Commercial Potato Cultivars Exhibit Distinct Susceptibility to the Root Lesion Nematode Pratylenchus penetrans
Abstract
1. Introduction
2. Materials and Methods
2.1. Nematode Culture
2.2. Plant Material
2.3. Root–Nematode Penetration Assay
2.4. Nematode Egression Assay
2.5. Nematode Reproduction Assay
2.6. Statistical Analysis
3. Results
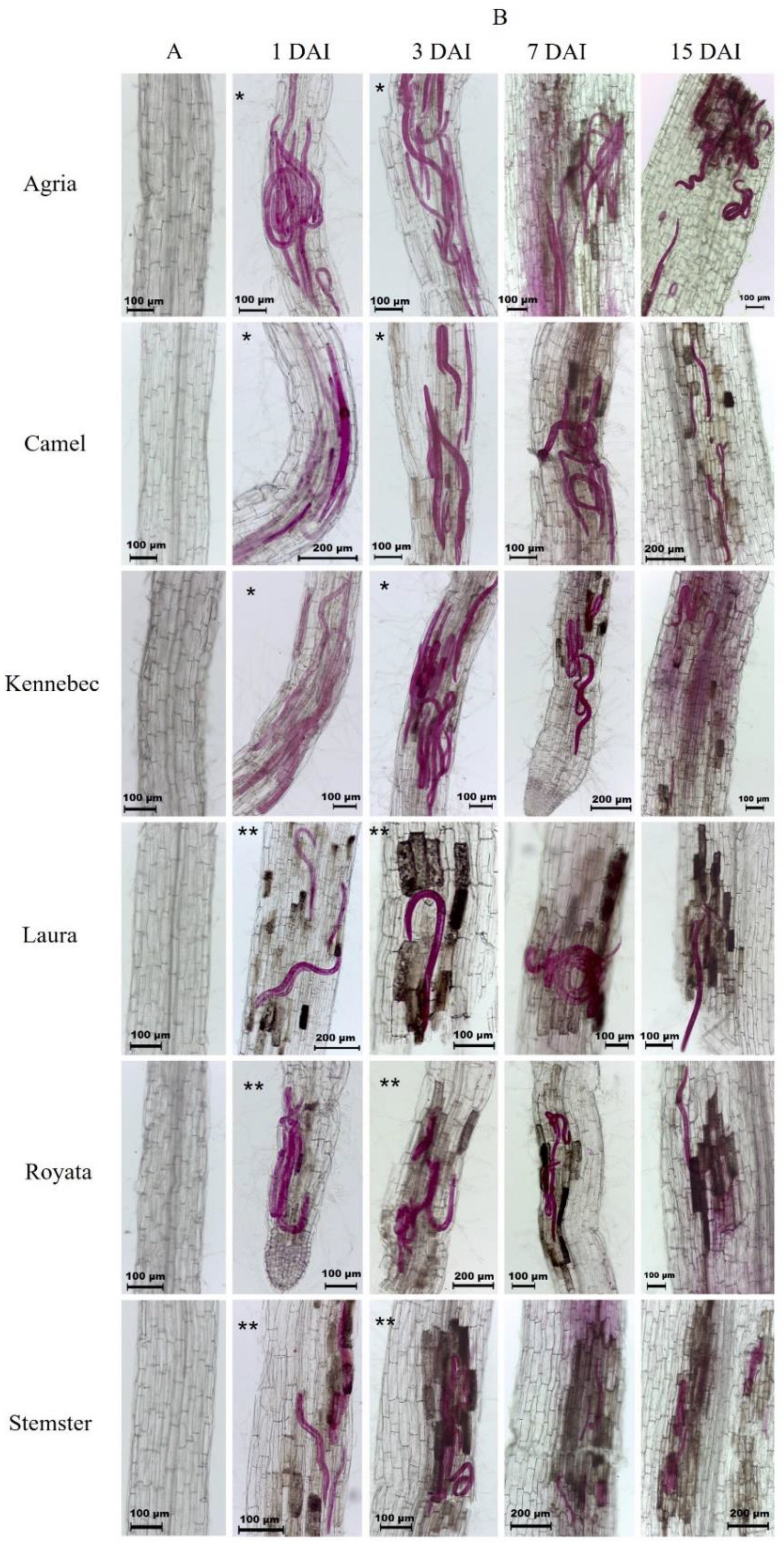
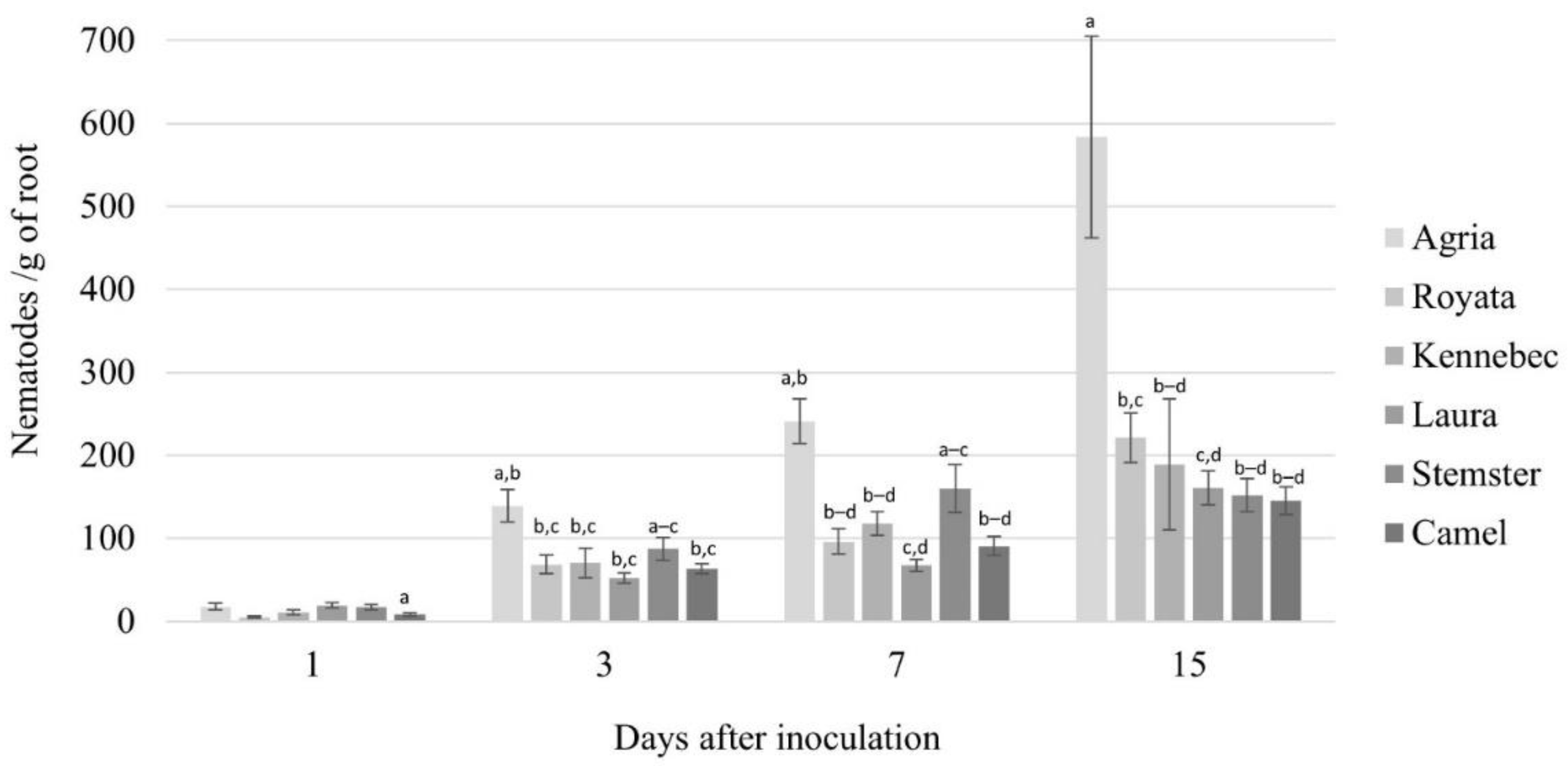
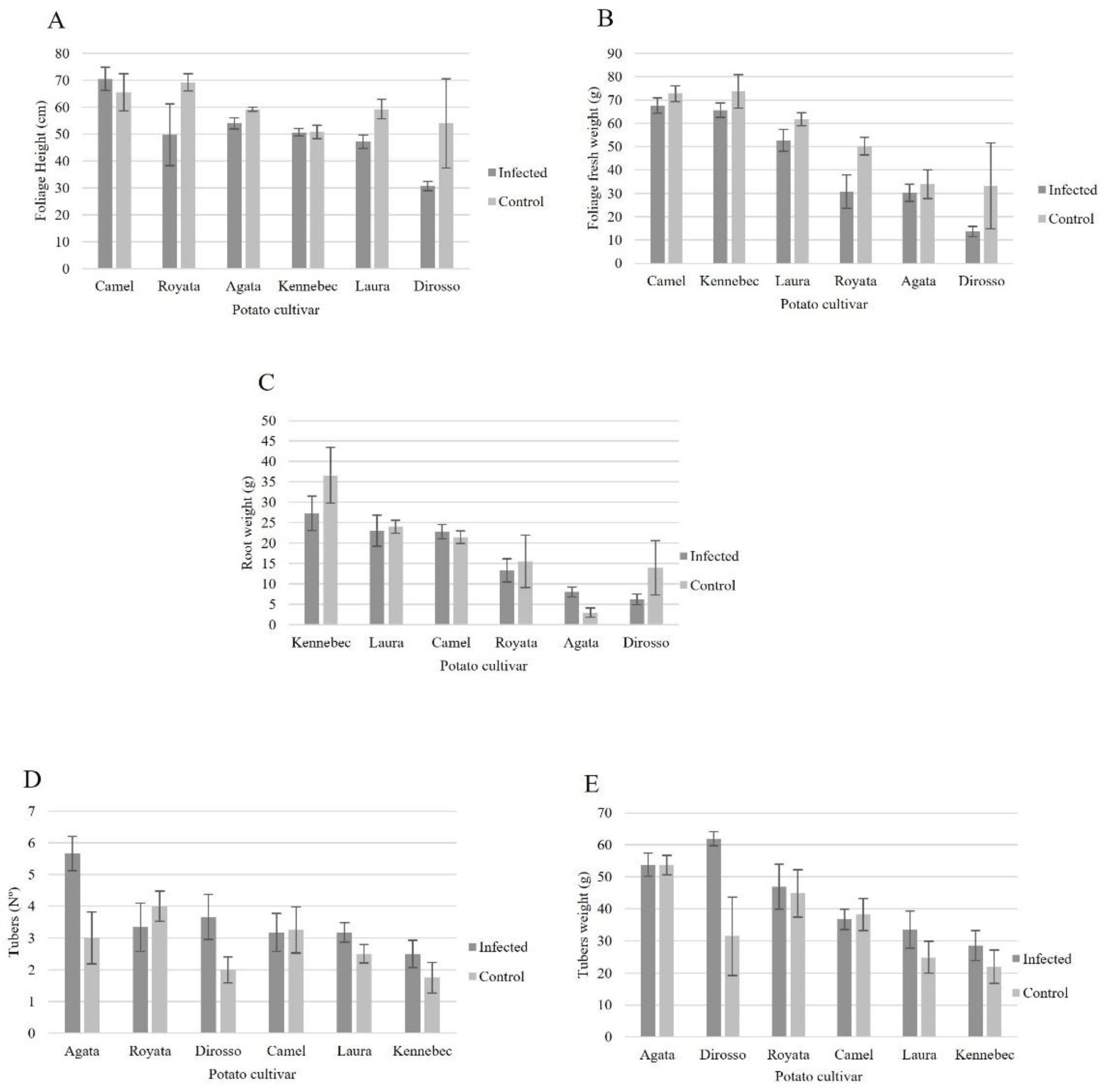
3.1. Root–Nematode Penetration Assay
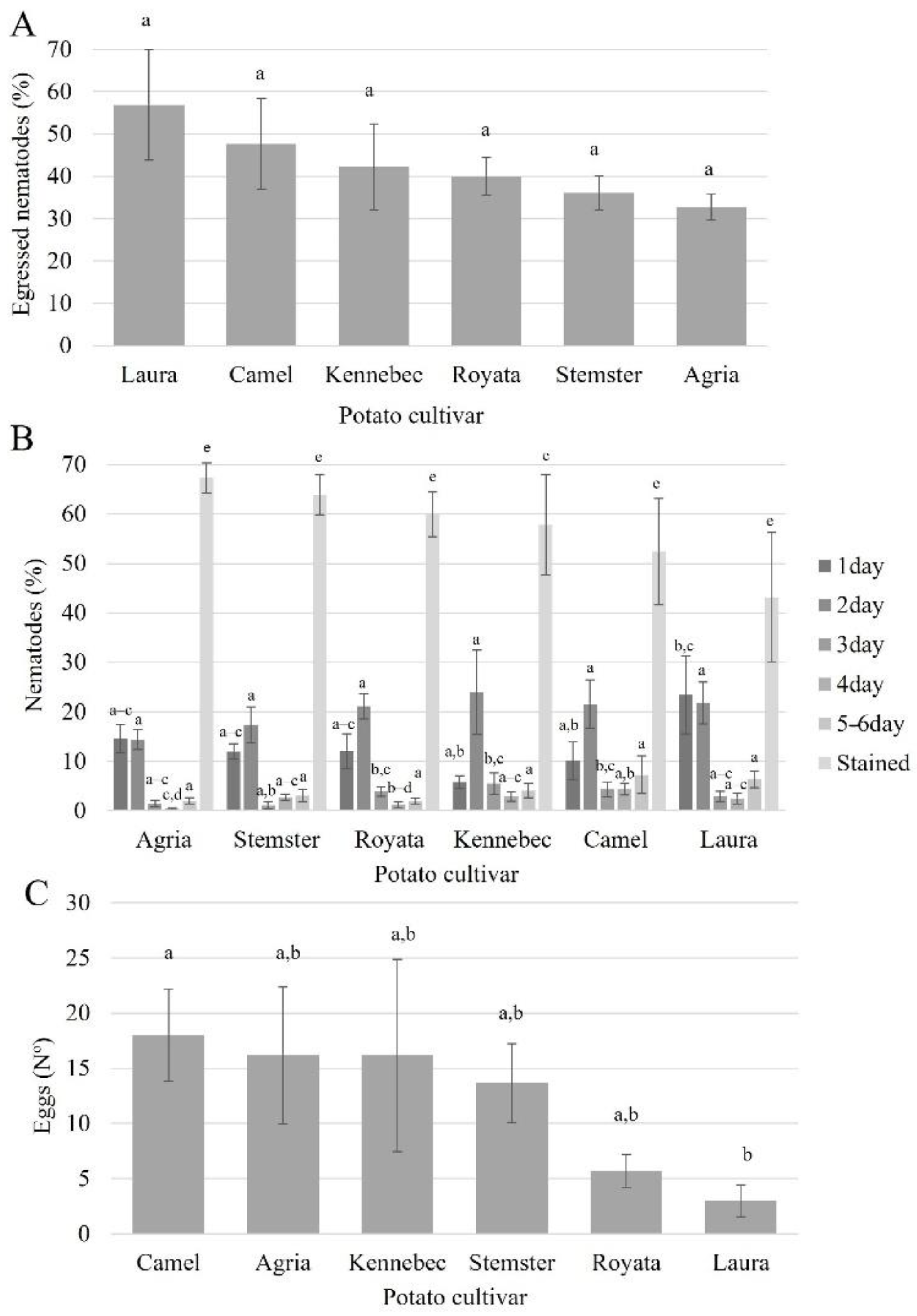
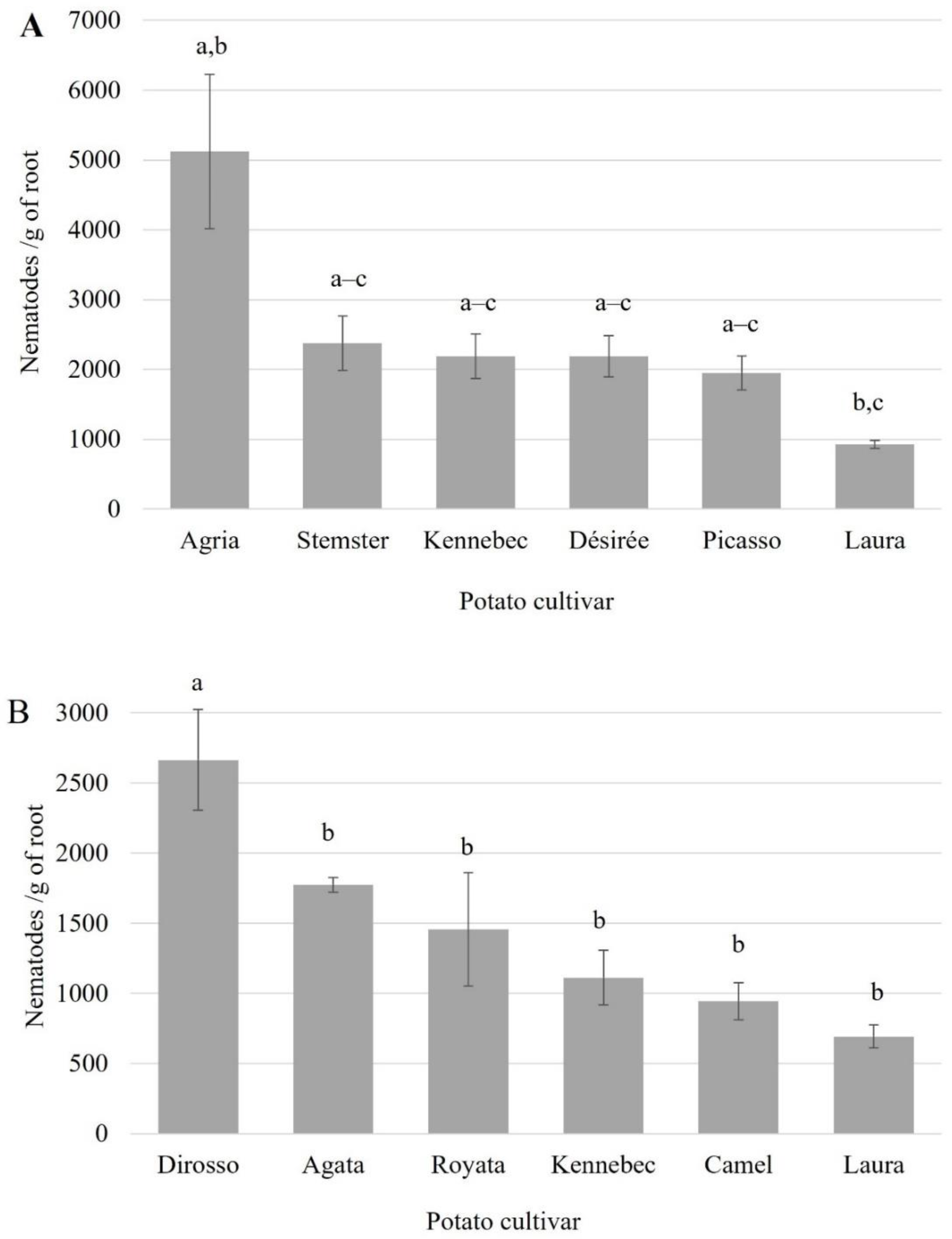
3.2. Nematode Egression Assay
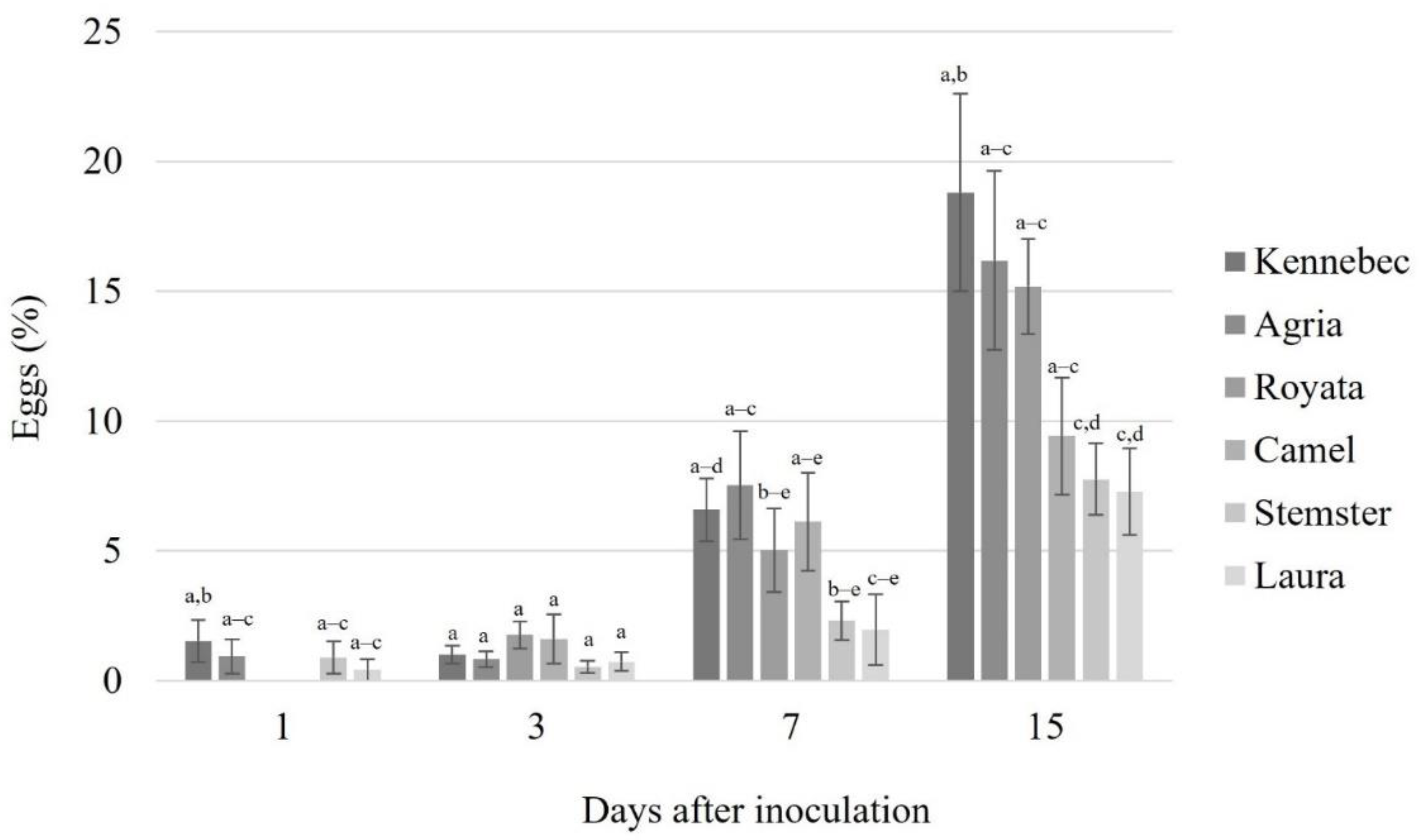
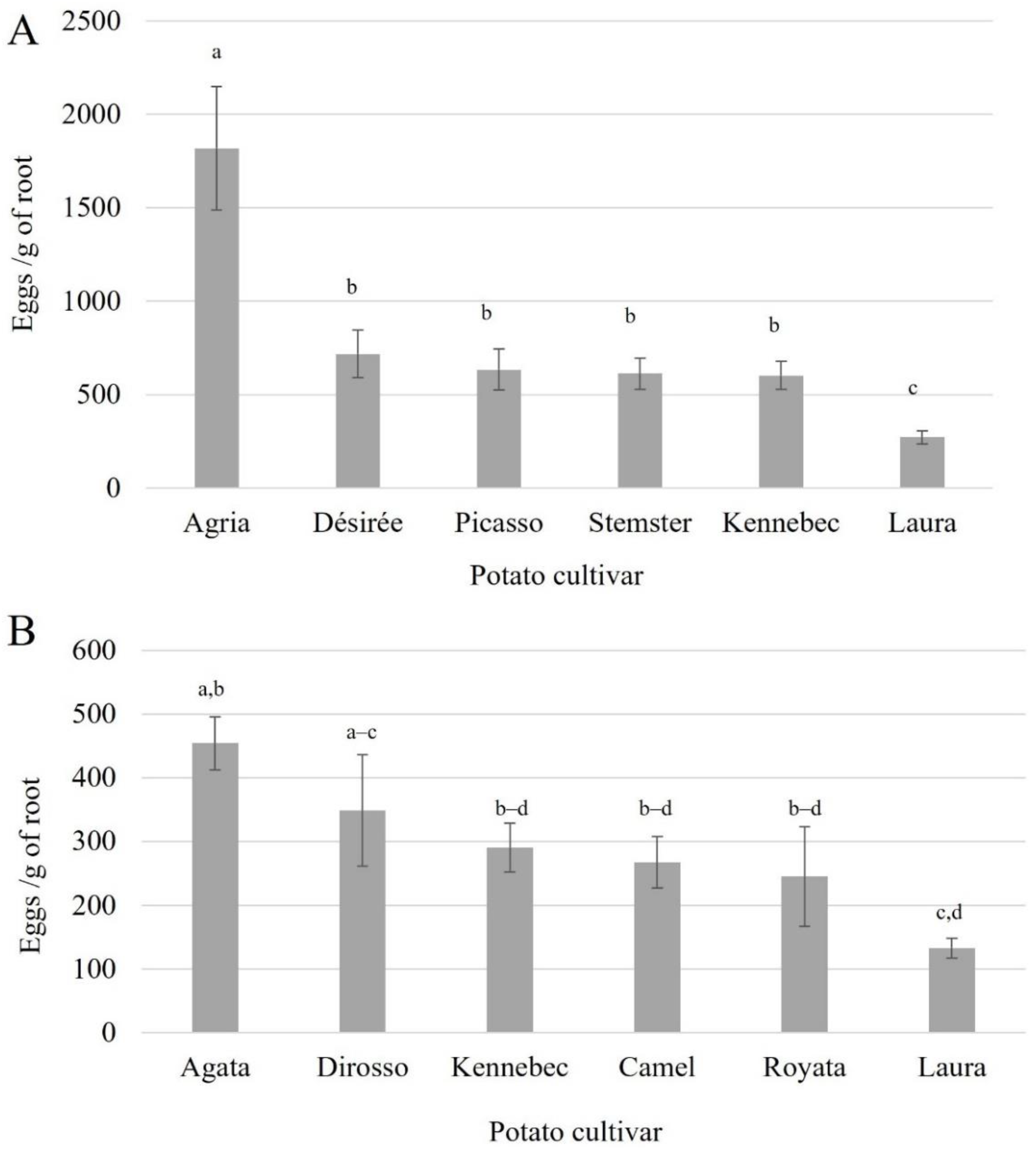
3.3. Nematode Reproduction Assay
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castillo, P.; Vovlas, N. Pratylenchus (Nematoda: Pratylenchidae): Diagnosis, Biology, Pathogenicity and Management; Brill: Leiden, The Netherlands, 2007; Volume 6, p. 529. [Google Scholar]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Cobb, N.A. A new parasitic nematode found infesting cotton and potatoes. J. Agric. Res. 1917, 11, 27–33. [Google Scholar]
- Moens, M.; Perry, R.N. Migratory plant endoparasitic nematodes: A group rich in contrasts and divergence. Annu. Rev. Phytopathol. 2009, 47, 313–332. [Google Scholar] [CrossRef]
- Jones, M.G.K.; Fosu-Nyarko, J. Molecular biology of root lesion nematodes (Pratylenchus spp.) and their interaction with host plants. Ann. Appl. Biol. 2014, 164, 163–181. [Google Scholar] [CrossRef]
- Zunke, U. Observations on the invasion and endoparasitic behavior of the root lesion nematode Pratylenchus penetrans. J. Nematol. 1990, 22, 309–320. [Google Scholar] [PubMed]
