Platanus hybrida’s Phenolic Profile, Antioxidant Power, and Antibacterial Activity against Methicillin-Resistant Staphylococcus aureus (MRSA)
Abstract
:1. Introduction
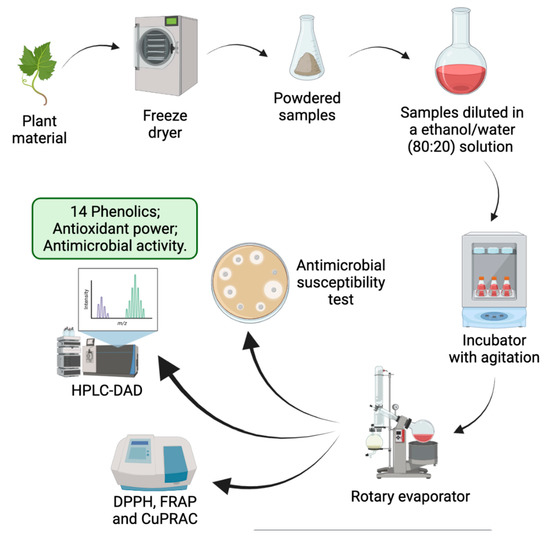
2. Materials and Methods
2.1. Plant Material
2.2. Phenolic Extracts
2.3. Phenolic Profile
2.4. Antioxidant Power
2.4.1. DPPH Assay
2.4.2. FRAP Assay
2.4.3. CuPRAC Assay
2.5. Antimicrobial Activity
2.5.1. Bacterial Strains
2.5.2. Antimicrobial Susceptibility Test
2.6. Statistical Analysis
3. Results
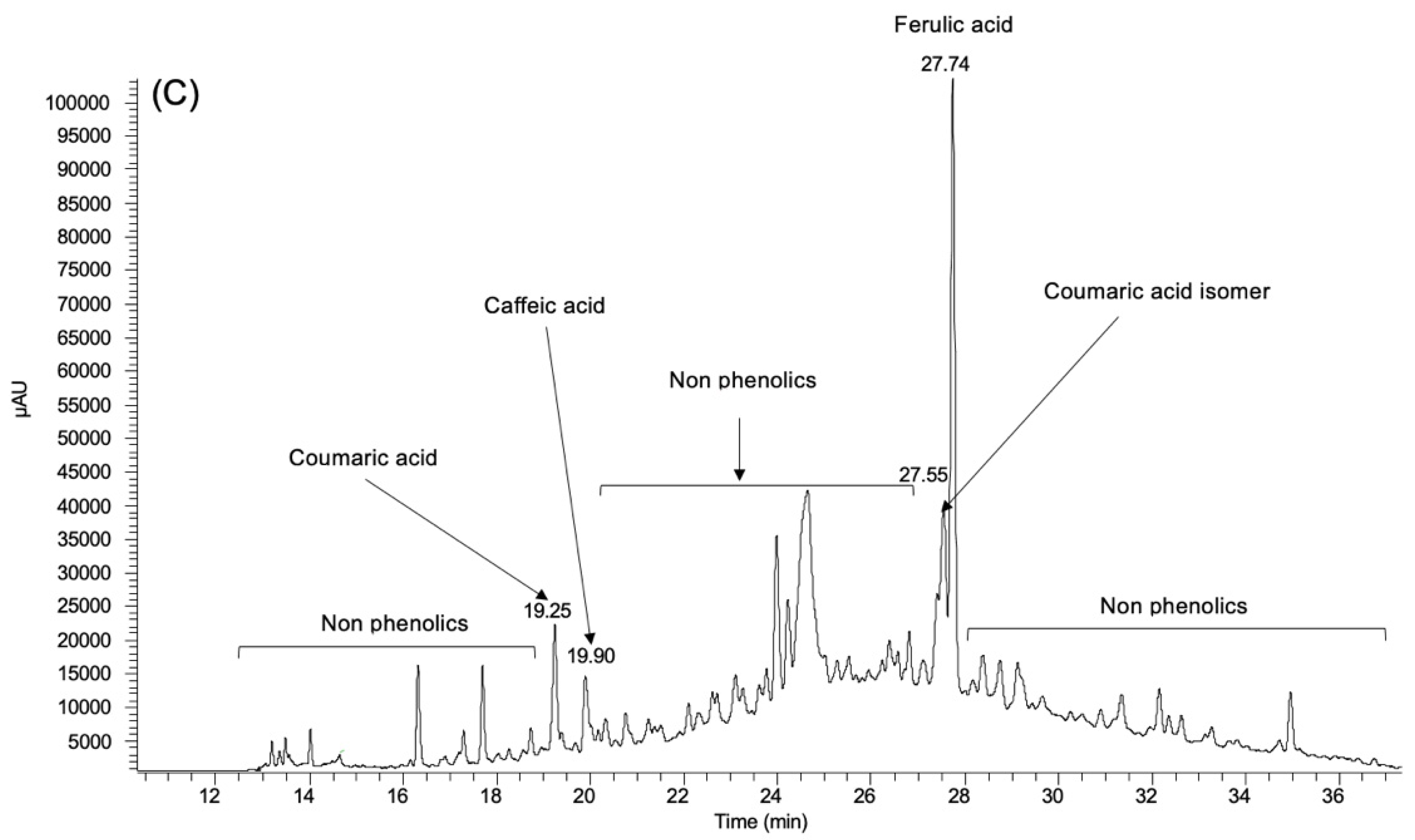
3.1. Phenolic Profile
3.2. Antioxidant Power
3.3. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, V.; Pereira, J.E.; Maltez, L.; Ferreira, E.; Manageiro, V.; Caniça, M.; Capelo, J.L.; Igrejas, G.; Poeta, P. Diversity of methicillin-resistant staphylococci among wild Lepus granatensis: First detection of mecA-MRSA in hares. FEMS Microbiol. Ecol. 2020, 96, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Algammal, A.M.; Hetta, H.F.; Elkelish, A.; Alkhalifah, D.H.H.; Hozzein, W.N.; Batiha, G.E.S.; El Nahhas, N.; Mabrok, M.A. Methicillin-resistant Staphylococcus aureus (MRSA): One health perspective approach to the bacterium epidemiology, virulence factors, antibiotic-resistance, and zoonotic impact. Infect. Drug Resist. 2020, 13, 3255–3265. [Google Scholar] [CrossRef] [PubMed]
- Amaro, F.; Martín-González, A. Microbial warfare in the wild—The impact of protists on the evolution and virulence of bacterial pathogens. Int. Microbiol. 2021, 24, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Bernier-Lachance, J.; Arsenault, J.; Usongo, V.; Parent, E.; Labrie, J.; Jacques, M.; Malouin, F.; Archambault, M. Prevalence and characteristics of livestock- associated methicillin-resistant Staphylococcus aureus (LA-MRSA) isolated from chicken meat in the province of Quebec, Canada. PLoS ONE 2020, 15, e0227183. [Google Scholar] [CrossRef] [PubMed]
- Collignon, P.J.; McEwen, S.A. One health-its importance in helping to better control antimicrobial resistance. Trop. Med. Infect. Dis. 2019, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Redican, K.J.; Akpinar-Elci, M. Integrating One Health into Professional Preparation Education for Public Health and Health Education Specialists. Am. J. Heal. Educ. 2021, 52, 11–17. [Google Scholar] [CrossRef]
- Tan, L.; Zhou, Z.; Liu, X.; Li, J.; Zheng, Y.; Cui, Z.; Yang, X.; Liang, Y.; Li, Z.; Feng, X.; et al. Overcoming Multidrug-Resistant MRSA Using Conventional Aminoglycoside Antibiotics. Adv. Sci. 2020, 7, 1902070. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Mumtaz, S.; Li, C.H.; Hussain, I.; Rotello, V.M. Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef]
- García, J.; García-Galán, M.J.; Day, J.W.; Boopathy, R.; White, J.R.; Wallace, S.; Hunter, R.G. A review of emerging organic contaminants (EOCs), antibiotic resistant bacteria (ARB), and antibiotic resistance genes (ARGs) in the environment: Increasing removal with wetlands and reducing environmental impacts. Bioresour. Technol. 2020, 307, 123228. [Google Scholar] [CrossRef]
