Comparison of Aroma Trait of the White-Fleshed Peach ‘Hu Jing Mi Lu’ and the Yellow-Fleshed Peach ‘Jin Yuan’ Based on Odor Activity Value and Odor Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Materials
2.2. HS-SPME Extraction of Volatiles
2.3. GC-MS Analysis of Volatiles
2.4. Volatiles Identification and Concentrations Calculation
2.5. Calculation of the Odor Activity Values (OAVs) of Volatile Compounds
2.6. Statistical Analysis
3. Results
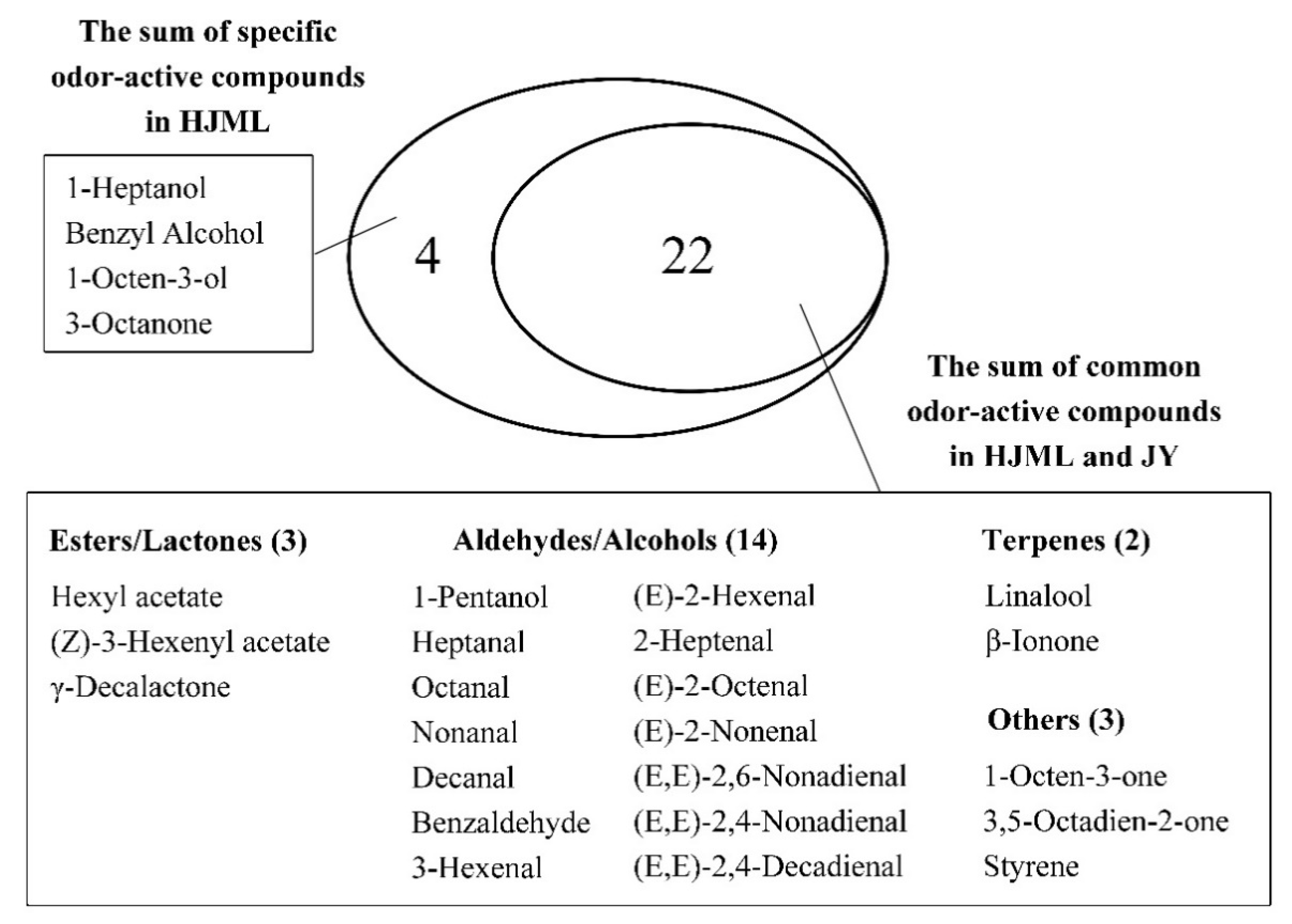
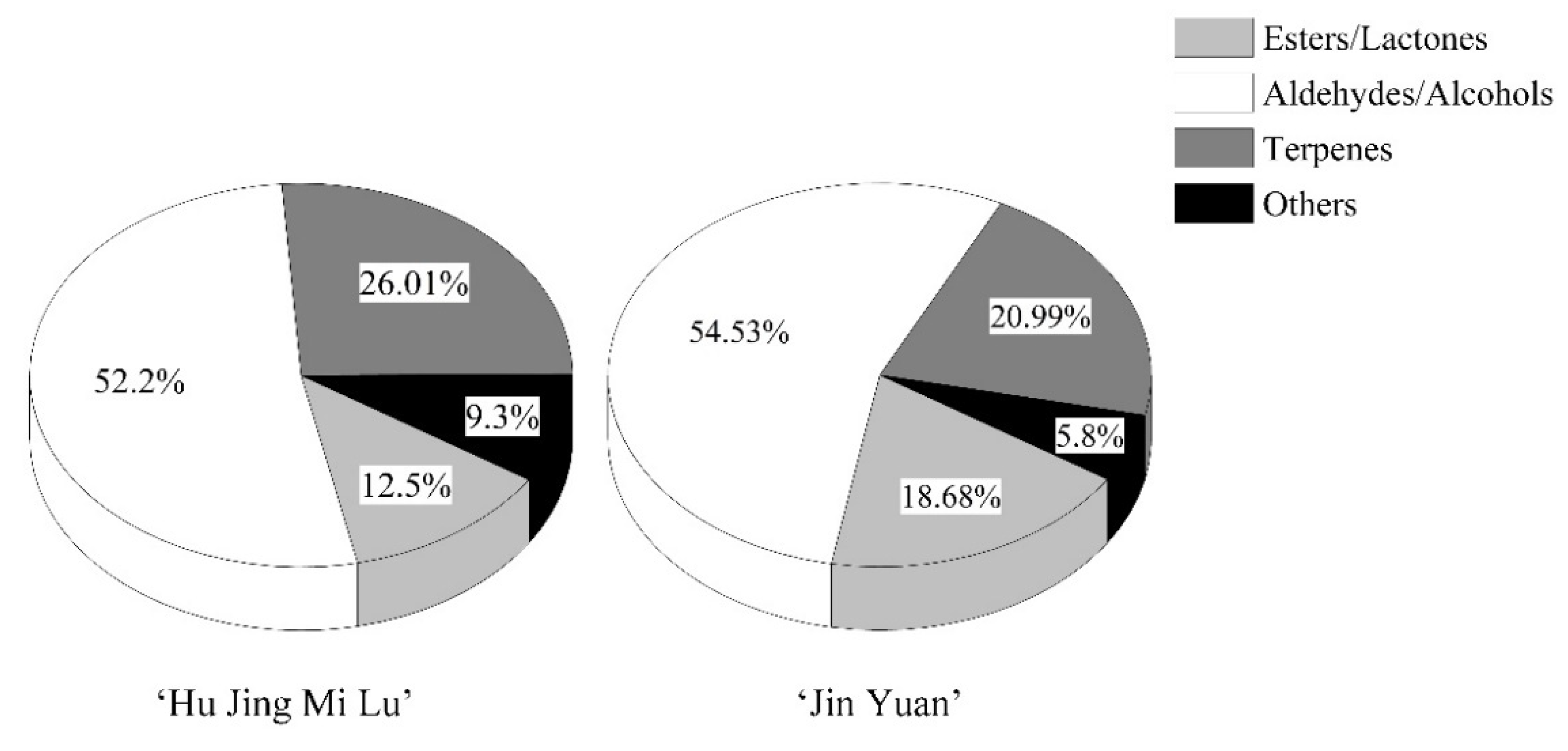
3.1. Chemical Groups of Volatile Compounds in the White- and Yellow-Fleshed Peach
3.2. Comparison of the Odor-Active Compounds in the White- and Yellow-Fleshed Peach
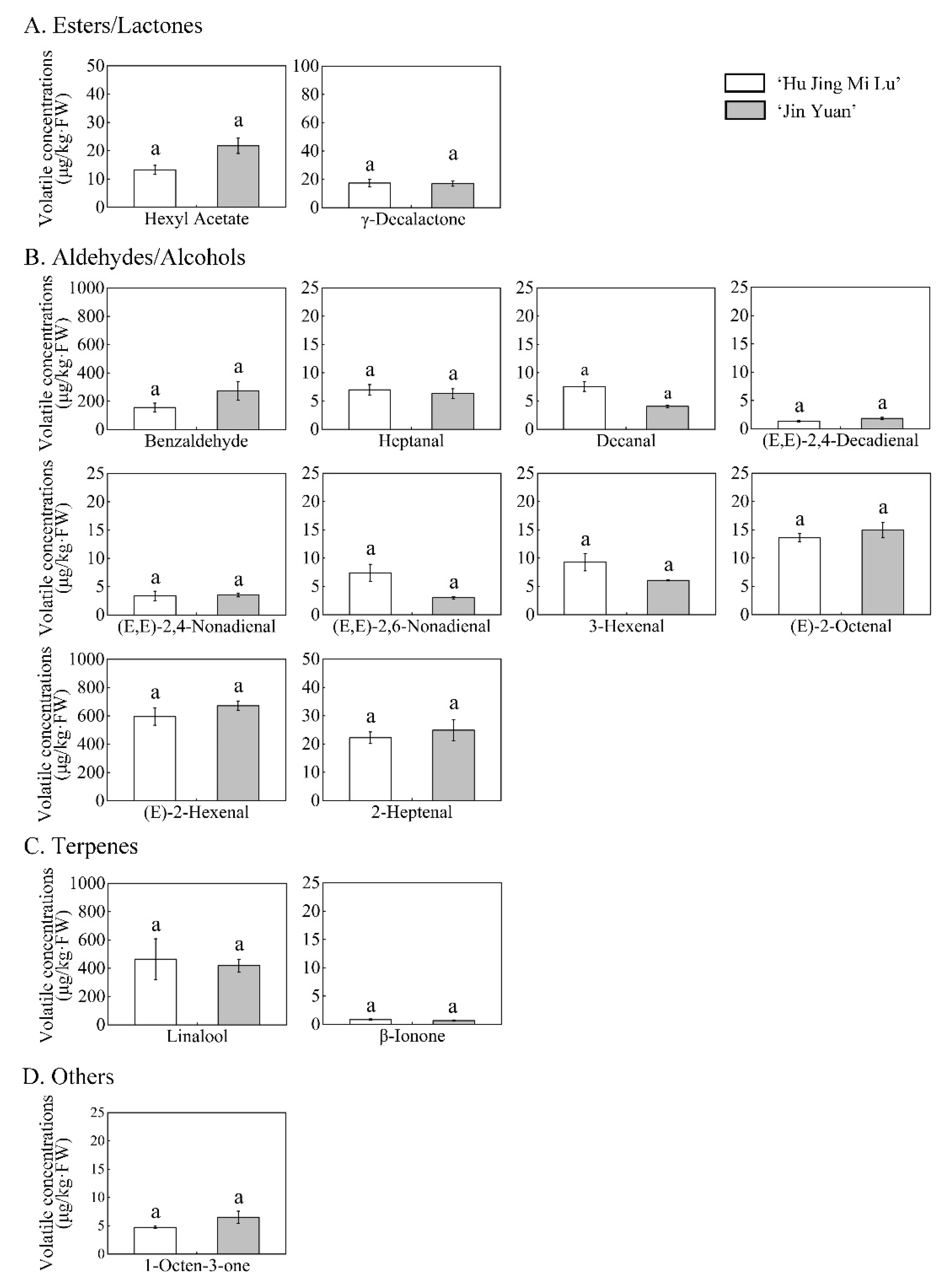
3.3. Concentrations of Odor-Active Compounds in the White- and Yellow-Fleshed Peach
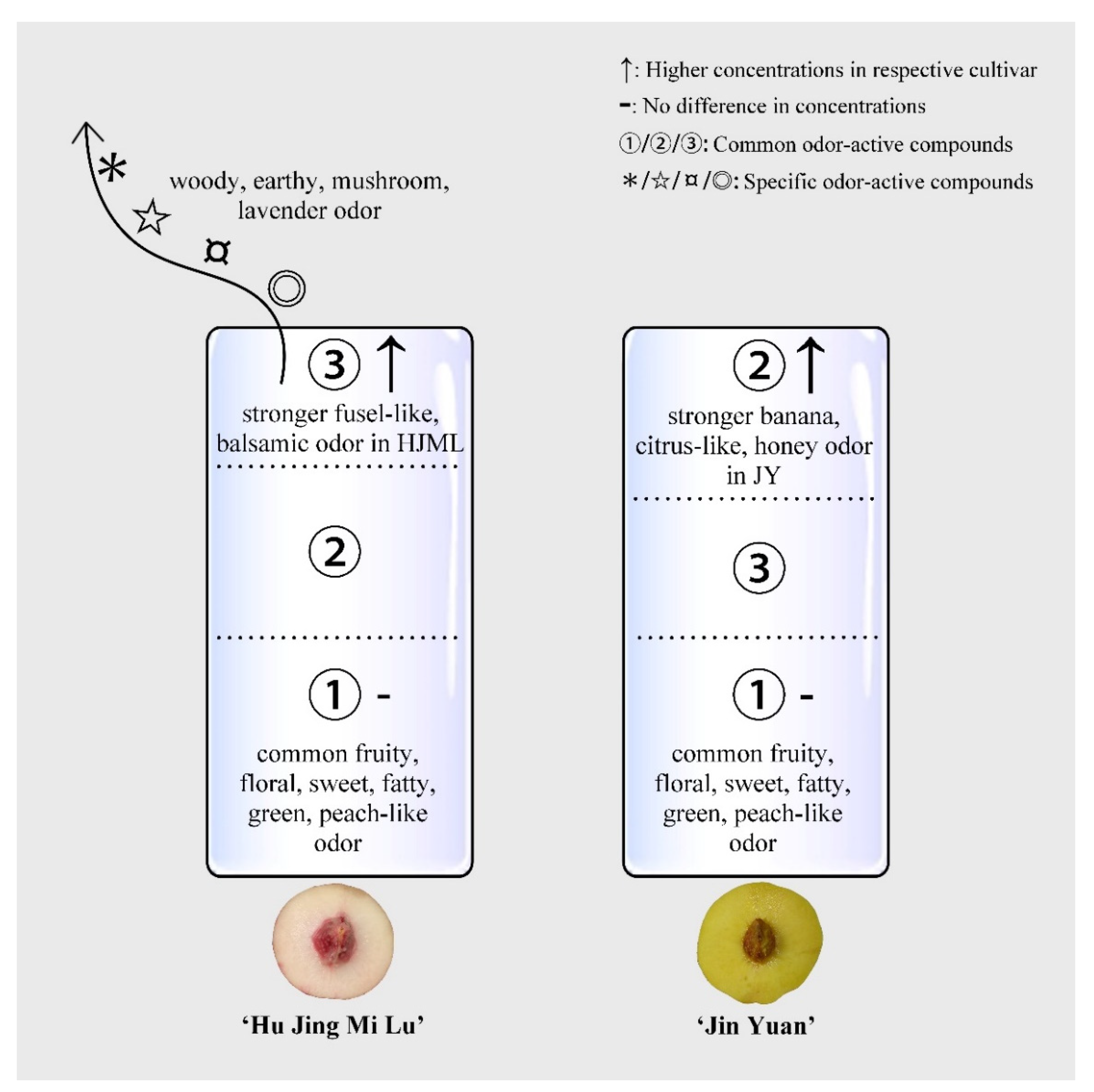
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cao, K.; Yang, X.W.; Li, Y.; Zhu, G.R.; Fang, W.C.; Chen, C.W.; Wang, X.W.; Wu, J.L.; Wang, L.R. New high-quality peach (Prunus persica L. Batsch) genome assembly to analyze the molecular evolutionary mechanism of volatile compounds in peach fruits. Plant J. 2021, 108, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.J.; Ma, R.J.; Cai, Z.X.; Xu, J.L. Establishment of peach primary core collection based on accessions conserved in National Fruit Germplasm Repository of Nanjing. Acta Hortic. Sin. 2013, 40, 125–134. [Google Scholar]
- Williamson, G.D.; Peace, C.P.; Bliss, F.A.; Garner, D.T.; Crisosto, C.H. Evidence for a single locus controlling flesh color, senescent leaf color, and hypanthium color in peach. J. Am. Soc. Hortic. Sci. 2006, 131, 256–260. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.Y.; Pan, H.F.; Gao, Z.H.; Shu, B.; Qi, Y.J.; Yi, X.K.; Qin, G.H.; Sheng, Y.; Chen, H.L.; Xu, Y.L. Transcriptome analysis of colouration-related genes in two white-fleshed nectarine varieties and their yellow-fleshed mutants. Biotechnol. Biotechnol. Equip. 2018, 32, 899–907. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.J.; Yu, Z.F.; Ye, Z.W.; Su, M.S. Multiplex analyses of the changes of aromatic compounds during the development of peach fruit using GC-MS and iTRAQ proteomic techniques. Sci. Hortic. 2018, 236, 96–105. [Google Scholar] [CrossRef]
