Insights into Factors Controlling Adventitious Root Formation in Apples
Abstract
1. Introduction
2. Developmental Phase of Excision Induced AR Formation in Stem Cuttings
3. Multiple Hormonal Pathways Mediate Adventitious Rooting
3.1. Auxin: A Master Regulator for ARs
3.2. Cytokinin: A Required Inhibitor
3.3. Ethylene: A Positive or Negative Regulator for ARs
3.4. Abscisic Acid: A Negative Regulator for ARs
3.5. Jasmonic Acid: A Positive or Negative Regulator for ARs
3.6. Melatonin: A Positive Regulator
3.7. Gibberellic Acid and Brassinosteroids: A Positive or Negative Regulator for ARs
4. Role of Phenolic Compound in the Regulation of ARs
5. Role of Sugars in the Regulation of ARs
6. Role of Polyamines in the Regulation of ARs
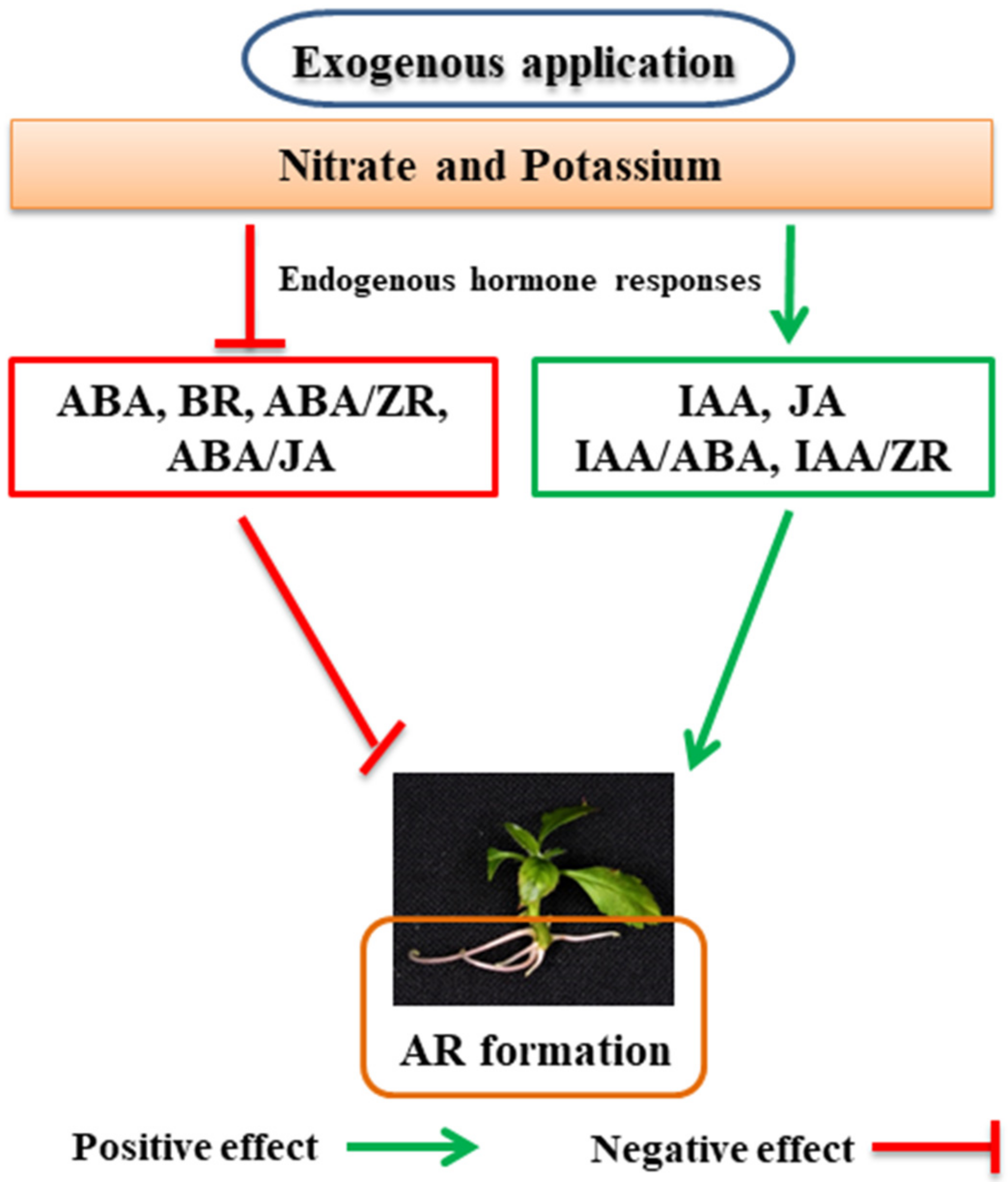
7. Role of Nutrients in the Life of ARs
7.1. Role of Nitrogen in the Formation of ARs
7.2. Role of Potassium in the Formation of ARs
8. Summary and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alvarez, R.; Nissen, S.J.; Sutter, E.G. Relationship between indole-3-acetic acid levels in apple (Malus pumila Mill) rootstocks cultured in vitro and adventitious root formation in the presence of indole-3-butyric acid. Plant Physiol. 1989, 89, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Xing, L.; Li, K.; Wei, Y.; Wang, H.; Mao, J.; Dong, F.; Ma, D.; Zhang, Z.; Han, M. Genome-wide identification, characterization and expression analysis of novel long non-coding RNAs that mediate IBA-induced adventitious root formation in apple rootstocks. Plant Growth Regul. 2019, 87, 287–302. [Google Scholar] [CrossRef]
- Lei, C.; Fan, S.; Li, K.; Meng, Y.; Mao, J.; Han, M.; Zhao, C.; Bao, L.; Zhang, D. iTRAQ-based proteomic analysis reveals potential regulation networks of IBA-induced adventitious root formation in apple. Int. J. Mol. Sci. 2018, 19, 667. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Dong, Z.; Zheng, X.; Song, S.; Jiao, J.; Wang, M.; Song, C. Auxin and its interaction with ethylene control adventitious root formation and development in apple rootstock. Front. Plant Sci. 2020, 11, 574881. [Google Scholar] [CrossRef] [PubMed]
- Patial, S.; Chandel, J.; Sharma, N.; Verma, P. Influence of Auxin on Rooting in Hardwood Cuttings of Apple (×Borkh.) Clonal Rootstock′M 116′under Malus domestica Mist Chamber Conditions. Indian J. Ecol. 2021, 48, 429–433. [Google Scholar]
- De Klerk, G.-J.; Brugge, J.T.; Marinova, S. Effectiveness of indoleacetic acid, indolebutyric acid and naphthaleneacetic acid during adventitious root formation in vitro in Malus ‘Jork 9’. Plant Cell Tissue Organ. Cult. 1997, 49, 39–44. [Google Scholar] [CrossRef]
- Li, K.; Liang, Y.; Xing, L.; Mao, J.; Liu, Z.; Dong, F.; Meng, Y.; Han, M.; Zhao, C.; Bao, L. Transcriptome analysis reveals multiple hormones, wounding and sugar signaling pathways mediate adventitious root formation in apple rootstock. Int. J. Mol. Sci. 2018, 19, 2201. [Google Scholar] [CrossRef]
- Guan, L.; Li, Y.; Huang, K.; Cheng, Z.-M.M. Auxin regulation and MdPIN expression during adventitious root initiation in apple cuttings. Hortic. Res. 2020, 7, 143. [Google Scholar] [CrossRef]
- Meng, Y.; Mao, J.; Tahir, M.M.; Wang, H.; Wei, Y.; Zhao, C.; Li, K.; Ma, D.; Zhao, C.; Zhang, D. Mdm-miR160 participates in auxin-induced adventitious root formation of apple rootstock. Sci. Hortic. 2020, 270, 109442. [Google Scholar] [CrossRef]
- Van der Krieken, W.; Breteler, H.; Visser, M. Uptake and metabolism of indolebutyric acid during root formation on Malus microcuttings. Acta Bot. Neerl. 1992, 41, 435–442. [Google Scholar] [CrossRef]
- Li, K.; Liu, Z.; Xing, L.; Wei, Y.; Mao, J.; Meng, Y.; Bao, L.; Han, M.; Zhao, C.; Zhang, D. miRNAs associated with auxin signaling, stress response, and cellular activities mediate adventitious root formation in apple rootstocks. Plant Physiol. Biochem. 2019, 139, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, X.; Hu, X.; Wu, T.; Wang, Y.; Xu, X.; Zhang, X.; Han, Z. High miR156 expression is required for auxin-induced adventitious root formation via MxSPL26 independent of PINs and ARFs in Malus xiaojinensis. Front. Plant Sci. 2017, 8, 1059. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Zhang, D.; Meng, Y.; Li, K.; Wang, H.; Han, M. Inhibition of adventitious root development in apple rootstocks by cytokinin is based on its suppression of adventitious root primordia formation. Physiol. Plant 2019, 166, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, Y.; Feng, C.; Wei, Y.; Peng, X.; Guo, X.; Guo, X.; Zhai, Z.; Li, J.; Shen, X. Overexpression of MsGH3. 5 inhibits shoot and root development through the auxin and cytokinin pathways in apple plants. Plant J. 2020, 103, 166–183. [Google Scholar] [CrossRef] [PubMed]
