Foliar Applications of Bacillus subtilis HA1 Culture Filtrate Enhance Tomato Growth and Induce Systemic Resistance against Tobacco mosaic virus Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Viral Source
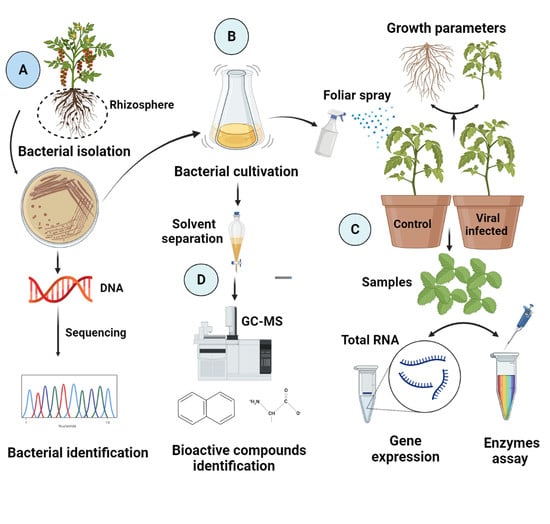
2.2. Bacterial Isolation
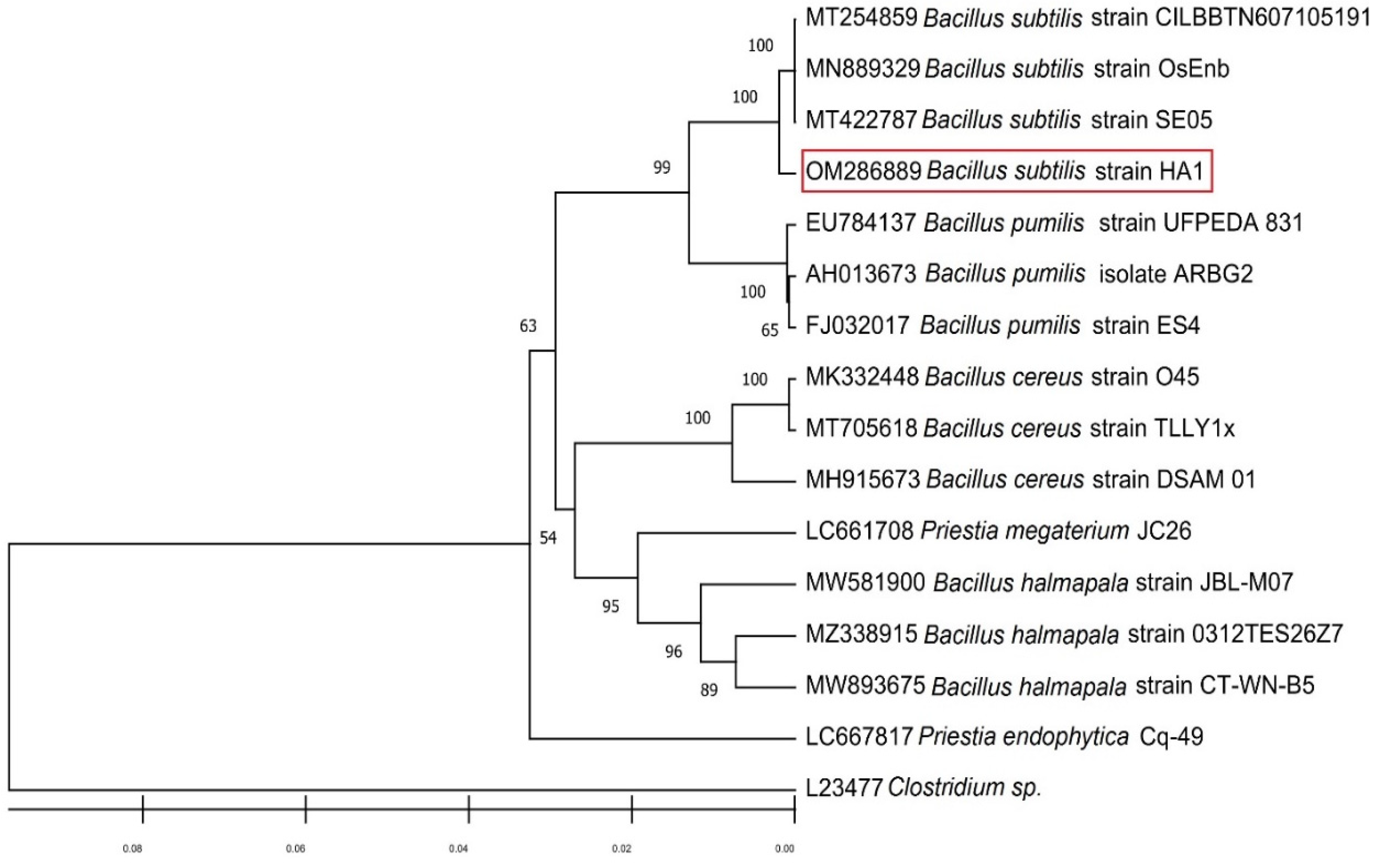
2.3. Isolate Identification through 16S rRNA Methodology
2.4. Greenhouse Experimental Design and Assessment of Growth Parameters
2.5. Oxidative Stress Markers
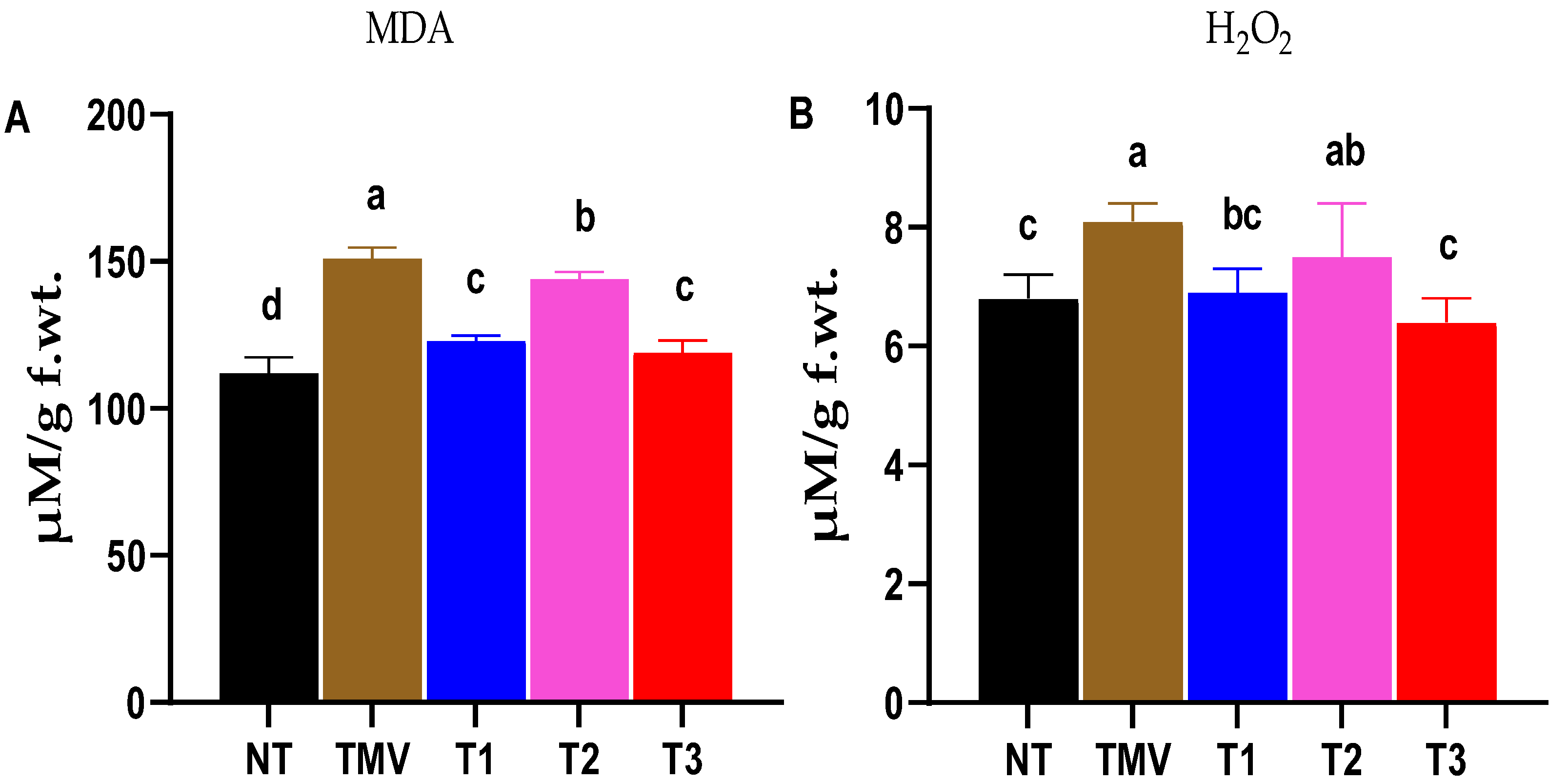
2.5.1. Malondialdehyde (MDA) Determination
2.5.2. Hydrogen Peroxide Determination
2.6. Determination of Antioxidant Enzymatic Activities
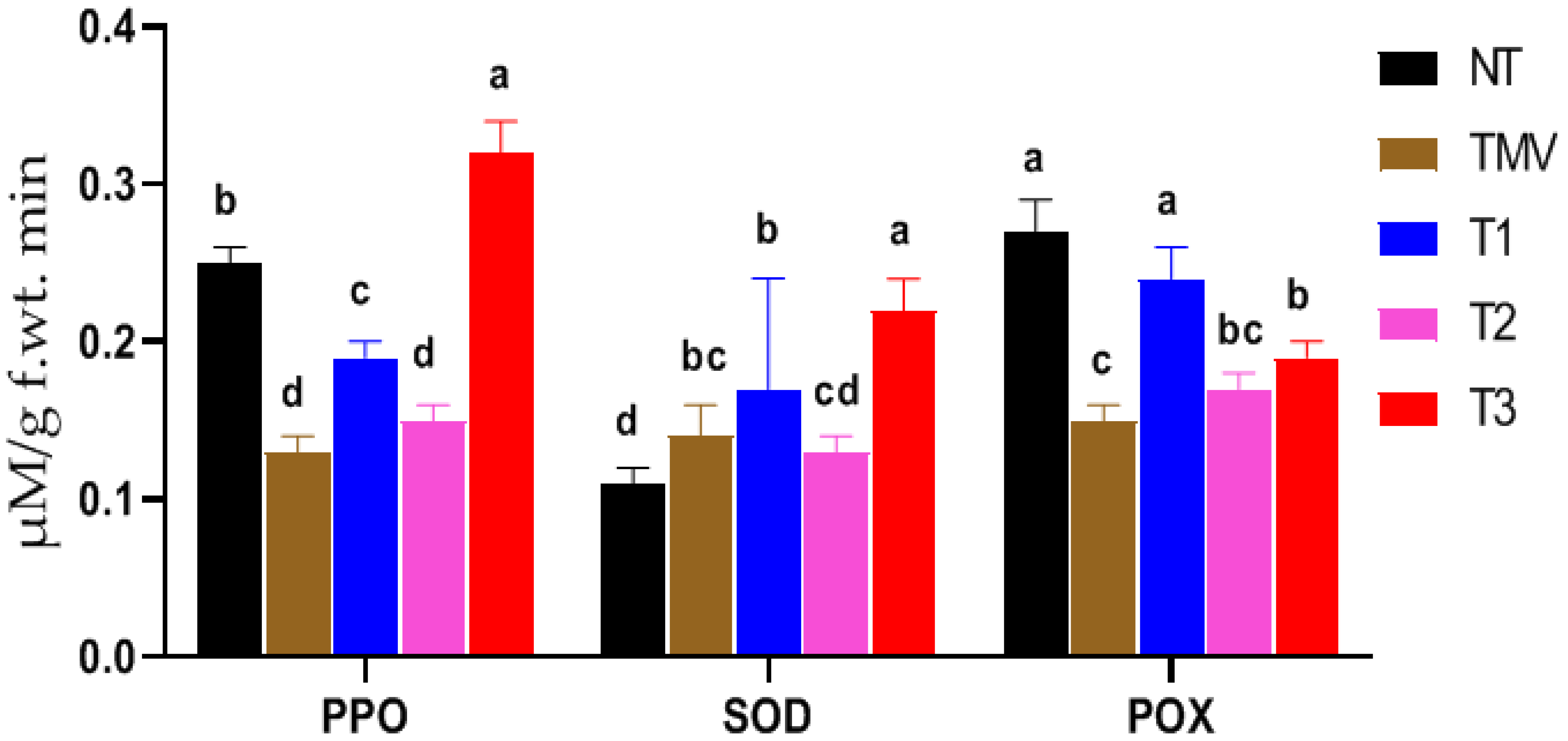
2.6.1. Polyphenol Oxidase (PPO)
2.6.2. Superoxide Dismutase (SOD)
2.6.3. Peroxidase (POX)
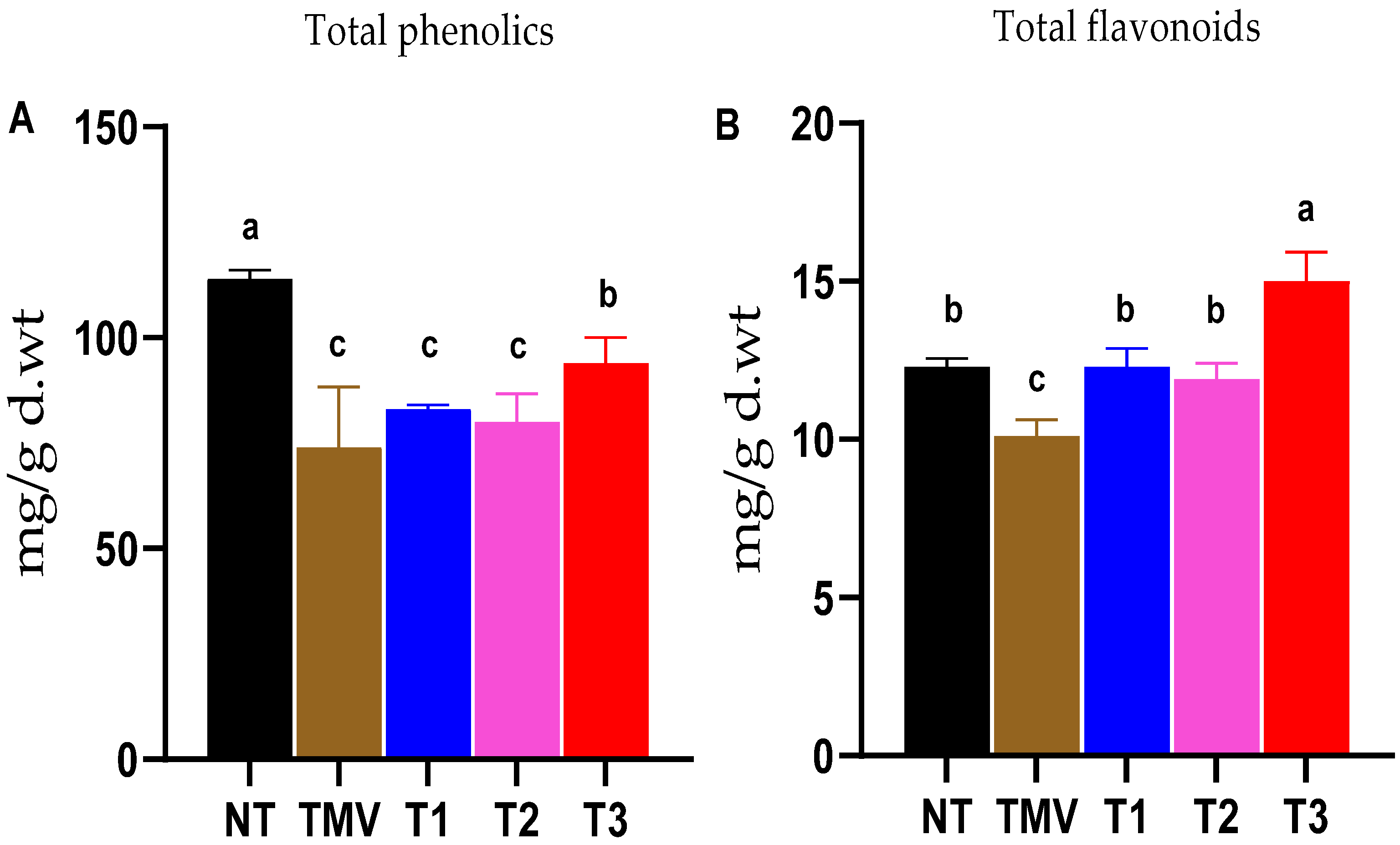
2.7. Determination of Total Phenolic Contents
2.8. Determination of Total Flavonoid Contents
2.9. Quantitative RT-PCR (qRT-PCR) Assay and Data Analysis
2.9.1. Total RNA Extraction and cDNA Synthesis
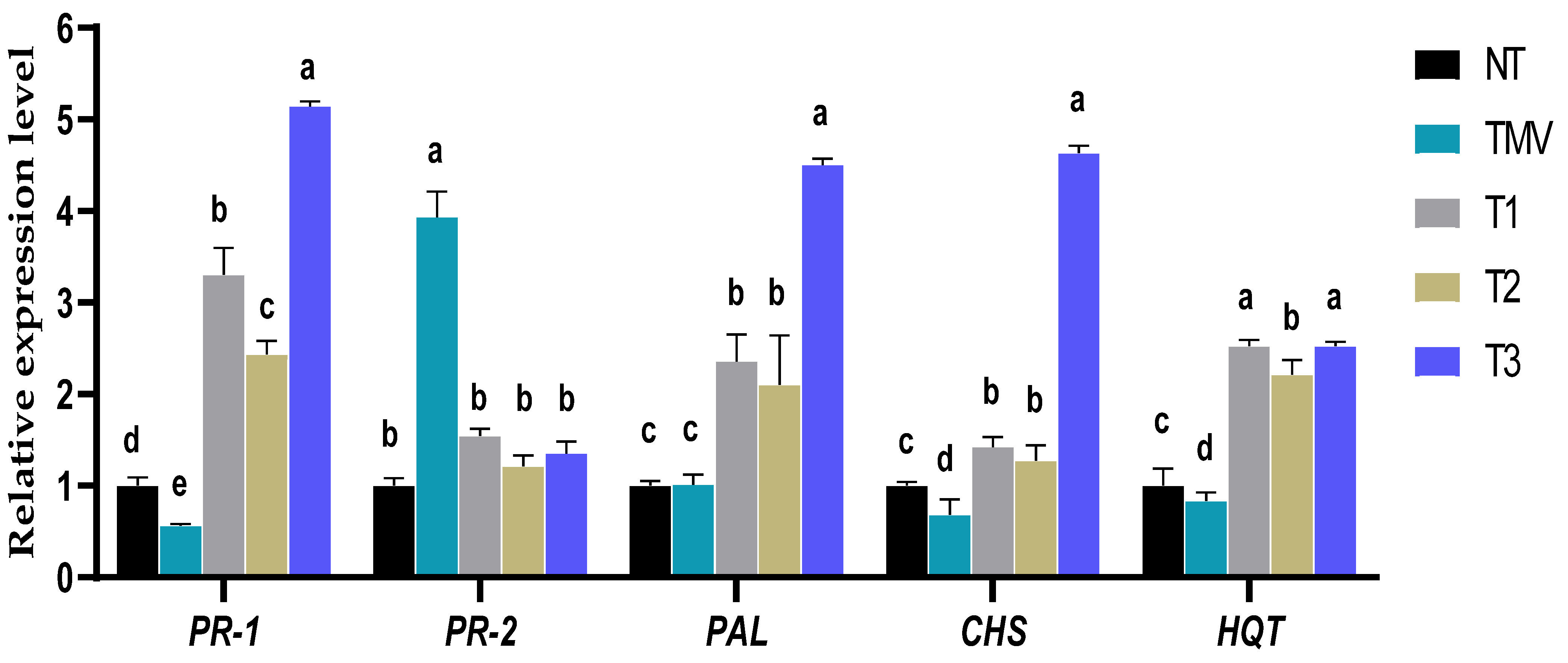
2.9.2. TMV-CP Accumulation and Defense Genes Expression Levels