- Vieira, P.; Mayer, T.; Eves-van den Akker, S.; Howe, D.K.; Zasada, I.; Baum, T.; Eisenback, J.D.; Kamo, K. Identification of candidate effector genes of Pratylenchus penetrans. Mol. Plant Pathol. 2018, 19, 1887–1907. [Google Scholar] [CrossRef]
- Holgado, R.; Oppen Skau, K.A.; Magnusson, C. Field damage in potato by lesion nematode Pratylenchus penetrans (Coob, 1917) Filipjev and Schuurmans Stekhoven, 1941, its association with tuber symptoms and its survival in storage. Nematol. Mediterr. 2009, 37, 25–29. [Google Scholar]
- Vieira, P.; Mowery, J.; Eisenback, J.D.; Shao, J.; Nemchinov, L.G. Cellular and transcriptional responses of resistant and susceptible cultivars of alfalfa to the root lesion nematode, Pratylenchus penetrans. Front. Plant Sci. 2019, 10, 971. [Google Scholar] [CrossRef]
- Vieira, P.; Mowery, J.; Kilcrease, J.; Eisenback, J.D.; Kamo, K. Characterization of Lilium longiflorum cv. ‘Nellie White’ infection with root-lesion nematode Pratylenchus penetrans by bright-field and transmission electron microscopy. J. Nematol. 2017, 49, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Lakshman, D.; Vieira, P.; Pandey, R.; Slovin, J.; Kamo, K. Symptom development in response to combined infection of in vitro growth Lilium longiflorum with the root lesion nematode Pratylenchus penetrans and soilborne fungi collected from disease roots field-grown lilies. Plant Dis. 2017, 101, 882–889. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rowe, R.C.; Riedel, R.M.; Martin, M.J. Synergistic interactions between Verticillium dahliae and Prantylenchus penetrans in potato early dying disease. Phytopathology 1985, 75, 412–418. [Google Scholar] [CrossRef]
- Chitwood, D.J. Phytochemical based strategies for nematode control. Annu. Rev. Phytopathol. 2002, 40, 221–249. [Google Scholar] [CrossRef] [PubMed]