- De Klerk, G.-J.; Hanecakova, J.; Jasik, J. The role of cytokinins in rooting of stem slices cut from apple microcuttings. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2001, 135, 79–84. [Google Scholar] [CrossRef]
- Harbage, J.F.; Stimart, D.P. Ethylene does not promote adventitious root initiation on apple microcuttings. J. Am. Soc. Hortic. Sci. 1996, 121, 880–885. [Google Scholar] [CrossRef]
- Sriskandarajah, S. Induction of Adventitious Roots in Some Scion Cultivars of Apple (Malus pumila Mill); University of Sydney: Sydney, Australia, 1984. [Google Scholar]
- Noiton, D.; Vine, J.H.; Mullins, M.G. Endogenous indole-3-acetic acid and abscisic acid in apple microcuttings in relation to adventitious root formation. Plant Growth Regul. 1992, 11, 63–67. [Google Scholar] [CrossRef]
- Mao, J.; Niu, C.; Li, K.; Chen, S.; Tahir, M.M.; Han, M.; Zhang, D. Melatonin promotes adventitious root formation in apple by promoting the function of MdWOX11. BMC Plant Biol. 2020, 20, 536. [Google Scholar] [CrossRef]
- Pawlicki, N.; Welander, M. The effects of benzyladenine and gibberellic acid on adventitious root formation in apple stem discs. Agronomie 1992, 12, 783–788. [Google Scholar] [CrossRef][Green Version]
- De Klerk, G.-J.; Guan, H.; Huisman, P.; Marinova, S. Effects of phenolic compounds on adventitious root formation and oxidative decarboxylation of applied indoleacetic acid in Malus ‘Jork 9’. Plant Growth Regul. 2011, 63, 175–185. [Google Scholar] [CrossRef]
- James, D.J.; Thurbon, I.J. Phenolic compounds and other factors controlling rhizogenesis in vitro in the apple rootstocks M. 9 and M. 26. Z. Für Pflanzenphysiol. 1981, 105, 11–20. [Google Scholar] [CrossRef]
- Jasik, J.; De Klerk, G.-J. Anatomical and ultrastructural examination of adventitious root formation in stem slices of apple. Biol. Plant 1997, 39, 79–90. [Google Scholar] [CrossRef]
- Pawlicki, N.; Welander, M. Influence of carbohydrate source, auxin concentration and time of exposure on adventitious rooting of the apple rootstock Jork 9. Plant Sci. 1995, 106, 167–176. [Google Scholar] [CrossRef]
- Calamar, A.; De Klerk, G.-J. Effect of sucrose on adventitious root regeneration in apple. Plant Cell Tissue Organ. Cult. 2002, 70, 207–212. [Google Scholar] [CrossRef]
- Kromer, K.; Gamian, A. Analysis of soluble carbohydrates, proteins and lipids in shoots of M 7 apple rootstock cultured in vitro during regeneration of adventitious roots. J. Plant Physiol. 2000, 156, 775–782. [Google Scholar] [CrossRef]
- Wang, H.; Tahir, M.M.; Nawaz, M.A.; Mao, J.; Li, K.; Wei, Y.; Ma, D.; Lu, X.; Zhao, C.; Zhang, D. Spermidine application affects the adventitious root formation and root morphology of apple rootstock by altering the hormonal profile and regulating the gene expression pattern. Sci. Hortic. 2020, 266, 109310. [Google Scholar] [CrossRef]
- Tahir, M.M.; Wang, H.; Ahmad, B.; Liu, Y.; Fan, S.; Li, K.; Lei, C.; Shah, K.; Li, S.; Zhang, D. Identification and characterization of NRT gene family reveals their critical response to nitrate regulation during adventitious root formation and development in apple rootstock. Sci. Hortic. 2021, 275, 109642. [Google Scholar] [CrossRef]
- Tahir, M.M.; Lu, Z.; Wang, C.; Shah, K.; Li, S.; Zhang, X.; Mao, J.; Liu, Y.; Shalmani, A.; Li, K. Nitrate Application Induces Adventitious Root Growth by Regulating Gene Expression Patterns in Apple Rootstocks. J. Plant Growth Regul. 2021, 1–12. [Google Scholar] [CrossRef]
- Tahir, M.M.; Li, S.; Mao, J.; Liu, Y.; Li, K.; Zhang, X.; Lu, X.; Ma, X.; Zhao, C.; Zhang, D. High nitrate inhibited adventitious roots formation in apple rootstock by altering hormonal contents and miRNAs expression profiles. Sci. Hortic. 2021, 286, 110230. [Google Scholar] [CrossRef]