- Zipperer, A.; Konnerth, M.C.; Laux, C.; Berscheid, A.; Janek, D.; Weidenmaier, C.; Burian, M.; Schilling, N.A.; Slavetinsky, C.; Marschal, M.; et al. Human commensals producing a novel antibiotic impair pathogen colonization. Nature 2016, 535, 511–516. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Henriques, M.; Silva, S.; Ferreira, I.C.F.R. Evaluation of bioactive properties and phenolic compounds in different extracts prepared from Salvia officinalis L. Food Chem. 2014, 170, 378–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uysal, A.; Zengin, G.; Mahomoodally, M.F.; Picot-Allain, C.; Jekő, J.; Cziáky, Z.; Rodrigues, M.J.; Ak, G.; Polat, R.; Urusan, Z.; et al. A comparative study on biological properties and chemical profiles of different solvent extracts from Centaurea bingoelensis, an endemic plant of Turkey. Process Biochem. 2021, 102, 315–324. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Sitarek, P.; Toma, M.; Rijo, P.; Domínguez-Martín, E.; Falcó, I.; Sánchez, G.; Śliwiński, T. Enhanced Accumulation of Betulinic Acid in Transgenic Hairy Roots of Senna obtusifolia Growing in the Sprinkle Bioreactor and Evaluation of Their Biological Properties in Various Biological Models. Chem. Biodivers. 2021, 18, e2100455. [Google Scholar] [CrossRef] [PubMed]
- Barral-Martinez, M.; Garcia-Oliveira, P.; Nuñez-Estevez, B.; Silva, A.; Finimundy, T.C.; Calhelha, R.; Nenadic, M.; Sokovic, M.; Barroso, F.; Simal-Gandara, J.; et al. Plants of the Family Asteraceae: Evaluation of Biological Properties and Identification of Phenolic Compounds. Chem. Proc. 2021, 5, 51. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Micol, V. Tackling antibiotic resistance with compounds of natural origin: A comprehensive review. Biomedicines 2020, 8, 405. [Google Scholar] [CrossRef]
- Silva, V.; Falco, V.; Dias, M.I.; Barros, L.; Silva, A.; Capita, R.; Alonso-Calleja, C.; Amaral, J.S.; Igrejas, G.; Ferreira, I.C.F.R.; et al. Evaluation of the phenolic profile of Castanea sativa mill. By-products and their antioxidant and antimicrobial activity against multiresistant bacteria. Antioxidants 2020, 9, 87. [Google Scholar] [CrossRef] [Green Version]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Tan, B.; Liu, Y.; Dunn, J.; Martorell Guerola, P.; Tortajada, M.; Cao, Z.; Ji, P. Chemical Composition and Antioxidant Properties of Essential Oils from Peppermint, Native Spearmint and Scotch Spearmint. Molecules 2019, 24, 2825. [Google Scholar] [CrossRef] [Green Version]
- Hajhashemi, V.; Ghannadi, A.; Mousavi, S. Antinociceptive study of extracts of Platanus orientalis leaves in mice. Res. Pharm. Sci. 2011, 6, 123–128. [Google Scholar]