2.10. Assessment of Active Biomolecules in the Bacterial Culture Filtrate through Gas Chromatography–Mass Spectrometry
2.11. Statistical Analysis
3. Results and Discussion
3.1. Bacterial Isolation and Molecular Characterization
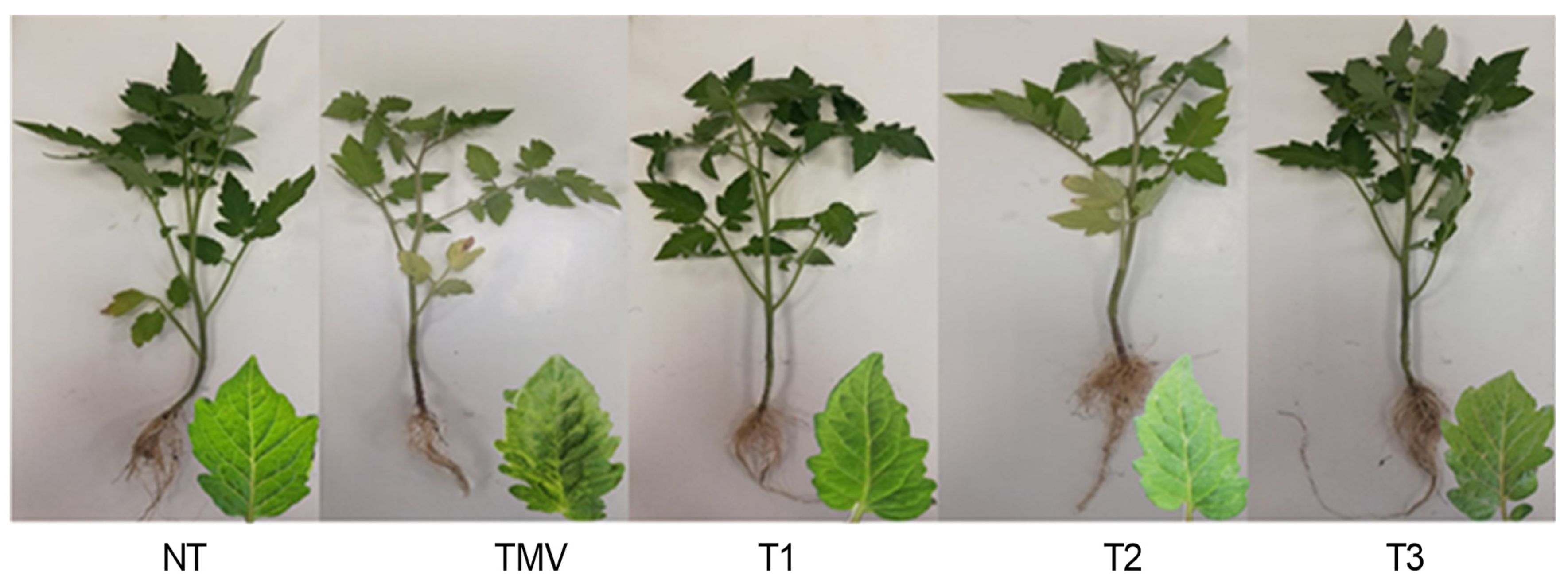
3.2. Effect of HA1-CF on Development of TMV Symptoms and Growth Parameters
3.3. Evaluation of Oxidative Stress Markers
3.4. Antioxidant Enzymatic Activities
3.5. Total Phenolic and Total Flavonoid Contents
3.6. Effect of HA1-CF on Systemic Accumulation of TMV
3.7. Effect of HA1-CF on Transcriptional Levels of Pathogenesis-Related Protein Genes
3.8. Effect of HA1-CF on the Transcript Levels of Polyphenolic Biosynthesis Genes
3.9. Identification of Bioactive Metabolites of HA1-CF by GC–MS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdelkhalek, A.; Hafez, E. Plant Viral Diseases in Egypt and Their Control. In Cottage Industry of Biocontrol Agents and Their Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 403–421. [Google Scholar]
- Mumford, R.A.; Macarthur, R.; Boonham, N. The role and challenges of new diagnostic technology in plant biosecurity. Food Secur. 2016, 8, 103–109. [Google Scholar] [CrossRef]