- Zhang, X.; Tahir, M.M.; Li, S.; Mao, J.; Nawaz, M.A.; Liu, Y.; Li, K.; Xing, L.; Niu, J.; Zhang, D. Transcriptome analysis reveals the inhibitory nature of high nitrate during adventitious roots formation in the apple rootstock. Physiol. Plant 2021, 173, 867–882. [Google Scholar] [CrossRef]
- Tahir, M.M.; Chen, S.; Ma, X.; Li, S.; Zhang, X.; Shao, Y.; Shalmani, A.; Zhao, C.; Bao, L.; Zhang, D. Transcriptome analysis reveals the promotive effect of potassium by hormones and sugar signaling pathways during adventitious roots formation in the apple rootstock. Plant Physiol. Biochem. 2021, 165, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Druege, U.; Franken, P.; Hajirezaei, M.R. Plant hormone homeostasis, signaling, and function during adventitious root formation in cuttings. Front. Plant Sci. 2016, 7, 381. [Google Scholar] [CrossRef] [PubMed]
- Agulló-Antón, M.Á.; Sánchez-Bravo, J.; Acosta, M.; Druege, U. Auxins or sugars: What makes the difference in the adventitious rooting of stored carnation cuttings? J. Plant Growth Regul. 2011, 30, 100–113. [Google Scholar] [CrossRef]
- de Klerk, G.-J.; van der Krieken, W.; de Jong, J.C. Review the formation of adventitious roots: New concepts, new possibilities. Vitr. Cell. Dev. Biol.-Plant 1999, 35, 189–199. [Google Scholar] [CrossRef]
- Caboni, E.; Tonelli, M.; Lauri, P.; Iacovacci, P.; Kevers, C.; Damiano, C.; Gaspar, T. Biochemical aspects of almond microcuttings related to in vitro rooting ability. Biol. Plant 1997, 39, 91–97. [Google Scholar] [CrossRef]
- Gatineau, F.; Fouché, J.G.; Kevers, C.; Hausman, J.-F.; Gaspar, T. Quantitative variations of indolyl compounds including IAA, IAA-aspartate and serotonin in walnut microcuttings during root induction. Biol. Plant 1997, 39, 131–137. [Google Scholar] [CrossRef]
- Label, P.; Sotta, B.; Miginiac, E. Endogenous levels of abscisic acid and indole-3-acetic acid during in vitro rooting of wild cherry explants produced by micropropagation. Plant Growth Regul. 1989, 8, 325–333. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Tang, Z. Effects of IAA and stimulated microgravity on formation of adventitious roots of Chinese cabbage. Shi Yan Sheng Wu Xue Bao 2000, 33, 179. [Google Scholar]
- Gray, W.M.; Kepinski, S.; Rouse, D.; Leyser, O.; Estelle, M. Auxin regulates SCF TIR1-dependent degradation of AUX/IAA proteins. Nature 2001, 414, 271–276. [Google Scholar] [CrossRef]
- Okushima, Y.; Overvoorde, P.J.; Arima, K.; Alonso, J.M.; Chan, A.; Chang, C.; Ecker, J.R.; Hughes, B.; Lui, A.; Nguyen, D. Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: Unique and overlapping functions of ARF7 and ARF19. Plant Cell 2005, 17, 444–463. [Google Scholar] [CrossRef]
- Wilmoth, J.C.; Wang, S.; Tiwari, S.B.; Joshi, A.D.; Hagen, G.; Guilfoyle, T.J.; Alonso, J.M.; Ecker, J.R.; Reed, J.W. NPH4/ARF7 and ARF19 promote leaf expansion and auxin-induced lateral root formation. Plant J. 2005, 43, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Okushima, Y.; Fukaki, H.; Onoda, M.; Theologis, A.; Tasaka, M. ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 2007, 19, 118–130. [Google Scholar] [CrossRef]
- Mao, J.; Niu, C.; Li, K.; Mobeen Tahir, M.; Khan, A.; Wang, H.; Li, S.; Liang, Y.; Li, G.; Yang, Z. Exogenous 6-benzyladenine application affects root morphology by altering hormone status and gene expression of developing lateral roots in Malus hupehensis. Plant Biol. 2020, 22, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-W.; Wang, L.-J.; Mao, Y.-B.; Cai, W.-J.; Xue, H.-W.; Chen, X.-Y. Control of root cap formation by microRNA-targeted auxin response factors in Arabidopsis. Plant Cell 2005, 17, 2204–2216. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, J.; Wang, L.; Li, Q.; Lu, Q.; Yu, Y.; Li, S.; Bai, M.Y.; Hu, Y.; Xiang, F. Repression of callus initiation by the mi RNA-directed interaction of auxin–cytokinin in Arabidopsis thaliana. Plant J. 2016, 87, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Sorin, C.; Bussell, J.D.; Camus, I.; Ljung, K.; Kowalczyk, M.; Geiss, G.; McKhann, H.; Garcion, C.; Vaucheret, H.; Sandberg, G. Auxin and light control of adventitious rooting in Arabidopsis require ARGONAUTE1. Plant Cell 2005, 17, 1343–1359. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.E.; Muto, H.; Higuchi, K.; Matamura, T.; Tatematsu, K.; Koshiba, T.; Yamamoto, K.T. Disruption and overexpression of auxin response factor 8 gene of Arabidopsis affect hypocotyl elongation and root growth habit, indicating its possible involvement in auxin homeostasis in light condition. Plant J. 2004, 40, 333–343. [Google Scholar] [CrossRef]