- Giberti, S.; Giovannini, D.; Forlani, G. Carotenoid cleavage in chromoplasts of white and yellow-fleshed peach varieties. J. Sci. Food Agric. 2019, 99, 1795–1803. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomä-S-Barberän, F.A.; Hess-Pierce, B.; Kader, A.A. Antioxidant capacities, phenolic compounds, carotenoids, and vitamin C contents of nectarine, peach, and plum cultivars from California. J. Agric. Food Chem. 2002, 50, 4976–4982. [Google Scholar] [CrossRef]
- Qin, J.; Yu, F.; Liu, L.; Zhu, T.T.; Chen, W.; Cao, S.F.; Yang, Z.F.; Shi, L.Y. Cloning of peach PpNAC19 and its regulation on the activity of PpCCD4 promoter. J. Nucl. Agric. Sci. 2021, 35, 1273–1280. [Google Scholar]
- Sun, T.H.; Li, L. Toward the ‘golden’ era: The status in uncovering the regulatory control of carotenoid accumulation in plants. Plant Sci. 2020, 290, 110331. [Google Scholar] [CrossRef]
- Penso, G.A.; Santos, C.E.M.D.; Bruckner, C.H.; Costa, J.C.F.D.; Citadin, I. Consumption, preferences and habits of purchasing consumers of peaches and nectarines. Rev. Bras. Frutic. 2018, 40. [Google Scholar] [CrossRef]
- Xin, R.; Liu, X.H.; Wei, C.Y.; Yang, C.; Liu, H.R.; Cao, X.M.; Wu, D.; Zhang, B.; Chen, K.S. E-Nose and GC-MS reveal a difference in the volatile profiles of white- and red-fleshed peach fruit. Sensors 2018, 18, 765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raffo, A.; Nardo, N.; Tabilio, M.R.; Paoletti, F. Effects of cold storage on aroma compounds of white- and yellow-fleshed peaches. Eur. Food Res. Technol. 2008, 226, 1503–1512. [Google Scholar] [CrossRef]
- Zhu, Y.Q.; Li, Q.W.; Zeng, W.F.; Niu, L.; Pan, L.; Cai, Z.G.; Cui, G.Z.; Wang, Z.Q. Analysis of volatile aromatic compounds of CN9 nectarine and its yellow-fleshed mutant. Jiangsu Agric. Sci. 2018, 46, 172–177. [Google Scholar]