- Abo-Zaid, G.A.; Matar, S.M.; Abdelkhalek, A. Induction of Plant Resistance against Tobacco Mosaic Virus Using the Biocontrol Agent Streptomyces cellulosae Isolate Actino 48. Agronomy 2020, 10, 1620. [Google Scholar] [CrossRef]
- McDaniel, L.; Maratos, M.; Farabaugh, J. Infection of plants by tobacco mosaic virus. Am. Biol. Teach. 1998, 60, 434–439. [Google Scholar] [CrossRef]
- Peng, J.; Song, K.; Zhu, H.; Kong, W.; Liu, F.; Shen, T.; He, Y. Fast detection of Tobacco Mosaic Virus infected tobacco using laser-induced breakdown spectroscopy. Sci. Rep. 2017, 7, 44551. [Google Scholar] [CrossRef]
- Abdelkhalek, A. Expression of tomato pathogenesis related genes in response to Tobacco mosaic virus. J. Anim. Plant Sci. 2019, 29, 1596–1602. [Google Scholar]
- Bazzini, A.A.; Hopp, H.E.; Beachy, R.N.; Asurmendi, S. Infection and coaccumulation of Tobacco Mosaic Virus proteins alter microRNA levels, correlating with symptom and plant development. Proc. Natl. Acad. Sci. USA 2007, 104, 12157–12162. