content showed a tendency to increase. However, the opposite trend was observed in the root part.
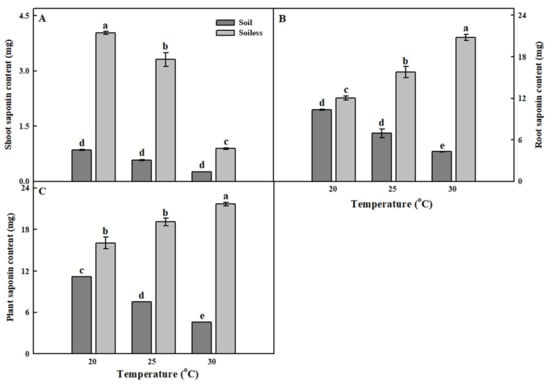
Figure 3.
The saponin contents of shoot (A), root (B), and plant (C) of balloon flower sprouts grown under soil culture and soilless culture systems with different temperatures at 17 days after transplanting. Different letters indicate significant difference at p < 0.05 (n = 30).
4. Discussion
The growth of balloon flower sprouts showed different trends for the shoot part and the root part. The results of this study showed that the soilless culture system and air temperature range of 20–30 °C were the optimal conditions for cultivating the shoot part of balloon flower in PFALs as compared with the soil culture system and temperatures below 20 °C. However, the root growth was highest at the low temperature of 15 °C, and a decreasing trend was observed as the temperature increased. In addition to the important effects of culture systems, plant growth and development are directly linked to a certain temperature range [16]. It is generally known that air temperature plays the most principal role among environmental conditions for controlling plant growth and development [17]. The enzyme activities in plants normally work in an optimal temperature range, and excessive fluctuation of the temperature range results in denaturation and inactivation of the enzymes and machinery, which disturb plant physiological and biochemical activities. Therefore, the speed of plant growth and development depends on the temperature surrounding the plant, and each plant species has a specific air temperature preference [18]. In general, plant biological processes accelerate at higher temperatures [19]. In the present study, the shoot growth of balloon flower sprouts was markedly enhanced as the temperature increased to 20 and 25 °C as compared with that of sprouts grown at 15 °C, and then, a slight decrease was observed in the shoot growth of sprouts grown at 30 °C. This result indicates that the optimal temperature for balloon flower sprouts growth is about 20 to 25 °C, with maximum growth expected at 25 °C. Meanwhile, the root growth was significantly increased as the temperature decreased to 15 °C as compared with the other temperature treatments. Different growth responses of the shoot and root parts to temperatures have been reported in previous studies. When the medicinal plant Dioscore dregeana was cultivated under various temperature conditions (10, 15, 20, 25, 30, 35, and 35/15 °C), its shoot dry weight was the highest in the 30/15 °C treatment, whereas its root dry weight was the highest in the 25/25 °C treatment [19].
In this study, balloon flower sprouts grown in a soilless culture system exhibited the highest growth characteristics (both of the shoot and root parts). It is well known that soil culture systems for crop production normally face many problems, such as loss of arable land, soil degradation impacts of climate change, and soil-borne pathogens. Therefore, soilless culture or hydroponic systems are currently one of the most favored and high production technologies in expanded agriculture development [20]. When growing plants in a homogenous nutrient solution, the soilless culture system can minimize the risk of soil-borne insect and pest attacks as well as efficiently manipulate nutrient composition, which leads to improvements in the quality and yield of plants. Plants grown in a soilless culture system normally develop superior root density, which is significantly involved in water and nutrient absorbance and oxygen consumption [21]. In addition, soilless-grown roots directly contact with water and nutrient solutions; therefore, they grow longer, more fibrous, and more fragile as compared with sturdy roots grown in soil culture systems [22,23]. In the present study, balloon flower sprouts were grown in soil and soilless culture systems with the same volume of containers. Although there were no clearly significant differences in the root fresh weight and root dry weight of plants between the two culture systems, the soilless-grown plants tended to exhibit increased root fresh and dry weights and displayed longer, whiter, and healthier roots as compared with the plants grown in soil culture. Root development can directly affect shoot growth. It is generally accepted that a 1% change in root development corresponds to a 2% change in plant yield [24]. In the present study, the shoot fresh and dry weights and plant heights of balloon flower sprouts in the soilless culture system were also higher than those of sprouts grown in the soil culture system, indicating that the application of a soilless culture system is more efficient for growing balloon flower in PFALs.