- Wang, G.Z.; Wang, G.X.; Liang, L.S.; Ma, Q.H. Recent progress in research on the composition and synthesis of aroma volatiles in peach fruits. Food Sci. 2014, 35, 278–284. [Google Scholar]
- Wang, Y.J.; Yang, C.X.; Li, S.H.; Yang, L.; Wang, Y.N.; Zhao, J.B.; Jiang, Q. Volatile characteristics of 50 peaches and nectarines evaluated by HP-SPME with GC-MS. Food Chem. 2009, 116, 356–364. [Google Scholar] [CrossRef]
- Xi, W.P.; Yu, S.L.; Zhou, Z.Q. Advances in aroma compounds biosynthesis of peach fruit. Acta Hortic. Sin. 2013, 40, 1679–1690. [Google Scholar]
- Pino, J.A.; Trujillo, R. Characterization of odour-active compounds of sour guava (Psidium acidum [DC.] Landrum) fruit by gas chromatography-olfactometry and odour activity value. Flavour Fragr. J. 2020, 36, 207–212. [Google Scholar] [CrossRef]
- Visai, C.; Vanoli, M. Volatile compound production during growth and ripening of peaches and nectarine. Sci. Hortic. 1997, 70, 15–24. [Google Scholar] [CrossRef]
- Zhang, B.; Shen, J.Y.; Wei, W.W.; Xi, W.P.; Xu, C.J.; Ferguson, I.; Chen, K.S. Expression of genes associated with aroma formation derived from the fatty acid pathway during peach fruit ripening. J. Agric. Food Chem. 2010, 58, 6157–6165. [Google Scholar] [CrossRef]
- Horvat, R.J.; Chapman, G.W.; Robertson, J.A.; Meredith, F.I.; Scorza, R.; Callahan, A.M.; Morgens, P. Comparison of the volatile compounds from several commercial peach cultivars. J. Agric. Food Chem. 1990, 38, 234–237. [Google Scholar] [CrossRef]
- Eduardo, I.; Chietera, G.; Bassi, D.; Rossini, L.; Vecchietti, A. Identification of key odor volatile compounds in the essential oil of nine peach accessions. J. Sci. Food Agric. 2010, 90, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Aubert, C.; Bony, P.; Chalot, G.; Landry, P.; Lurol, S. Effects of storage temperature, storage duration, and subsequent ripening on the physicochemical characteristics, volatile compounds, and phytochemicals of Western Red nectarine (Prunus persica L. Batsch). J. Agric. Food Chem. 2014, 62, 4707–4724. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.F.; An, X.J.; Han, S.; Jiang, L.; Yu, M.L.; Ma, R.J.; Yu, Z.F. Effect of 1-MCP on the production of volatiles and biosynthesis-related gene expression in peach fruit during cold storage. Postharvest Biol. Technol. 2018, 141, 50–57. [Google Scholar] [CrossRef]
- Cai, H.F.; Han, S.; Jiang, L.; Yu, M.L.; Ma, R.J.; Yu, Z.F. 1-MCP treatment affects peach fruit aroma metabolism as revealed by transcriptomics and metabolite analyses. Food Res. Int. 2019, 122, 573–584. [Google Scholar] [CrossRef]
- Leng, P.; Hu, H.W.; Cui, A.H.; Tang, H.J.; Liu, Y.G. HS-GC-IMS with PCA to analyze volatile flavor compounds of honey peach packaged with different preservation methods during storage. LWT-Food Sci. Technol. 2021, 149, 111963. [Google Scholar] [CrossRef]
- Yang, C.; Duan, W.Y.; Xie, K.L.; Ren, C.H.; Zhu, C.Q.; Chen, K.S.; Zhang, B. Effect of salicylic acid treatment on sensory quality, flavor-related chemicals and gene expression in peach fruit after cold storage. Postharvest Biol. Technol. 2020, 161, 111089. [Google Scholar] [CrossRef]
- Zhou, H.J.; Du, J.H.; Zhang, X.N.; Su, M.S.; Xiong, W.; Ye, Z.W. Effects of UVC pretreatment on composition and content of volatile substances of ‘Jinxiang’ yellow peach during cold storage and shelf-life. Acta Agric. Shanghai 2021, 37, 91–99. [Google Scholar]
- Wei, S.H.; Xiao, X.M.; Wei, L.J.; Li, L.S.; Li, G.C.; Liu, F.H.; Xie, J.M.; Yu, J.H.; Zhong, Y. Development and comprehensive HS-SPME/GC-MS analysis optimization, comparison, and evaluation of different cabbage cultivars (Brassica oleracea L. var. capitata L.) volatile components. Food Chem. 2021, 340, 128166. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Boca Raton, FL, USA, 2010. [Google Scholar]
- Ceccarelli, A.; Farneti, B.; Frisina, C.; Allen, D.; Donati, I.; Cellini, A.; Costa, G.; Spinelli, F.; Stefanelli, D. Harvest maturity stage and cold storage length influence on flavour development in peach fruit. Agronomys 2018, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.X.; Wang, G.X.; Liang, L.S. The changes of volatile compositions of ‘Okubo’ peach and its relationship with ethylene and relevant enzymes stored at ambient temperature. Acta Hortic. Sin. 2009, 38, 35–42. [Google Scholar]