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A.; Alsubaie, M.M.; Behiry, S.I. First Report of Protective Activity of Paronychia argentea Extract against Tobacco Mosaic Virus Infection. Plants 2021, 10, 2435. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, A.; Al-Askar, A.A.; Behiry, S.I. Bacillus licheniformis strain POT1 mediated polyphenol biosynthetic pathways genes activation and systemic resistance in potato plants against Alfalfa mosaic virus. Sci. Rep. 2020, 10, 16120. [Google Scholar] [CrossRef] [PubMed]
- Sorokan, A.; Cherepanova, E.; Burkhanova, G.; Veselova, S.; Rumyantsev, S.; Alekseev, V.; Mardanshin, I.; Sarvarova, E.; Khairullin, R.; Benkovskaya, G.; et al. Endophytic Bacillus spp. as a Prospective Biological Tool for Control of Viral Diseases and Non-vector Leptinotarsa decemlineata Say. in Solanum tuberosum L. Front. Microbiol. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Takishita, Y.; Zhou, G.; Smith, D.L. Plant Associated Rhizobacteria for Biocontrol and Plant Growth Enhancement. Front. Plant Sci. 2021, 12, 420. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, R.; You, M.P.; Barbetti, M.J.; Chen, Y. Pathogen biocontrol using plant growth-promoting bacteria (PGPR): Role of bacterial diversity. Microorganisms 2021, 9, 1988. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Pagnani, G.; Bernardi, M.; Mattedi, A.; Spera, D.M.; Del Gallo, M. Cell-free supernatants of plant growth-promoting bacteria: A review of their use as biostimulant and microbial biocontrol agents in sustainable agriculture. Sustainability 2020, 12, 9917. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Hashem, A.; Abd Allah, E.F. Bacillus: A biological tool for crop improvement through bio-molecular changes in adverse environments. Front. Physiol. 2017, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of plant growth promoting rhizobacteria in agricultural sustainability—A review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef] [PubMed]
- Saeed, Q.; Xiukang, W.; Haider, F.U.; Kučerik, J.; Mumtaz, M.Z.; Holatko, J.; Naseem, M.; Kintl, A.; Ejaz, M.; Naveed, M.; et al. Rhizosphere bacteria in plant growth promotion, biocontrol, and bioremediation of contaminated sites: A comprehensive review of effects and mechanisms. Int. J. Mol. Sci. 2021, 22, 10529. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, D.; Mhamdi, R. Microbial inoculants and their impact on soil microbial communities: A review. BioMed Res. Int. 2013, 2013, 863240. [Google Scholar] [CrossRef]
- Vitti, A.; Pellegrini, E.; Nali, C.; Lovelli, S.; Sofo, A.; Valerio, M.; Scopa, A.; Nuzzaci, M. Trichoderma harzianum T-22 induces systemic resistance in tomato infected by Cucumber mosaic virus. Front. Plant Sci. 2016, 7, 1520. [Google Scholar] [CrossRef] [PubMed]
- Abo-Zaid, G.; Abdelkhalek, A.; Matar, S.; Darwish, M.; Abdel-Gayed, M. Application of Bio-Friendly Formulations of Chitinase-Producing Streptomyces cellulosae Actino 48 for Controlling Peanut Soil-Borne Diseases Caused by Sclerotium rolfsii. J. Fungi 2021, 7, 167. [Google Scholar] [CrossRef]
- Heflish, A.A.; Abdelkhalek, A.; Al-Askar, A.A.; Behiry, S.I. Protective and Curative Effects of Trichoderma asperelloides Ta41 on Tomato Root Rot Caused by Rhizoctonia solani Rs33. Agronomy 2021, 11, 1162. [Google Scholar] [CrossRef]