Determining the optimal cultivation conditions for improving both the yield and biochemical compounds of plants is an important task for the successful production of plants in controlled environment conditions. The soilless culture system enables one to use a suitable nutrient solution composition and to prevent the nutrient deficiency of some important nutrient elements, which can contribute to the stimulation of bioactive compound accumulation in plants as compared with soil cultivation [25]. In addition, for most plants, especially medicinal plants, the accumulation of phytochemicals critically depends on environmental conditions, such as light intensity, light quality, temperature, etc. These factors significantly affect some physiological processes involved in growth and development and the biochemical processes involved in the synthesis of secondary metabolites [26]. Temperature, as one of the major environmental variables, can significantly influence the composition and concentrations of secondary metabolites. Many studies have reported mixed results for the effect of high or low temperatures on secondary metabolite accumulation. For example, the contents of secondary metabolites have been reported to have been increased in high temperatures in the root, leaves, and flowers of Astracantha compacta [27], and elevated temperatures also have been reported to have increased the root ginsenoside contents in Panax quinquefolius [28]. Meanwhile, low temperatures, between 15 and 20 °C, have been reported to have been beneficial to the accumulation of saponins in the roots of P. notoginseng [29]. In the present study, similar to the growth results, there was an optimal temperature range in which the saponin concentration of balloon flowers was increased. Changes in bioactive compounds according to temperatures have been reported in previous papers. When green perilla was exposed to various temperature conditions (15/10, 20/15, 25/20, 30/25, and 35/30 °C), DPPH radical scavenging activity and total phenolic content were significantly increased at 35/30 °C as compared with other temperature conditions [30]. In the present study, as the temperature of the day increased, the concentration of bioactive compounds showed a tendency to increase. The concentrations of total and individual saponin in the shoot and root parts of balloon flower were significantly affected by the culture system and temperature. In both culture systems, the temperatures of 20 and 25 °C stimulated the individual and total saponin concentrations in the shoot part of the plant, while a slightly different pattern for these concentrations was observed for the root parts. These results suggest that the secondary metabolites generated in different plant parts may vary depending on environmental factors. It has been reported that secondary metabolites occurring in Tithonia diversifolia depend on the plant part (leaves, stems, roots and inflorescences) and environmental factors (humidity, rainfall, temperature, solar radiation, and minerals) [31].
Regarding the growth results, there existed a temperature condition that maximized the concentrations of individual and total saponins. The total saponin concentration per plant (shoot and root) showed a similar trend to that of the saponin content (per unit of leaf dry weight) (Figure 3). In general, when medicinal crops such as balloon flower plants are used as sprouts or microgreens, both the shoot and root parts are consumed. Therefore, for the growth of whole plants, 20 °C is considered to be the optimal growth temperature.
5. Conclusions
In conclusion, first, this study demonstrated the effects of cultivation systems and air temperature on the growth of and saponin concentrations in balloon flower. As far as we know, there is no information available on the effect of either culture system or temperature on the accumulation of saponin compounds in balloon flower sprouts. A soilless culture system and an air temperature of 20 °C were defined as the optimal conditions for enhancing plant growth and increasing individual and total saponin concentrations in balloon flower. Based on the results, these cultivation conditions can be applied for efficiently growing balloon flower sprouts in controlled environment conditions such as PFALs in order to increase the plant’s economic and industrial value. Overall, these results suggest that a soilless culture system with temperature conditions at 20 and 25 °C are suitable for improving the growth and saponin concentration of balloon flower cultivated in PFALs. Therefore, cultivation of balloon flower sprouts with a short cultivation period in PFALs has the potential to increase the plants’ economic and industrial value by producing higher saponin contents. Furthermore, the results show that environmental conditions can be used to improve the yield and the quality of balloon flower spouts and can be applied to food and natural products in the future.