- Fan, X.; Cui, X.P. Based on HS-SPME/GC-MS and electronic nose technology to study the aroma compounds of peach of different flesh types during the July postharvest storage in Jiangsu Province. Food Sci. 2021, 42, 222–229. [Google Scholar]
- Schieberle, P.; Grosch, W. Potent odorants of the wheat bread crumb—Differences to the crust and effect of a longer dough fermentation. Z. Lebensm.-Unters. Forsch. 1991, 192, 130–135. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Dong, J.; Wang, G.X. Formation of aroma volatiles in strawberry fruit and aroma breeding. Sci. Agric. Sin. 2004, 37, 1039–1044. [Google Scholar]
- Maggi, F.; Bílek, T.; Lucarini, D.; Papa, F.; Sagratini, G.; Vittori, S. Melittis melissophyllum L. subsp. melissophyllum (Lamiaceae) from central Italy: A new source of a mushroom-like flavour. Food Chem. 2009, 113, 216–221. [Google Scholar]
- Elsharif, S.A.; Buettner, A. Structure-odor relationship study on geraniol, nerol, and their synthesized oxygenated derivatives. J. Agric. Food Chem. 2018, 66, 2324–2333. [Google Scholar] [CrossRef]
- Beltran, J.C.; Stange, C. Apocarotenoids: A new carotenoid-derived pathway. Carotenoids Nat. 2016, 79, 239–272. [Google Scholar]
- Zhu, J.C.; Xiao, Z.B. Characterization of the key aroma compounds in peach by gas chromatography-olfactometry, quantitative measurements and sensory analysis. Eur. Food Res. Technol. 2018, 245, 129–141. [Google Scholar] [CrossRef]
- Cao, S.F.; Liang, M.H.; Shi, L.Y.; Shao, J.R.; Song, C.B.; Bian, K.; Chen, W.; Yang, Z.F. Accumulation of carotenoids and expression of carotenogenic genes in peach fruit. Food Chem. 2017, 214, 137–146. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.Q.; Duan, Y.R.; Wu, J.K.; Zhang, L.B. Research progress on color formation mechanism of peach flesh. Hebei Fruits 2018, 6, 1–4. [Google Scholar]