- McCarter, J.P. Molecular approaches toward resistance to plant-parasitic nematodes. In Cell Biology of Plant Nematode Parasitism. Plant Cell Monographs; Berg, R.H., Taylor, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 15, pp. 239–267. [Google Scholar]
- Molinari, S. Natural genetic and induced plant resistance, as a control strategy to plant-parasitic nematodes alternative to pesticides. Plant Cell Rep. 2011, 30, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Trudgill, D.L. Resistance to and tolerance of plant parasitic nematodes in plants. Annu. Rev. Phytopathol. 1991, 29, 167–192. [Google Scholar] [CrossRef]
- Peng, Y.; Moens, M. Host resistance and tolerance to migratory plant-parasitic nematodes. Nematology 2002, 5, 145–177. [Google Scholar]
- Mwaura, P.; Niere, B.; Vidal, S. Resistance and tolerance of potato varieties to potato rot nematode (Ditylenchus destructor) and stem nematode (Ditylenchus dipsaci). Ann. Appl. Biol. 2015, 166, 257–270. [Google Scholar] [CrossRef]
- Brown, M.J.; Riedel, R.M.; Rowe, R.C. Species of Pratylenchus associated with cv. Superior in Ohio. J. Nematol. 1980, 12, 189–192. [Google Scholar] [PubMed]
- Esteves, I.; Maleita, C.; Abrantes, I. Root-lesion and root-knot nematodes parasitizing potato. Eur. J. Plant Pathol. 2015, 141, 397–406. [Google Scholar] [CrossRef]
- Hafez, S.L.; Sundararaj, P.; Handoo, Z.; Siddiqi, M.R. Occurrence and distribution of nematodes in Idaho crops. Int. J. Nematol. 2010, 20, 91–98. [Google Scholar]
- Harding, R.B.; Wicks, T.J. Verticillium dahliae and Pratylenchus spp.: Populations in potato soils and plants in Australia. Australas. Plant Pathol. 2007, 36, 62–67. [Google Scholar] [CrossRef]