- Kandan, A.; Ramiah, M.; Vasanthi, V.J.; Radjacommare, R.; Nandakumar, R.; Ramanathan, A.; Samiyappan, R. Use of Pseudomonas fluorescens-based formulations for management of tomato spotted wilt virus (TSWV) and enhanced yield in tomato. Biocontrol Sci. Technol. 2005, 15, 553–569. [Google Scholar] [CrossRef]
- Deng, Z.S.; Zhao, L.F.; Kong, Z.Y.; Yang, W.Q.; Lindström, K.; Wang, E.T.; Wei, G.H. Diversity of endophytic bacteria within nodules of the Sphaerophysa salsula in different regions of Loess Plateau in China. FEMS Microbiol. Ecol. 2011, 76, 463–475. [Google Scholar] [CrossRef] [PubMed]
- El-Dougdoug, K.A.; Ghaly, M.F.; Taha, M.A. Biological control of Cucumber mosaic virus by certain local Streptomyces isolates: Inhibitory effects of selected five Egyptian isolates. Intl. J. Virol. 2012, 8, 151–164. [Google Scholar] [CrossRef][Green Version]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Dong, J.; Hu, Z.; Li, S.; Su, X.; Zhang, J.; Yin, Y.; Xu, T.; Zhang, Z.; Chen, H. Anti-TMV activity and functional mechanisms of two sesquiterpenoids isolated from Tithonia diversifolia. Pestic. Biochem. Physiol. 2017, 140, 24–29. [Google Scholar] [CrossRef]