- Lara, B.; Rojo, J.; Fernández-González, F.; Pérez-Badia, R. Prediction of airborne pollen concentrations for the plane tree as a tool for evaluating allergy risk in urban green areas. Landsc. Urban Plan. 2019, 189, 285–295. [Google Scholar] [CrossRef]
- Dogan, A.; Anuk, O.O. Investigation of the phytochemical composition and antioxidant properties of chinar (Platanus orientalis L.) leaf infusion against ethanol-induced oxidative stress in rats. Mol. Biol. Rep. 2019, 46, 3049–3061. [Google Scholar] [CrossRef] [PubMed]
- Chatzigeorgiou, S.; Thai, Q.D.; Tchoumtchoua, J.; Tallas, K.; Tsakiri, E.N.; Papassideri, I.; Halabalaki, M.; Skaltsounis, A.L.; Trougakos, I.P. Isolation of natural products with anti-ageing activity from the fruits of Platanus orientalis. Phytomedicine 2017, 33, 53–61. [Google Scholar] [CrossRef]
- Ribeiro, J.; Silva, V.; Aires, A.; Carvalho, R.; Igrejas, G.; Poeta, P. Antimicrobial Activity of Phenolic Compounds Extracted from Platanus hybrida: Exploring Alternative Therapies for a Post-Antibiotic Era. Proceedings 2021, 66, 18. [Google Scholar] [CrossRef]
- Vrinceanu, D.; Berghi, O.; Cergan, R.; Dumitru, M.; Ciuluvica, R.; Giurcaneanu, C.; Neagos, A. Urban allergy review: Allergic rhinitis and asthma with plane tree sensitization (Review). Exp. Ther. Med. 2021, 21, 275. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Huang, J.; Chen, L.; Bao, M. Plant regeneration from excised hypocotyl explants of Platanus acerifolia willd. Vitr. Cell. Dev. Biol.—Plant 2002, 38, 558–563. [Google Scholar] [CrossRef]
- Kutbay, I.; Akfırat, F.Ş. Mapping of Biochemical Constituents in Platanus acerifolia Leaves By Analytical Techniques. Procedia—Soc. Behav. Sci. 2015, 195, 1719–1727. [Google Scholar] [CrossRef] [Green Version]
- Thai, Q.D.; Tchoumtchoua, J.; Makropoulou, M.; Boulaka, A.; Meligova, A.K.; Mitsiou, D.J.; Mitakou, S.; Michel, S.; Halabalaki, M.; Alexis, M.N.; et al. Phytochemical study and biological evaluation of chemical constituents of Platanus orientalis and Platanus × acerifolia buds. Phytochemistry 2016, 130, 170–181. [Google Scholar] [CrossRef]
- Silva, V.; Igrejas, G.; Falco, V.; Santos, T.P.; Torres, C.; Oliveira, A.M.P.; Pereira, J.E.; Amaral, J.S.; Poeta, P. Chemical composition, antioxidant and antimicrobial activity of phenolic compounds extracted from wine industry by-products. Food Control 2018, 92, 516–522. [Google Scholar] [CrossRef] [Green Version]
- Aires, A.; Carvalho, R. Kiwi fruit residues from industry processing: Study for a maximum phenolic recovery yield. J. Food Sci. Technol. Technol 2020, 57, 4265–4276. [Google Scholar] [CrossRef]