- Meng, Y.; Huang, F.; Shi, Q.; Cao, J.; Chen, D.; Zhang, J.; Ni, J.; Wu, P.; Chen, M. Genome-wide survey of rice microRNAs and microRNA–target pairs in the root of a novel auxin-resistant mutant. Planta 2009, 230, 883–898. [Google Scholar] [CrossRef]
- Marin, E.; Jouannet, V.; Herz, A.; Lokerse, A.S.; Weijers, D.; Vaucheret, H.; Nussaume, L.; Crespi, M.D.; Maizel, A. miR390, Arabidopsis TAS3 tasiRNAs, and their AUXIN RESPONSE FACTOR targets define an autoregulatory network quantitatively regulating lateral root growth. Plant Cell 2010, 22, 1104–1117. [Google Scholar] [CrossRef]
- Levy, A.; Szwerdszarf, D.; Abu-Abied, M.; Mordehaev, I.; Yaniv, Y.; Riov, J.; Arazi, T.; Sadot, E. Profiling microRNAs in Eucalyptus grandis reveals no mutual relationship between alterations in miR156 and miR172 expression and adventitious root induction during development. BMC Genom. 2014, 15, 524. [Google Scholar] [CrossRef]
- Grieneisen, V.A.; Xu, J.; Marée, A.F.; Hogeweg, P.; Scheres, B. Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature 2007, 449, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Ioio, R.D.; Nakamura, K.; Moubayidin, L.; Perilli, S.; Taniguchi, M.; Morita, M.T.; Aoyama, T.; Costantino, P.; Sabatini, S. A genetic framework for the control of cell division and differentiation in the root meristem. Science 2008, 322, 1380–1384. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.J.; Marchant, A.; Green, H.G.; May, S.T.; Ward, S.P.; Millner, P.A.; Walker, A.R.; Schulz, B.; Feldmann, K.A. Arabidopsis AUX1 gene: A permease-like regulator of root gravitropism. Science 1996, 273, 948–950. [Google Scholar] [CrossRef] [PubMed]
- Marchant, A.; Bhalerao, R.; Casimiro, I.; Eklöf, J.; Casero, P.J.; Bennett, M.; Sandberg, G. AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell 2002, 14, 589–597. [Google Scholar] [CrossRef]
- Liu, B.; Wang, L.; Zhang, J.; Li, J.; Zheng, H.; Chen, J.; Lu, M. WUSCHEL-related Homeobox genes in Populus tomentosa: Diversified expression patterns and a functional similarity in adventitious root formation. BMC Genom. 2014, 15, 296. [Google Scholar] [CrossRef]
- Tian, H.; Jia, Y.; Niu, T.; Yu, Q.; Ding, Z. The key players of the primary root growth and development also function in lateral roots in Arabidopsis. Plant Cell Rep. 2014, 33, 745–753. [Google Scholar] [CrossRef]
- Hu, X.; Xu, L. Transcription factors WOX11/12 directly activate WOX5/7 to promote root primordia initiation and organogenesis. Plant Physiol. 2016, 172, 2363–2373. [Google Scholar] [CrossRef]
- Mashiguchi, K.; Tanaka, K.; Sakai, T.; Sugawara, S.; Kawaide, H.; Natsume, M.; Hanada, A.; Yaeno, T.; Shirasu, K.; Yao, H. The main auxin biosynthesis pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 18512–18517. [Google Scholar] [CrossRef]
- Sozzani, R.; Cui, H.; Moreno-Risueno, M.; Busch, W.; Van Norman, J.; Vernoux, T.; Brady, S.; Dewitte, W.; Murray, J.A.H.; Benfey, P. Spatiotemporal regulation of cell-cycle genes by SHORTROOT links patterning and growth. Nature 2010, 466, 128–132. [Google Scholar] [CrossRef]
- Liu, J.; Sheng, L.; Xu, Y.; Li, J.; Yang, Z.; Huang, H.; Xu, L. WOX11 and 12 are involved in the first-step cell fate transition during de novo root organogenesis in Arabidopsis. Plant Cell 2014, 26, 1081–1093. [Google Scholar] [CrossRef]
- Mähönen, A.P.; Higuchi, M.; Törmäkangas, K.; Miyawaki, K.; Pischke, M.S.; Sussman, M.R.; Helariutta, Y.; Kakimoto, T. Cytokinins regulate a bidirectional phosphorelay network in Arabidopsis. Curr. Biol. 2006, 16, 1116–1122. [Google Scholar] [CrossRef]