- Ingham, R.E.; Hamm, P.B.; Riga, E.; Merrifield, K.J. First report of stunting and root rot of potato associated with Pratylenchus penetrans in the Columbia basin of Washington. Plant Dis. 2005, 89, 207. [Google Scholar] [CrossRef] [PubMed]
- Olthof, T.H.A.; Anderson, R.V.; Squire, S. Plant-parasitic nematodes associated with potatoes (Solanum tuberosum L.) in Simcoe County, Ontario. Can. J. Plant Pathol. 1982, 4, 389–391. [Google Scholar] [CrossRef]
- Olthof, T.H.A.; Potter, J.W. The relationship between population densities of Pratylenchus penetrans and crop losses in summer-maturing vegetables in Ontario. Phytopathology 1973, 63, 577–582. [Google Scholar] [CrossRef]
- Olthof, T.H.A.; Wolynetz, M.S. Pratylenchus penetrans and P. neglectus in tubers of potato (Solanum tuberosum) in Ontario. Can. J. Plant Sci. 1991, 71, 1251–1256. [Google Scholar] [CrossRef]
- Philis, J. Presence and control of Pratylenchus penetrans on potato in Cyprus. Nematol. Medit. 1995, 23, 235–238. [Google Scholar]
- Bernard, E.C.; Laughlin, C.W. Relative susceptibility of selected cultivars of potato to Pratylenchus penetrans. J. Nematol. 1976, 8, 239–242. [Google Scholar] [PubMed]
- Kotcon, J.B.; Loria, R. Tolerance of potato cultivars to damage by Pratylenchus penetrans. In Proceedings of the First International Congress of Nematology 45, Guelph, ON, Canada, 5–10 August 1984. [Google Scholar]
- Kimpinski, J.; McRae, K.B. Relationship of yield and Pratylenchus spp. population densities in Superior and Russet Burbank potato. Ann. Appl. Nematol. 1988, 2, 34–37. [Google Scholar]
- Olthof, T.H.A. Reaction of six Solanum tuberosum cultivars to Pratylenchus penetrans. J. Nematol. 1986, 18, 54–58. [Google Scholar]