- Aires, A.; Carvalho, R.; Matos, M.; Carnide, V.; Silva, A.P.; Gonçalves, B. Variation of chemical constituents, antioxidant activity, and endogenous plant hormones throughout different ripening stages of highbush blueberry (Vaccinium corymbosum L.) cultivars produced in centre of Portugal. J. Food Biochem. 2017, 41, e12414. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, V.; Almeida, F.; Carvalho, J.A.; Castro, A.P.; Ferreira, E.; Manageiro, V.; Tejedor-Junco, M.T.; Caniça, M.; Igrejas, G.; Poeta, P. Emergence of community-acquired methicillin-resistant Staphylococcus aureus EMRSA-15 clone as the predominant cause of diabetic foot ulcer infections in Portugal. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Almeida, F.; Silva, A.; Correia, S.; Carvalho, J.A.; Castro, A.P.; Ferreira, E.; Manageiro, V.; Caniça, M.; Igrejas, G.; et al. First report of linezolid-resistant cfr-positive methicillin-resistant Staphylococcus aureus in humans in Portugal. J. Glob. Antimicrob. Resist. 2019, 17, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; De Sousa, T.; Gómez, P.; Sabença, C.; Vieira-Pinto, M.; Capita, R.; Alonso-Calleja, C.; Torres, C.; Capelo, J.L.; Igrejas, G.; et al. Livestock-associated methicillin-resistant Staphylococcus aureus (MRSA) in purulent subcutaneous lesions of farm rabbits. Foods 2020, 9, 439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahromi, S.G. Extraction Techniques of Phenolic Compounds from Plants; Woodhead Publishing: Thorston, UK, 2019. [Google Scholar]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef] [PubMed]
- Sik, B.; Hanczné, E.L.; Kapcsándi, V.; Ajtony, Z. Conventional and nonconventional extraction techniques for optimal extraction processes of rosmarinic acid from six Lamiaceae plants as determined by HPLC-DAD measurement. J. Pharm. Biomed. Anal. 2020, 184, 113173. [Google Scholar] [CrossRef] [PubMed]
- Kaderides, K.; Papaoikonomou, L.; Serafim, M.; Goula, A.M. Microwave-assisted extraction of phenolics from pomegranate peels: Optimization, kinetics, and comparison with ultrasounds extraction. Chem. Eng. Process.—Process Intensif. 2019, 137, 1–11. [Google Scholar] [CrossRef]
- Zabad, O.M.; Samra, Y.A.; Eissa, L.A. P-Coumaric acid alleviates experimental diabetic nephropathy through modulation of Toll like receptor-4 in rats. Life Sci. 2019, 238, 116965. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Sinha, B.; Choudhury, B.P.; Jha, N.K.; Palit, P.; Kundu, S.; Mandal, S.C.; Kolesarova, A.; Yousef, M.I.; Ruokolainen, J.; et al. Scavenging properties of plant-derived natural biomolecule para-coumaric acid in the prevention of oxidative stress-induced diseases. Antioxidants 2021, 10, 1205. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Hu, C.; Chen, Y.; Cao, Y.; Jia, Y.; Zhang, J. Metabolomics analysis reveals the protective effect of quercetin-3-O-galactoside (Hyperoside) on liver injury in mice induced by acetaminophen. J. Food Biochem. 2020, 44, e13420. [Google Scholar] [CrossRef] [PubMed]
- Al-Naib, F.A.-G.; Rice, E.L. Allelopathic Effects of Platanus occidentalis. Torrey Bot. Soc. 1971, 98, 75–82. [Google Scholar] [CrossRef]