- Atkinson, J.A.; Rasmussen, A.; Traini, R.; Voß, U.; Sturrock, C.; Mooney, S.J.; Wells, D.M.; Bennett, M.J. Branching out in roots: Uncovering form, function, and regulation. Plant Physiol. 2014, 166, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Carvajal, G.A.; Morse, A.M.; Dervinis, C.; Davis, J.M. The cytokinin type-B response regulator PtRR13 is a negative regulator of adventitious root development in Populus. Plant Physiol. 2009, 150, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Jinxiang, W.; Xiaolong, Y.; Ruichi, P. Relationship between adventitious root formation and plant hormones. Plant Physiol. Commun. 2005, 41, 133–142. [Google Scholar]
- Amiri, E.M.; Elahinia, A. Optimization of medium composition for apple rootstocks. Afr. J. Biotechnol. 2011, 10, 3594–3601. [Google Scholar]
- Hutchison, C.E.; Li, J.; Argueso, C.; Gonzalez, M.; Lee, E.; Lewis, M.W.; Maxwell, B.B.; Perdue, T.D.; Schaller, G.E.; Alonso, J.M. The Arabidopsis histidine phosphotransfer proteins are redundant positive regulators of cytokinin signaling. Plant Cell 2006, 18, 3073–3087. [Google Scholar] [CrossRef]
- Chen, L.; Tong, J.; Xiao, L.; Ruan, Y.; Liu, J.; Zeng, M.; Huang, H.; Wang, J.-W.; Xu, L. YUCCA-mediated auxin biogenesis is required for cell fate transition occurring during de novo root organogenesis in Arabidopsis. J. Exp. Bot. 2016, 67, 4273–4284. [Google Scholar] [CrossRef]
- Zhao, Y.; Cheng, S.; Song, Y.; Huang, Y.; Zhou, S.; Liu, X.; Zhou, D.-X. The interaction between rice ERF3 and WOX11 promotes crown root development by regulating gene expression involved in cytokinin signaling. Plant Cell 2015, 27, 2469–2483. [Google Scholar] [CrossRef]
- Chandler, J.W.; Werr, W. Cytokinin–auxin crosstalk in cell type specification. Trends Plant Sci. 2015, 20, 291–300. [Google Scholar] [CrossRef]
- Da Costa, C.T.; De Almeida, M.R.; Ruedell, C.M.; Schwambach, J.; Maraschin, F.D.S.; Fett-Neto, A.G. When stress and development go hand in hand: Main hormonal controls of adventitious rooting in cuttings. Front. Plant Sci. 2013, 4, 133. [Google Scholar] [CrossRef]
- Zimmerman, P.W. Several chemical growth substances which cause initiation of roots and other responses in plants. Contrib. Boyce Thompson Inst. 1935, 7, 209–229. [Google Scholar]
- Růžička, K.; Ljung, K.; Vanneste, S.; Podhorská, R.; Beeckman, T.; Friml, J.; Benková, E. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 2007, 19, 2197–2212. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.M. Abscisic acid: Hidden architect of root system structure. Plants 2015, 4, 548–572. [Google Scholar] [CrossRef] [PubMed]
- Petricka, J.J.; Benfey, P.N. Root layers: Complex regulation of developmental patterning. Curr. Opin. Genet. Dev. 2008, 18, 354–361. [Google Scholar] [CrossRef]
- Heidstra, R.; Sabatini, S. Plant and animal stem cells: Similar yet different. Nat. Rev. Mol. Cell Biol. 2014, 15, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Han, W.; De Smet, I.; Talboys, P.; Loya, R.; Hassan, A.; Rong, H.; Jürgens, G.; Paul Knox, J.; Wang, M.H. ABA promotes quiescence of the quiescent centre and suppresses stem cell differentiation in the Arabidopsis primary root meristem. Plant J. 2010, 64, 764–774. [Google Scholar] [CrossRef]
- Pelese, F.; Megnegneau, B.; Sotta, B.; Sossountzov, L.; Caboche, M.; Miginiac, E. Hormonal characterization of a nonrooting naphthalene-acetic acid tolerant tobacco mutant by an immunoenzymic method. Plant Physiol. 1989, 89, 86–92. [Google Scholar] [CrossRef]
- De Smet, I.; Signora, L.; Beeckman, T.; Inzé, D.; Foyer, C.H.; Zhang, H. An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. Plant J. 2003, 33, 543–555. [Google Scholar] [CrossRef]
- Zhang, X.; Tahir, M.M.; Li, S.; Tang, T.; Mao, J.; Li, K.; Shao, Y.; Yang, W.; Niu, J.; Zhang, D. Effect of exogenous abscisic acid (ABA) on the morphology, phytohormones, and related gene expression of developing lateral roots in ‘Qingzhen 1’apple plants. Plant Cell Tissue Organ. Cult. (PCTOC) 2022, 148, 23–34. [Google Scholar] [CrossRef]
- Li, S.; Tahir, M.M.; Wu, T.; Xie, L.; Zhang, X.; Mao, J.; Ayyoub, A.; Xing, L.; Zhang, D.; Shao, Y. Transcriptome Analysis Reveals Multiple Genes and Complex Hormonal-Mediated Interactions with PEG during Adventitious Root Formation in Apple. Int. J. Mol. Sci. 2022, 23, 976. [Google Scholar] [CrossRef]
- Wasternack, C.; Song, S. Jasmonates: Biosynthesis, metabolism, and signaling by proteins activating and repressing transcription. J. Exp. Bot. 2017, 68, 1303–1321. [Google Scholar] [CrossRef] [PubMed]