- Brodie, B.B.; Plaisted, R.L. Resistance in potato to Pratylenchus penetrans. J. Nematol. 1993, 25, 466–471. [Google Scholar]
- Mai, W.F.; Bloom, J.R.; Chen, T.A. Biology and Ecology of the Plant-Parasitic Nematode Pratylenchus penetrans; Bulletin 810; The Pennsylvania State University, College of Agriculture: University Park, PA, USA, 1977. [Google Scholar]
- France, R.A.; Brodie, B.B. Differentiation of two New York isolates of Pratylenchus penetrans based on their reaction on potato. J. Nematol. 1995, 27, 339–345. [Google Scholar] [PubMed]
- France, R.A.; Brodie, B.B. Characterization of Pratylenchus penetrans from ten geographically isolated populations based on their reaction on potato. J. Nematol. 1996, 28, 520–526. [Google Scholar]
- Figueiredo, J.; Vieira, P.; Abrantes, I.; Esteves, I. Detection of the root lesion nematode Pratylenchus penetrans in potato tubers. Plant Pathol. 2021, 70, 1960–1968. [Google Scholar] [CrossRef]
- Orlando, V.; Grove, I.G.; Edwards, S.G.; Prior, T.; Roberts, D.; Neilson, R.; Back, M. Root-lesion nematodes of potato: Current status of diagnostics, pathogenicity and management. Plant Pathol. 2020, 69, 405–417. [Google Scholar] [CrossRef]
- Al-Banna, L.; Ploeg, A.T.; Williamson, V.M.; Kaloshian, I. Discrimination of six Pratylenchus species using PCR and species-specific primers. J. Nematol. 2004, 36, 142–146. [Google Scholar] [PubMed]
- Castillo, P.; Trapero-Casas, J.L.; Jiménez-Díaz, R.M. Effect of time, temperature and inoculum density on reproduction of Pratylenchus thornei in carrot disks cultures. J. Nematol. 1995, 27, 120–124. [Google Scholar] [PubMed]
- Byrd, D.W.; Kirkpatrick, T., Jr.; Barker, K.R. An improved technique for clearing and staining plant tissue for detection of nematodes. J. Nematol. 1983, 15, 142–143. [Google Scholar]