- Tuli, H.S.; Aggarwal, V.; Kaur, J.; Aggarwal, D.; Parashar, G.; Parashar, N.C.; Tuorkey, M.; Kaur, G.; Savla, R.; Sak, K.; et al. Baicalein: A metabolite with promising antineoplastic activity. Life Sci. 2020, 259, 118183. [Google Scholar] [CrossRef]
- Lin, M.C.; Tsai, M.J.; Wen, K.C. Supercritical fluid extraction of flavonoids from Scutellariae Radix. J. Chromatogr. A 1999, 830, 387–395. [Google Scholar] [CrossRef]
- Georgieva, Y.; Katsarova, M.; Stoyanov, P.; Mladenov, R.; Denev, P.; Teneva, D.; Plotnikov, E.; Bozov, P.; Dimitrova, S. Metabolite profile and antioxidant activity of some species of genus Scutellaria growing in bulgaria. Plants 2021, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Singh, R.K.; Gomes, N.; Soares, B.G.; Silva, A.; Falco, V.; Capita, R.; Alonso-Calleja, C.; Pereira, J.E.; Amaral, J.S.; et al. Comparative insight upon chitosan solution and chitosan nanoparticles application on the phenolic content, antioxidant and antimicrobial activities of individual grape components of Sousão variety. Antioxidants 2020, 9, 178. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Rui, Y.X.; Guo, S.D.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef]
- López-Froilán, R.; Hernández-Ledesma, B.; Cámara, M.; Pérez-Rodríguez, M.L. Evaluation of the Antioxidant Potential of Mixed Fruit-Based Beverages: A New Insight on the Folin-Ciocalteu Method. Food Anal. Methods 2018, 11, 2897–2906. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Devaki, M. The ferric reducing/antioxidant power (FRAP) assay for non-enzymatic antioxidant capacity: Concepts, procedures, limitations and applications. Meas. Antioxid. Act. Capacit. Recent Trends Appl. 2018, 5, 77–106. [Google Scholar] [CrossRef]
- Aires, A.; Carvalho, R.; Rosa, E.A.S.; Saavedra, M.J. Phytochemical characterization and antioxidant properties of baby-leaf watercress produced under organic production system. CYTA—J. Food 2013, 11, 343–351. [Google Scholar] [CrossRef]
- Mercado-Mercado, G.; de la Rosa, L.A.; Alvarez-Parrilla, E. Effect of pectin on the interactions among phenolic compounds determined by antioxidant capacity. J. Mol. Struct. 2020, 1199, 126967. [Google Scholar] [CrossRef]
- Parikh, B.; Patel, V.H. Total phenolic content and total antioxidant capacity of common Indian pulses and split pulses. J. Food Sci. Technol. 2018, 55, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Olszowy-Tomczyk, M. Synergistic, antagonistic and additive antioxidant effects in the binary mixtures. Phytochem. Rev. 2020, 19, 63–103. [Google Scholar] [CrossRef]
- Velásquez, P.; Giordano, A.; Valenzuela, L.M.; Montenegro, G. Combined antioxidant capacity of Chilean bee hive products using mixture design methodology. Lwt 2022, 155, 112982. [Google Scholar] [CrossRef]
- Yamazaki, E.; Inagaki, M.; Kurita, O.; Inoue, T. Antioxidant activity of Japanese pepper (Zanthoxylum piperitum DC.) fruit. Food Chem. 2007, 100, 171–177. [Google Scholar] [CrossRef]