- Schilmiller, A.L.; Howe, G.A. Systemic signaling in the wound response. Curr. Opin. Plant Biol. 2005, 8, 369–377. [Google Scholar] [CrossRef]
- Ahkami, A.H.; Lischewski, S.; Haensch, K.T.; Porfirova, S.; Hofmann, J.; Rolletschek, H.; Melzer, M.; Franken, P.; Hause, B.; Druege, U. Molecular physiology of adventitious root formation in Petunia hybrida cuttings: Involvement of wound response and primary metabolism. New Phytol. 2009, 181, 613–625. [Google Scholar] [CrossRef]
- Lischweski, S.; Muchow, A.; Guthörl, D.; Hause, B. Jasmonates act positively in adventitious root formation in petunia cuttings. BMC Plant Biol. 2015, 15, 229. [Google Scholar] [CrossRef]
- Światek, A.; Lenjou, M.; Van Bockstaele, D.; Inzé, D.; Van Onckelen, H. Differential effect of jasmonic acid and abscisic acid on cell cycle progression in tobacco BY-2 cells. Plant Physiol. 2002, 128, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, L.; Morreel, K.; De Witte, E.; Lammertyn, F.; Van Montagu, M.; Boerjan, W.; Inzé, D.; Goossens, A. Mapping methyl jasmonate-mediated transcriptional reprogramming of metabolism and cell cycle progression in cultured Arabidopsis cells. Proc. Natl. Acad. Sci. USA 2008, 105, 1380–1385. [Google Scholar] [CrossRef]
- Park, W.J. Melatonin as an endogenous plant regulatory signal: Debates and perspectives. J. Plant Biol. 2011, 54, 143–149. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin promotes adventitious-and lateral root regeneration in etiolated hypocotyls of Lupinus albus L. J. Pineal Res. 2007, 42, 147–152. [Google Scholar] [CrossRef]
- Sarropoulou, V.; Dimassi-Theriou, K.; Therios, I.; Koukourikou-Petridou, M. Melatonin enhances root regeneration, photosynthetic pigments, biomass, total carbohydrates and proline content in the cherry rootstock PHL-C (Prunus avium × Prunus cerasus). Plant Physiol. Biochem. 2012, 61, 162–168. [Google Scholar] [CrossRef]
- Li, C.; Liang, B.; Chang, C.; Wei, Z.; Zhou, S.; Ma, F. Exogenous melatonin improved potassium content in Malus under different stress conditions. J. Pineal Res. 2016, 61, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Qi, W.-b.; Reiter, R.J.; Wei, W.; Wang, B.-m. Exogenously applied melatonin stimulates root growth and raises endogenous indoleacetic acid in roots of etiolated seedlings of Brassica juncea. J. Plant Physiol. 2009, 166, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Pelagio-Flores, R.; Muñoz-Parra, E.; Ortiz-Castro, R.; López-Bucio, J. Melatonin regulates Arabidopsis root system architecture likely acting independently of auxin signaling. J. Pineal Res. 2012, 53, 279–288. [Google Scholar] [CrossRef]
- Müssig, C.; Shin, G.-H.; Altmann, T. Brassinosteroids promote root growth in Arabidopsis. Plant Physiol. 2003, 133, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Gorter, C.J. Further Experiments on Auxin-Synergists. Physiol. Plant 1962, 15, 88–95. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Riadh, K.; Gopi, R.; Manivannan, P.; Ines, J.; Al-Juburi, H.J.; Chang-Xing, Z.; Hong-Bo, S.; Panneerselvam, R. Antioxidant defense responses: Physiological plasticity in higher plants under abiotic constraints. Acta Physiol. Plant 2009, 31, 427–436. [Google Scholar] [CrossRef]
- Murphy, A.; Peer, W.A.; Taiz, L. Regulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta 2000, 211, 315–324. [Google Scholar] [CrossRef]
- Buer, C.S.; Imin, N.; Djordjevic, M.A. Flavonoids: New roles for old molecules. J. Integr. Plant Biol. 2010, 52, 98–111. [Google Scholar] [CrossRef]
- Couée, I.; Hummel, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of polyamines in root development. Plant Cell Tissue Organ. Cult. 2004, 76, 1–10. [Google Scholar] [CrossRef]
- Hausman, J.F.; Gevers, C.; Gaspar, T. Involvement of putrescine in the inductive rooting phase of poplar shoots raised in vitro. Physiol. Plant 1994, 92, 201–206. [Google Scholar] [CrossRef]
- Kevers, C.; Hausman, J.-F.; Faivre-Rampant, O.; Evers, D.; Gaspar, T. Hormonal control of adventitious rooting: Progress and questions. J. Appl. Bot. 1997, 71, 71–79. [Google Scholar]