- Whitehead, A.G.; Hemming, J.R. A comparison of some quantitative methods of extracting small vermiform nematode from soil. Ann. Appl. Biol. 1965, 55, 25–38. [Google Scholar] [CrossRef]
- Baermann, G. A simple method to recover larvae of Ancylostomus (Nematoda) from soil samples. Geneeskd. Tijdschr. Voor Ned.-Indie 1917, 57, 131–137. [Google Scholar]
- Fosu-Nyarko, J.; Jones, M.G.K. Advances in understanding the molecular mechanisms of root lesion nematode host interactions. Annu. Rev. Phytopathol. 2016, 54, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Birch, P.R.J.; Bryan, G.; Fenton, B.; Gilroy, E.; Hein, I.; Jones, J.T.; Prashar, A.; Taylor, M.A.; Torrance, L.; Toth, I.K. Crops that feed the world 8: Potato: Are the trends of increased global production sustainable? Food Secur. 2012, 4, 477–508. [Google Scholar] [CrossRef]
- Dahal, K.; Li, X.-Q.; Tai, H.; Creelman, A.; Bizimungu, B. Improving potato stress tolerance and tuber yield under a climate change scenario—A current overview. Front. Plant Sci. 2019, 10, 563. [Google Scholar] [CrossRef] [PubMed]
- Holgado, R.; Magnusson, C. Nematodes as a limiting factor in potato production in Scandinavia. Potato Res. 2012, 55, 269–278. [Google Scholar] [CrossRef][Green Version]
- Lima, F.S.O.; Mattos, V.S.; Silva, E.S.; Carvalho, M.A.S.; Teixeira, R.A.; Silva, J.C.; Correa, V.R. Nematodes affecting potato and sustainable practices for their management. In Potato: From Incas to All over the World; Yildiz, M., Ed.; IntechOpen: London, UK, 2018; pp. 107–121. [Google Scholar]
- Linsell, K.J.; Riley, I.T.; Davies, K.A.; Oldach, K.H. Characterization of resistance to Pratylenchus thornei (Nematoda) in wheat (Triticum aestivum); attraction, penetration, motility and reproduction. Phytopathology 2014, 104, 174–187. [Google Scholar] [CrossRef]