- Conforti, F.; Statti, G.A.; Tundis, R.; Menichini, F.; Houghton, P. Antioxidant activity of methanolic extract of Hypericum triquetrifolium Turra aerial part. Fitoterapia 2002, 73, 479–483. [Google Scholar] [CrossRef]
- Dutta, S.; Kundu, A. Macroporous resin-assisted enrichment, characterizations, antioxidant and anticandidal potential of phytochemicals from Trachyspermum ammi. J. Food Biochem. 2021, e13847. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Li, H. Reassessment of Antioxidant Activity of Baicalein in vitro. Asian J. Pharm. Biol. Res. 2011, 1, 186–194. [Google Scholar]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Yang, G.; Hong, S.; Yang, P.; Sun, Y.; Wang, Y.; Zhang, P.; Jiang, W.; Gu, Y. Discovery of an ene-reductase for initiating flavone and flavonol catabolism in gut bacteria. Nat. Commun. 2021, 12, 790. [Google Scholar] [CrossRef] [PubMed]
- Woodman, O.L.; Meeker, W.F.; Boujaoude, M. Vasorelaxant and antioxidant activity of flavonols and flavones: Structure-activity relationships. J. Cardiovasc. Pharmacol. 2005, 46, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidant properties of ferulic acid and its related compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Mansoor, A.A.; Gross, A.; Ashfaq, M.K.; Jacob, M.; Khan, S.I.; Hamann, M.T. Methicillin-resistant Staphylococcus aureus (MRSA)-active metabolites from Platanus occidentalis (American sycamore). J. Nat. Prod. 2009, 72, 2141–2144. [Google Scholar] [CrossRef] [Green Version]
- Jalil, M.T.M.; Zakaria, N.A.; Ibrahim, D. Effect of culture medium incorporated with ocimum sanctum extract in enhancing anti-mrsa activity of endophytic fungus, Lasiodiplodia pseudotheobromae. J. Pure Appl. Microbiol. 2021, 15, 1398–1408. [Google Scholar] [CrossRef]
- Tayel, A.A.; Shaban, S.M.; Moussa, S.H.; Elguindy, N.M.; Diab, A.M.; Mazrou, K.E.; Ghanem, R.A.; El-Sabbagh, S.M. Bioactivity and application of plant seeds’ extracts to fight resistant strains of Staphylococcus aureus. Ann. Agric. Sci. 2018, 63, 47–53. [Google Scholar] [CrossRef]
- Oliveira, D.M.; Oliveira, D.B.C.; Nunes, Y.R.F.; Almeida Alves, T.M.; Kohlhoff, M.; Andrade, A.A.; Cota, B.B. Natural Occurring Phenolic Derivatives from Mauritia flexuosa (Buriti) Stems and Their Potential Antibacterial Activity against Methicillin-Resistant Staphylococcus aureus (MRSA). Chem. Biodivers. 2022, 19, e202100788. [Google Scholar] [CrossRef] [PubMed]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Wang, X.; Han, L.; Li, S.; Zhou, W. Antioxidant and antimicrobial polyvinyl alcohol electrospun nanofibers containing baicalein-hydroxypropyl-β-cyclodextrin inclusion complex. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 614, 126135. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial activity of polyphenols and alkaloids in middle eastern plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- Jubair, N.; Rajagopal, M.; Chinnappan, S.; Abdullah, N.B.; Fatima, A. Review on the Antibacterial Mechanism of Plant-Derived Compounds against Multidrug-Resistant Bacteria (MDR). Evidence-based Complement. Altern. Med. 2021, 2021, 3663315. [Google Scholar] [CrossRef] [PubMed]