- Geiss, G.; Gutierrez, L.; Bellini, C. Adventitious root formation: New insights and perspectives. Annu. Plant Rev. Online 2009, 37, 127–156. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Hsu, P.-K.; Tsay, Y.-F. Uptake, allocation and signaling of nitrate. Trends Plant Sci. 2012, 17, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef]
- O′Brien, J.A.; Vega, A.; Bouguyon, E.; Krouk, G.; Gojon, A.; Coruzzi, G.; Gutiérrez, R.A. Nitrate transport, sensing, and responses in plants. Mol. Plant 2016, 9, 837–856. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.M.; Zhang, X.; Shah, K.; Hayat, F.; Li, S.; Mao, J.; Liu, Y.; Shao, Y.; Zhang, D. Nitrate application affects root morphology by altering hormonal status and gene expression patterns in B9 apple rootstock nursery plants. Fruit Res. 2021, 1, 1–11. [Google Scholar] [CrossRef]
- López-Bucio, J.; Cruz-Ramırez, A.; Herrera-Estrella, L. The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol. 2003, 6, 280–287. [Google Scholar] [CrossRef]
- Crawford, N.M.; Glass, A.D. Molecular and physiological aspects of nitrate uptake in plants. Trends Plant Sci. 1998, 3, 389–395. [Google Scholar] [CrossRef]
- Zhang, H.; Jennings, A.; Barlow, P.W.; Forde, B.G. Dual pathways for regulation of root branching by nitrate. Proc. Natl. Acad. Sci. USA 1999, 96, 6529–6534. [Google Scholar] [CrossRef]
- Sriskandarajah, S.; Skirvin, R.; Abu-Qaoud, H. The effect of some macronutrients on adventitious root development on scion apple cultivars in vitro. Plant Cell Tissue Organ. Cult. 1990, 21, 185–189. [Google Scholar] [CrossRef]
- Bhat, K. Nutrient inflows into apple roots. Plant Soil 1983, 71, 371–380. [Google Scholar] [CrossRef]
- Hilo, A.; Shahinnia, F.; Druege, U.; Franken, P.; Melzer, M.; Rutten, T.; von Wirén, N.; Hajirezaei, M.-R. A specific role of iron in promoting meristematic cell division during adventitious root formation. J. Exp. Bot. 2017, 68, 4233–4247. [Google Scholar] [CrossRef] [PubMed]
- Véry, A.-A.; Nieves-Cordones, M.; Daly, M.; Khan, I.; Fizames, C.; Sentenac, H. Molecular biology of K+ transport across the plant cell membrane: What do we learn from comparison between plant species? J. Plant Physiol. 2014, 171, 748–769. [Google Scholar] [CrossRef]
- Römheld, V.; Kirkby, E.A. Research on potassium in agriculture: Needs and prospects. Plant Soil 2010, 335, 155–180. [Google Scholar] [CrossRef]
- Yamasaki, A.; Yano, T. In Effect of Supplemental Application of Fertilizers on Flower Bud Initiation and Development of Strawberry; Possible Role of Nitrogen. In Proceedings of the VI International Strawberry Symposium 842, Huelva, Spain, 3 March 2008; pp. 765–768. [Google Scholar]
- Hartz, T.; Johnstone, P.; Francis, D.; Miyao, E. Processing tomato yield and fruit quality improved with potassium fertigation. HortScience 2005, 40, 1862–1867. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, G.; Huang, G. Promotive effect of potassium on adventitious root formation in some plants. Plant Sci. 1991, 79, 47–50. [Google Scholar] [CrossRef]
- Kannan, C.G.; Perumalsamy, P.; Thangavelu, M. Influences of potassium chloride fertilization on mycorrhizal formation in a tropical alfisol. Commun. Soil Sci. Plant Anal. 2017, 48, 524–538. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.-H. Potassium transport and signaling in higher plants. Annu. Rev. Plant Biol. 2013, 64, 451–476. [Google Scholar] [CrossRef]

| Application | Test Material | Culture | Reported Effect | Reference |
|---|---|---|---|---|
| IBA | M26 and M9 | In vitro tissueculture | IBA promotes AR formation by increasing free IAA accumulation at stem basal parts | [1] |
| IBA | M26 | In vitro tissueculture | IBA treatment increased endogenous IAA content at the primordia formation stage | [2] |
| IBA | T337 | In vitro tissueculture | IBA-treated cuttings produced more ARs by hormone signaling and protein homeostasis | [3] |
| IBA and ethylene | M9-T337 | In vitro tissueculture | IBA boosted ethylene and auxin production during AR formation, reducing AR elongation as indicated by ethylene | [4] |
| IBA and NAA | M116 | Mist chamber conditions | Increasing auxin levels correlated positively with rooting success | [5] |
| IAA, IBA, NAA | Jork9 | In vitro tissueculture | IAA is the more preferred auxin for increasing rooting in vitro | [6] |