- Mendes-Pinto, M.M. Carotenoid breakdown products the-norisoprenoids-in wine aroma. Arch Biochem. Biophys. 2009, 483, 236–245. [Google Scholar] [CrossRef]
- Serra, S. Recent advances in the synthesis of carotenoid-derived flavours and fragrances. Molecules 2015, 20, 12817–12840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, M.H.; Strack, D. Carotenoids and their cleavage products: Biosynthesis and functions. Nat. Prod. Rep. 2011, 28, 663–692. [Google Scholar] [CrossRef] [PubMed]
- Tieman, D.M.; Zeigler, M.; Schmelz, E.A.; Taylor, M.G.; Bliss, P.; Kirst, M.; Klee, H.J. Identification of loci affecting flavour volatile emissions in tomato fruits. J. Exp. Bot. 2006, 57, 887–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.; Huang, N.; Jiang, S.Z.; Li, K.; Zhuang, Z.; Wang, Q.; Lu, S. Cloning and functional characterization of two carotenoid cleavage dioxygenases for ionone biosynthesis in chili pepper (Capsicum annuum L.) fruits. Sci. Hortic. 2021, 288, 110368. [Google Scholar] [CrossRef]
- Ilg, A.; Bruno, M.; Beyer, P.; Al-Babili, S. Tomato carotenoid cleavage dioxygenases 1A and 1B: Relaxed double bond specificity leads to a plenitude of dialdehydes, mono-apocarotenoids and isoprenoid volatiles. FEBS Open Bio 2014, 4, 584–593. [Google Scholar] [CrossRef] [Green Version]
- Bouvier, F.; Isner, J.C.; Dogbo, O.; Camara, B. Oxidative tailoring of carotenoids: A prospect towards novel functions in plants. Trends Plant Sci. 2005, 10, 187–194. [Google Scholar] [CrossRef]
- Paparella, A.; Shaltiel-Harpaza, L.; Ibdah, M. β-Ionone: Its occurrence and biological function and metabolic engineering. Plants 2021, 10, 754. [Google Scholar] [CrossRef]
- Lewinsohn, E.; Sitrit, Y.; Bar, E.; Azulay, Y.; Meir, A.; Zamir, D.; Tadmor, Y. Carotenoid pigmentation affects the volatile composition of tomato and watermelon fruits, as revealed by comparative genetic analyses. J. Agric. Food Chem. 2005, 53, 3142–3148. [Google Scholar] [CrossRef]