- Kathiresan, T.; Mehta, U.K. Penetration, multiplication and histopathological response of Pratylenchus zeae in resistant and susceptible sugarcane clones. Int. J. Nematol. 2002, 12, 189–196. [Google Scholar]
- Channale, S.; Kalavikatte, D.; Thompson, J.P.; Kudapa, H.; Bajaj, P.; Varshney, R.K.; Zwart, R.S.; Thidi, M. Transcriptome analysis reveals key genes associated with root-lesion nematode Pratylenchus thornei resistance in chickpea. Sci. Rep. 2021, 11, 17491. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Sharma, S.; Sharma, R.; Pundir, S.; Singh, V.K.; Chaturvedi, D.; Singh, B.; Kumar, S.; Sharma, S. Genome-wide association study in hexaploid wheat identifies novel genomic regions associated with resistance to root lesion nematode (Pratylenchus thornei). Sci. Rep. 2021, 11, 35723. [Google Scholar] [CrossRef]
- Miller, P.M. Reproduction, penetration, and pathogenicity of Pratylenchus penetrans on tobacco, vegetables, and cover crops. Phytopathology 1978, 68, 1502–1504. [Google Scholar] [CrossRef]
- Townshend, J.L.; Stobbs, L. Histopathology and histochemistry of lesions caused by Pratylenchus penetrans in roots of forage legumes. Can. J. Plant Pathol. 1981, 3, 123–128. [Google Scholar] [CrossRef]
- Kurppa, S.; Vrain, T.C. Penetration and feeding behaviour of Pratylenchus penetrans in strawberry roots. Rev. Nematol. 1985, 8, 273–276. [Google Scholar]
- Motalaote, B.; Starr, J.L.; Frederiksen, R.A.; Miller, F.R. Host status and susceptibility of sorghum to Pratylenchus species. Rev. Nematol. 1987, 10, 81–86. [Google Scholar]
- Castillo, P.; Vovlas, N.; Jimenez-Diaz, R.M. Pathogenicity and histopathology of Pratylenchus thornei populations on selected chickpea genotypes. Plant Pathol. 1998, 47, 370–376. [Google Scholar] [CrossRef]