Author Contributions
Conceptualization, methodology, software, formal analysis, investigation, resources, data curation, and writing—original draft preparation, T.K.L.N.; data curation, formal analysis, investigation, and methodology, G.O.L., D.Y.C. and J.-H.L.; supervision, validation, funding acquisition, and writing—review and editing, K.M.C. and K.-H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET), through the Technology Commercialization Support Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (821037031HD020), and a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (no. 2021R1F1A1047454).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data are available in the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ji, M.-Y.; Bo, A.; Yang, M.; Xu, J.-F.; Jiang, L.-L.; Zhou, B.-C.; Li, M.-H. The pharmacological effects and health benefits of Platycodon grandiflorus—A medicine food homology species. Foods 2020, 9, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.H.; Yoo, D.S.; Cha, M.-R.; Choi, C.W.; Kim, Y.S.; Choi, S.-U.; Lee, K.R.; Ryu, S.Y. Antiproliferative effects of saponins from the roots of Platycodon grandiflorum on cultured human tumor cells. J. Nat. Prod. 2010, 73, 1863–1867. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Yoon, J.-W.; Kim, C.-T.; Lim, S.-T. Antioxidant activity of phenylpropanoid esters isolated and identified from Platycodon grandiflorum A. DC. Phytochemistry 2004, 65, 3033–3039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Yang, D.; Zhang, C.; Zhang, N.; Li, M.; Liu, Y. Platycodon grandiflorus–An Ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol 2015, 164, 147–161. [Google Scholar] [CrossRef]
- Han, S.B.; Park, S.H.; Lee, K.H.; Lee, C.W.; Lee, S.H.; Kim, H.C.; Kim, Y.S.; Lee, H.S.; Kim, H.M. Polysaccharide isolated from the radix of Platycodon grandiflorum selectively activates B cells and macrophages but not T cells. Int. Immunopharmacol. 2001, 1, 1969–1978. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.S.; Choi, S.-U.; Kim, J.S.; Lee, H.S.; Roh, S.H.; Jeong, Y.C.; Kim, Y.-K.; Ryu, S.Y. Isolation of a new saponin and cytotoxic effect of saponins from the root of Platycodon grandiflorum on human tumor cell lines. Planta Med. 2005, 71, 566–568. [Google Scholar] [CrossRef]
- Yoon, Y.D.; Han, S.B.; Kang, J.S.; Lee, C.W.; Park, S.-K.; Lee, H.S.; Kang, J.S.; Kim, H.M. Toll-like receptor 4-dependent activation of macrophages by polysaccharide isolated from the radix of Platycodon grandiflorum. Int. Immunopharmacol. 2003, 3, 1873–1882. [Google Scholar] [CrossRef]
- Goto, E. Plant production in a closed plant factory with artificial lighting. In Proceedings of the Seventh International Symposium on Light in Horticultural Systems, Wageningen, The Netherlands, 14–18 October 2012. [Google Scholar] [CrossRef]
- Son, J.E.; Kim, H.J.; Ahn, T.I. Chapter 20–Hydroponic systems. In Plant Factory, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 273–283. [Google Scholar] [CrossRef]
- Abrami, G. Optimum mean temperature for a plant growth calculated by a new method of summation. Ecology 1972, 53, 893–900. [Google Scholar] [CrossRef]
- Guy, C.L.; Niemi, K.J.; Brambl, R. Altered gene expression during cold acclimation of spinach. Proc. Nat. Acad. Sci. USA 1985, 82, 3673–3677. [Google Scholar] [CrossRef] [Green Version]
- Holaday, A.S.; Martindale, W.; Alred, R.; Brooks, A.L.; Leegood, R.C. Changes in activities of enzymes of carbon metabolism in leaves during exposure of plants to low temperature. Plant. Physiol. 1992, 98, 1105–1114. [Google Scholar] [CrossRef] [Green Version]