- Vogel, J.T.; Tan, B.C.; McCarty, D.R.; Klee, H.J. The carotenoid cleavage dioxygenase 1 enzyme has broad substrate specificity, cleaving multiple carotenoids at two different bond positions. J. Biol. Chem. 2008, 283, 11364–11373. [Google Scholar] [CrossRef] [Green Version]


| Classification | Volatile Compounds | Odor Description 1 | Concentrations (μg/kg·FW) | Odor Threshold in Water (μg·kg−1) 1 | OAV | ||
|---|---|---|---|---|---|---|---|
| HJML | JY | HJML | JY | ||||
| Esters and Lactones | Hexyl acetate | Fruity, apple, cherry, pear, floral | 13.23 ± 1.61 a | 21.73 ± 2.72 a | 2 | 6.61 | 10.86 |
| (Z)-3-Hexenyl Acetate | Green, fruity, floral, banana | 192.44 ± 22.83 b | 318.83 ± 11.25 a | 7.8 | 24.67 | 40.88 | |
| γ-Decalactone | Fruity, peach-like | 17.39 ± 2.71 a | 16.94 ± 1.84 a | 1 | 17.38 | 16.94 | |
| Alcohols and Aldehydes | 1-Pentanol | Fusel-like | 7.17 ± 0.77 a | 4.68 ± 0.45 b | 1.6 | 4.48 | 2.93 |
| 1-Heptanol | Fragrant, woody, fatty | 4.09 ± 0.24 a | 2.08 ± 0.03 b | 3 | 1.36 | <1 | |
| Benzyl Alcohol | Fruity | 2.43 ± 0.44 | - | 1.2 | 2.02 | - | |
| Heptanal | Fatty | 6.99 ± 0.92 a | 6.33 ± 0.85 a | 3 | 2.33 | 2.11 | |
| Octanal | Fatty, citrus, honey | 3.35 ± 0.14 b | 5.15 ± 0.37 a | 1.4 | 2.39 | 3.68 | |
| Nonanal | Fatty, citrus-like | 7.79 ± 0.49 b | 10.41 ± 0.61 a | 1 | 7.79 | 10.41 | |
| Decanal | Sweet, waxy, floral, Citrus, fatty | 7.55 ± 0.85 a | 4.06 ± 0.23 a | 0.1 | 75.52 | 40.60 | |
| Benzaldehyde | Almond, cherry, nutty | 155.76 ± 31.23 a | 272.93 ± 64.39 a | 100 | 1.56 | 2.73 | |
| 3-Hexenal | Green, fruity, apple-like | 9.28 ± 1.52 a | 6.07 ± 0.09 a | 0.25 | 37.10 | 24.27 | |
| (E)-2-Hexenal | Sweet, almond, fruity Green, apple, plum | 594.95 ± 60.89 a | 671.70 ± 33.84 a | 30 | 19.83 | 22.39 | |
| 2-Heptenal | Fatty | 22.21 ± 2.07 a | 24.88 ± 3.72 a | 13 | 1.71 | 1.91 | |
| (E)-2-Octenal | Green | 13.59 ± 0.75 a | 14.93 ± 1.34 a | 3 | 4.53 | 4.98 | |
| (E)-2-Nonenal | Fatty | 21.04 ± 4.48 a | 8.02 ± 0.57 b | 0.1 | 210.37 | 80.16 | |
| (E,E)-2,6-Nonadienal | Green, vegetable | 7.40 ± 1.51 a | 3.01 ± 0.20 a | 0.09 | 82.19 | 33.45 | |