| IBA | T337 | In vitro tissueculture | Endogenous and exogenous auxins both influence AR development through homologous signaling pathways | [7] |
| IAA | M9 | Hydroponic culture in a growth chamber | IAA promotes AR founder cell division and elongation via upregulation of PINs | [8] |
| IBA | MP, M26, SH6, T337 | In vitro tissueculture | The mode of IBA during AR formation is species-specific | [9] |
| IBA | M9-Jork | In vitro tissueculture | IBA induced more roots than IAA | [10] |
| IBA | M9-T337 | In vitro tissueculture | Several miRNAs and their targets collaborated with hormone signaling pathways to contribute ARs | [11] |
| IBA | Malus xiaojinesis | Sand culture in a greenhouse | miR156 high expression is required for auxin-induced AR formation | [12] |
| 6BA | M26 | In vitro tissueculture | CK prevents AR primordia formation by increasing the expression of CK signaling pathway genes | [13] |
| IAA and 6BA | Malus sieversii | Hydroponic culture in a growth chamber | Auxin and CK are both important regulators of AR formation | [14] |
| 6BA | Jork9 | In vitro tissueculture | CK is essential for AR formation | [15] |
| Ethylene | Gala and Triple Red Delicious | In vitro tissueculture | AR in apples was not related to ethylene | [16] |
| ABA | Malus pumila | In vitro tissueculture | ABA negatively regulates AR formation | [17] |
| IAA and ABA | Jonathan | In vitro tissueculture | IBA increases adventitious rooting | [18] |
| Melatonin | Malus prunifolia | In vitro tissueculture | MT promotes ARs’ initiation stage by IAA homeostasis | [19] |
| GA3 | M9 cv Jork | In vitro tissueculture | GA3 treatments limit AR formation from the initial to final stages of AR formation | [20] |
| Phenolic compounds | Jork9 | In vitro tissueculture | Phenolic compounds may act as antioxidants, preventing auxin oxidation and, thus, contributing to AR formation | [21] |
| Phenolic compounds | M9 and M26 | In vitro tissueculture | Auxin and PG stimulated ARs more than auxin-alone controls | [22] |
| IBA and sugar | Jork9 | In vitro tissueculture | Starch grains provide energy for AR growth | [23] |
| Carbohydrates | Jork9 | In vitro tissueculture | Sugar promotes root regeneration, and sucrose content affects AR density | [24] |
| Sucrose | Jork9 | In vitro tissueculture | Sucrose and auxin interacted to mediate AR formation | [25] |
| Sucrose and IBA | M7 | In vitro tissueculture | Soluble saccharides are crucial for primordia formation but not essential for later stages | [26] |
| Spermidine | Malus prunifolia | In vitro tissueculture | Spd promotes AR formation by interacting with IAA and regulating different gene sets | [27] |
| Nitrate | B9 | In vitro tissueculture | Nitrate promotes ARs at lower-medium levels and inhibits them at higher levels | [28] |
| Nitrate | B9 | In vitro tissueculture | Nitrate promotes AR’s length by upregulating the expression of different gene sets | [29] |
| Nitrate | B9 | In vitro tissueculture | High nitrate inhibited ARs by ABA signaling miRNA | [30] |
| Nitrate | B9 | In vitro tissueculture | The high availability of nitrate delays AR initiation and emergence stages | [31] |
| Potassium | B9 | In vitro tissueculture | KCl-treated cuttings produced more ARs than control cuttings | [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahir, M.M.; Mao, J.; Li, S.; Li, K.; Liu, Y.; Shao, Y.; Zhang, D.; Zhang, X. Insights into Factors Controlling Adventitious Root Formation in Apples. Horticulturae 2022, 8, 276. https://doi.org/10.3390/horticulturae8040276
Tahir MM, Mao J, Li S, Li K, Liu Y, Shao Y, Zhang D, Zhang X. Insights into Factors Controlling Adventitious Root Formation in Apples. Horticulturae. 2022; 8(4):276. https://doi.org/10.3390/horticulturae8040276
Chicago/Turabian StyleTahir, Muhammad Mobeen, Jiangping Mao, Shaohuan Li, Ke Li, Yu Liu, Yun Shao, Dong Zhang, and Xiaoyun Zhang. 2022. "Insights into Factors Controlling Adventitious Root Formation in Apples" Horticulturae 8, no. 4: 276. https://doi.org/10.3390/horticulturae8040276
APA StyleTahir, M. M., Mao, J., Li, S., Li, K., Liu, Y., Shao, Y., Zhang, D., & Zhang, X. (2022). Insights into Factors Controlling Adventitious Root Formation in Apples. Horticulturae, 8(4), 276. https://doi.org/10.3390/horticulturae8040276