- Maevskaya, S.; Egorova, E.; Bukhov, N. Effect of elevated temperature on nitrite and nitrate reduction in leaves and intact chloroplasts. Russ. J. Plant. Physiol. 2003, 50, 599–603. [Google Scholar] [CrossRef]
- Rokka, A.; Aro, E.-M.; Herrmann, R.G.; Andersson, B.; Vener, A.V. Dephosphorylation of photosystem II reaction center proteins in plant photosynthetic membranes as an immediate response to abrupt elevation of temperature. Plant. Physiol. 2000, 123, 1525–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, I.-S.; Kang, E.M.; Kim, N. High-performance liquid chromatographic analysis of saponin compounds in Bupleurum falcatum. J. Chromatogr. Sci. 2000, 38, 229–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant. Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Went, F. The effect of temperature on plant growth. Annu. Rev. Plant. Physiol. 1953, 4, 347–362. [Google Scholar] [CrossRef]
- Chaitanya, K.; Sundar, D.; Masilamani, S.; Ramachandra Reddy, A. Variation in heat stress-induced antioxidant enzyme activities among three mulberry cultivars. Plant. Growth Regul. 2002, 36, 175–180. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Savvas, D.; Gruda, N. Application of soilless culture technologies in the modern greenhouse industry—A review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- Tüzel, Y.; Balliu, A. Advances in liquid-and solid-medium soilless culture systems. In Advances in Horticultural Soilless Culture, 1st ed.; Gruda, N.S., Ed.; Burleigh Dodds Science: Cambridge, UK, 2021; pp. 213–248. [Google Scholar] [CrossRef]
- Sankhalkar, S.; Komarpant, R.; Dessai, T.R.; Simoes, J.; Sharma, S. Effects of soil and soil-less culture on morphology, physiology and biochemical studies of vegetable plants. Curr. Agric. Res. J. 2019, 7, 181. [Google Scholar] [CrossRef]
- Sardare, M.D.; Admane, S.V. A review on plant without soil-hydroponics. Int. J. Eng Res. Technol. 2013, 2, 299–304. [Google Scholar] [CrossRef]
- Bláha, L. Importance of root-shoot ratio for crops production. J. Agron. Crop. Sci. 2019, 2, 12. [Google Scholar] [CrossRef]
- Sgherri, C.; Cecconami, S.; Pinzino, C.; Navari-Izzo, F.; Izzo, R. Levels of antioxidants and nutraceuticals in basil grown in hydroponics and soil. Food Chem. 2010, 123, 416–422. [Google Scholar] [CrossRef]
- Verma, N.; Shukla, S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Naghiloo, S.; Movafeghi, A.; Delazar, A.; Nazemiyeh, H.; Asnaashari, S.; Dadpour, M.R. Ontogenetic variation of total phenolics and antioxidant activity in roots, leaves and flowers of Astragalus compactus Lam. (Fabaceae). BioImpacts BI 2012, 2, 105. [Google Scholar] [CrossRef]
- Jochum, G.M.; Mudge, K.W.; Thomas, R.B. Elevated temperatures increase leaf senescence and root secondary metabolite concentrations in the understory herb Panax quinquefolius (Araliaceae). Am. J. Bot. 2007, 94, 819–826. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Wang, Y.; Zhou, Y.; Zhu, L.; Zheng, G.; Ma, X.; Ji, P.; Xu, F. Effect of different temperature on saponin content in different parts of three-year-old Panax notoginseng. Southwest China J. Agric. Sci. 2019, 32, 2802–2806. [Google Scholar]
- Lin, K.-H.; Jhou, Y.-J.; Wu, C.-W.; Chang, Y.-S. Growth, physiological, and antioxidant characteristics in green and red Perilla frutescens varieties as affected by temperature-and water-stressed conditions. Sci. Hortic. 2020, 274, 109682. [Google Scholar] [CrossRef]
- Sampaio, B.L.; Edrada-Ebel, R.; Da Costa, F.B. Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: A model for environmental metabolomics of plants. Sci. Rep. 2016, 6, 29265. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).