| (E,E)-2,4-Nonadienal | Fatty, floral | 3.35 ± 0.84 a | 3.52 ± 0.34 a | 0.05 | 66.97 | 70.47 | |
| (E,E)-2,4-Decadienal | Oily, chicken fat | 1.33 ± 0.12 a | 1.84 ± 0.19 a | 0.07 | 19.06 | 26.25 | |
| Terpenes | 1-Octen-3-ol | Sweet, earthy, herbaceous, rose, mushroom | 25.76 ± 3.85 a | 7.87 ± 0.64 b | 14 | 1.84 | <1 |
| Linalool | Floral | 464.28 ± 143.93 a | 418.74 ± 45.02 a | 4 | 116.07 | 104.68 | |
| β-Ionone | Floral | 0.83 ± 0.13 a | 0.67 ± 0.12 a | 0.007 | 119.20 | 95.52 | |
| Others | 1-Octen-3-one | Mushroom | 4.72 ± 0.20 a | 6.51 ± 1.08 a | 0.05 | 94.40 | 130.21 |
| 3,5-Octadien-2-one | Herbaceous | 2.48 ± 0.06 b | 3.07 ± 0.00 a | 0.15 | 16.50 | 20.44 | |
| Styrene | Sweet, balsamic, floral | 8.22 ± 0.82 a | 4.01 ± 0.12 b | 3.6 | 2.28 | 1.11 | |
| 3-Octanone | Fruity, lavender | 22.46 ± 2.19 a | 11.54 ± 0.23 b | 21 | 1.07 | <1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Zhang, Y.; Ma, R.; Yu, M. Comparison of Aroma Trait of the White-Fleshed Peach ‘Hu Jing Mi Lu’ and the Yellow-Fleshed Peach ‘Jin Yuan’ Based on Odor Activity Value and Odor Characteristics. Horticulturae 2022, 8, 245. https://doi.org/10.3390/horticulturae8030245
Liu W, Zhang Y, Ma R, Yu M. Comparison of Aroma Trait of the White-Fleshed Peach ‘Hu Jing Mi Lu’ and the Yellow-Fleshed Peach ‘Jin Yuan’ Based on Odor Activity Value and Odor Characteristics. Horticulturae. 2022; 8(3):245. https://doi.org/10.3390/horticulturae8030245
Chicago/Turabian StyleLiu, Wenjing, Yuanyuan Zhang, Ruijuan Ma, and Mingliang Yu. 2022. "Comparison of Aroma Trait of the White-Fleshed Peach ‘Hu Jing Mi Lu’ and the Yellow-Fleshed Peach ‘Jin Yuan’ Based on Odor Activity Value and Odor Characteristics" Horticulturae 8, no. 3: 245. https://doi.org/10.3390/horticulturae8030245
APA StyleLiu, W., Zhang, Y., Ma, R., & Yu, M. (2022). Comparison of Aroma Trait of the White-Fleshed Peach ‘Hu Jing Mi Lu’ and the Yellow-Fleshed Peach ‘Jin Yuan’ Based on Odor Activity Value and Odor Characteristics. Horticulturae, 8(3), 245. https://doi.org/10.3390/horticulturae8030245