- Macêdo, S.K.S.; Almeida, T.S.; Alencar Filho, J.M.T.; Lima, K.S.B.; Libório, R.C.; Costa, M.M.; Rolim Neto, P.J.; Rolim, L.A.; Nunes, X.P. Phytochemical identification and quantification of quercetin in Triplaris gardneriana wedd. leaves by HPLC-DAD with evaluation of antibacterial activity. Nat. Prod. Res. 2021, 35, 3083–3088. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, N.K.; Yogendra Kumar, M.S.; Gupta, A. Antioxidant, cytoprotective and antibacterial effects of Sea buckthorn (Hippophae rhamnoides L.) leaves. Food Chem. Toxicol. 2010, 48, 3443–3448. [Google Scholar] [CrossRef] [PubMed]
- Ibitoye, O.B.; Ajiboye, T.O. Ferulic acid potentiates the antibacterial activity of quinolone-based antibiotics against Acinetobacter baumannii. Microb. Pathog. 2019, 126, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [Green Version]

| Source | Bacterial Strain | Resistance | Reference | |
|---|---|---|---|---|
| Phenotype | Genotype | |||
| Rabbits | VS2746 | PEN-FOX-ERY-DA-TET | mecA, tetK, ermC | [34] |
| VS2747 | PEN-FOX-CIP-ERY-DA | mecA, ermC | [34] | |
| VS2752 | CIP-CN-TOB-ERY-DA-TET | ermC, aac(6′)-Ie-aph(2″)-Ia | [34] | |
| Hares | VS2730 | PEN-FOX-ERY | blaZ, ermC, ermB | [1] |
| VS2731 | PEN-FOX-ERY-DA | ermC, mphC | [1] | |
| VS2732 | PEN-FOX-CN-ERY-DA | ermC, mphC, aac(6′)-le-aph(2″)-la | [1] | |
| Humans | VS2704 | PEN-FOX-CIP-LNZ-CN-ERY-DA-TET-STX | blaZ, aac(6′)-Ie-aph(2″)-Ia, tetK-teto-tetL, cfr, dfrA, dfrG, dfrK | [33] |
| VS2705 | PEN-FOX-CIP-CN-TOB-KAN-ERY | blaZ, ermA, aac(6′)-Ie-aph(2′′)-Ia, ant(4′)-Ia, aph(3′)-IIIa | [32] | |
| VS2713 | PEN-FOX-CN-TOB-ERY-DA | blaZ, ermC, aac(6′)-Ie-aph(2″)-Ia, msr(A/B), ant(4′)-Ia, mphC, linA | [32] | |
| Phenolics | Leaf | Fruit | Stem Bark |
|---|---|---|---|
| Neochlorogenic acid | 0.57 ± 0.037 | 0.72 ± 0.106 | nd |
| Chlorogenic acid | 3.02 ± 0.001 | 0.92 ± 0.105 | nd |
| Coumaric acid | 0.92 ± 0.025 | 2.17 ± 0.008 | 0.21 ± 0.018 |
| Coumaric acid isomer | nd | nd | 0.30 ± 0.040 |
| Caffeic acid | nd | nd | 0.16 ± 0.029 |
| Ferulic acid | nd | nd | 0.99 ± 0.053 |
| Quercetin-3-O-rutinoside | 1.08 ± 0.022 | nd | nd |
| Quercetin-3-O-galactoside | 6.06 ± 0.143 | nd | nd |
| Baicalein | nd | 8.87 ± 0.198 | nd |
| Luteolin-7-O-glucoside | 0.43 ± 0.072 | nd | nd |
| Luteolin-4-O-glucoside | 0.32 ± 0.054 | nd | nd |
| Luteolin | 1.23 ± 0.162 | nd | nd |
| Luteolin isomer 1 | 0.85 ± 0.055 | nd | nd |
| Luteolin isomer 2 | 1.17 ± 0.044 | nd | nd |
| Total | 15.65 ± 1.631 | 12.68 ± 2.374 | 1.66 ± 0.269 |
| Assay | Leaf | Fruit | Stem Bark |
|---|---|---|---|
| DPPH | 1.13 ± 0.004 a | 1.65 ± 0.112 b | 1.17 ± 0.021 a |