- Rahaman, M.M.; Zwart, R.S.; Rupasinghe, T.W.T.; Hayden, H.L.; Thompson, J.P. Metabolomic profiling of wheat genotypes resistant and susceptible to root-lesion nematode Pratylenchus thornei. Plant Mol. Biol. 2021, 106, 381–406. [Google Scholar] [CrossRef]

| Assay | Potato Cultivar | Susceptibility Index (SI) |
|---|---|---|
| 1 | Laura | 0.4 b,c |
| Picasso | 0.9 a,b,c | |
| Kennebec | 1.0 a,b,c | |
| Désirée | 1.0 a,b,c | |
| Stemster | 1.1 a,b,c | |
| Agria | 2.3 a,b | |
| 2 | Laura | 0.6 b |
| Camel | 0.8 b | |
| Kennebec | 1.0 a | |
| Royata | 1.3 b | |
| Agata | 1.6 b | |
| Dirosso | 2.4 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueiredo, J.; Vieira, P.; Abrantes, I.; Esteves, I. Commercial Potato Cultivars Exhibit Distinct Susceptibility to the Root Lesion Nematode Pratylenchus penetrans. Horticulturae 2022, 8, 244. https://doi.org/10.3390/horticulturae8030244
Figueiredo J, Vieira P, Abrantes I, Esteves I. Commercial Potato Cultivars Exhibit Distinct Susceptibility to the Root Lesion Nematode Pratylenchus penetrans. Horticulturae. 2022; 8(3):244. https://doi.org/10.3390/horticulturae8030244
Chicago/Turabian StyleFigueiredo, Joana, Paulo Vieira, Isabel Abrantes, and Ivânia Esteves. 2022. "Commercial Potato Cultivars Exhibit Distinct Susceptibility to the Root Lesion Nematode Pratylenchus penetrans" Horticulturae 8, no. 3: 244. https://doi.org/10.3390/horticulturae8030244
APA StyleFigueiredo, J., Vieira, P., Abrantes, I., & Esteves, I. (2022). Commercial Potato Cultivars Exhibit Distinct Susceptibility to the Root Lesion Nematode Pratylenchus penetrans. Horticulturae, 8(3), 244. https://doi.org/10.3390/horticulturae8030244