| FRAP | 1.03 ± 0.003 a | 1.11 ± 0.013 a | 1.05 ± 0.004 a |
| CuPRAC | 1.05 ± 0.004 a | 1.10 ± 0.012 a | 1.07 ± 0.008 a |
| DPPH | FRAP | CuPRAC | |
|---|---|---|---|
| HPLC-DAD | 0.25 b | 0.25 b | −0.27 b |
| DPPH | 0.98 a | 0.94 a | |
| FRAP | 0.99 a |
| Source | Bacterial Strain | Extract Concentration (mg/mL) | Diameter of Inhibition Zone (mm) | ||
|---|---|---|---|---|---|
| Leaf | Fruit | Stem Bark | |||
| Rabbits | VS2746 | 100 | 14 | 14 | - |
| 75 | 13 | 13 | - | ||
| 50 | 11 | 12 | - | ||
| 25 | 8 | 10 | - | ||
| 10 | - | - | - | ||
| VS2747 | 100 | 13 | 14 | 12 | |
| 75 | 13 | 14 | 11 | ||
| 50 | 11 | 14 | 11 | ||
| 25 | 8 | 10 | 10 | ||
| 10 | - | 9 | 9 | ||
| VS2752 | 100 | 11 | 14 | - | |
| 75 | 11 | 14 | - | ||
| 50 | 11 | 13 | - | ||
| 25 | 8 | 10 | - | ||
| 10 | - | - | - | ||
| Hares | VS2730 | 100 | 12 | 12 | - |
| 75 | 12 | 11 | - | ||
| 50 | 11 | 10 | - | ||
| 25 | 9 | 9 | - | ||
| 10 | - | - | - | ||
| VS2731 | 100 | 12 | 14 | - | |
| 75 | 12 | 14 | - | ||
| 50 | 11 | 13 | - | ||
| 25 | 8 | 11 | - | ||
| 10 | - | - | - | ||
| VS2732 | 100 | 14 | 14 | 10 | |
| 75 | 13 | 14 | - | ||
| 50 | 12 | 13 | - | ||
| 25 | 12 | 11 | - | ||
| 10 | 10 | 9 | - | ||
| Humans | VS2704 | 100 | 10 | 15 | - |
| 75 | 10 | 13 | - | ||
| 50 | 10 | 12 | - | ||
| 25 | - | 11 | - | ||
| 10 | - | 9 | - | ||
| VS2705 | 100 | 13 | - | - | |
| 75 | 12 | - | - | ||
| 50 | 9 | - | - | ||
| 25 | - | - | - | ||
| 10 | - | - | - | ||
| VS2713 | 100 | 13 | 14 | - | |
| 75 | 13 | 13 | - | ||
| 50 | 10 | 13 | - | ||
| 25 | - | 12 | - | ||
| 10 | - | - | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, J.; Silva, V.; Aires, A.; Carvalho, R.; Barros, L.; Gaivão, I.; Igrejas, G.; Poeta, P. Platanus hybrida’s Phenolic Profile, Antioxidant Power, and Antibacterial Activity against Methicillin-Resistant Staphylococcus aureus (MRSA). Horticulturae 2022, 8, 243. https://doi.org/10.3390/horticulturae8030243
Ribeiro J, Silva V, Aires A, Carvalho R, Barros L, Gaivão I, Igrejas G, Poeta P. Platanus hybrida’s Phenolic Profile, Antioxidant Power, and Antibacterial Activity against Methicillin-Resistant Staphylococcus aureus (MRSA). Horticulturae. 2022; 8(3):243. https://doi.org/10.3390/horticulturae8030243
Chicago/Turabian StyleRibeiro, Jessica, Vanessa Silva, Alfredo Aires, Rosa Carvalho, Lillian Barros, Isabel Gaivão, Gilberto Igrejas, and Patrícia Poeta. 2022. "Platanus hybrida’s Phenolic Profile, Antioxidant Power, and Antibacterial Activity against Methicillin-Resistant Staphylococcus aureus (MRSA)" Horticulturae 8, no. 3: 243. https://doi.org/10.3390/horticulturae8030243
APA StyleRibeiro, J., Silva, V., Aires, A., Carvalho, R., Barros, L., Gaivão, I., Igrejas, G., & Poeta, P. (2022). Platanus hybrida’s Phenolic Profile, Antioxidant Power, and Antibacterial Activity against Methicillin-Resistant Staphylococcus aureus (MRSA). Horticulturae, 8(3), 243. https://doi.org/10.3390/horticulturae8030243