- Kavroulakis, N.; Ehaliotis, C.; Ntougias, S.; Zervakis, G.I.; Papadopoulou, K.K. Local and systemic resistance against fungal pathogens of tomato plants elicited by a compost derived from agricultural residues. Physiol. Mol. Plant Pathol. 2005, 66, 163–174. [Google Scholar] [CrossRef]
- André, C.M.; Schafleitner, R.; Legay, S.; Lefèvre, I.; Aliaga, C.A.A.; Nomberto, G.; Hoffmann, L.; Hausman, J.-F.; Larondelle, Y.; Evers, D. Gene expression changes related to the production of phenolic compounds in potato tubers grown under drought stress. Phytochemistry 2009, 70, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Sagi, M.; Davydov, O.; Orazova, S.; Yesbergenova, Z.; Ophir, R.; Stratmann, J.W.; Fluhr, R. Plant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum. Plant Cell 2004, 16, 616–628. [Google Scholar] [CrossRef]
- Hafez, E.E.; El-Morsi, A.A.; El-Shahaby, O.A.; Abdelkhalek, A.A. Occurrence of iris yellow spot virus from onion crops in Egypt. VirusDisease 2014, 25, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Junglee, S.; Urban, L.; Sallanon, H.; Lopez-Lauri, F. Optimized Assay for Hydrogen Peroxide Determination in Plant Tissue Using Potassium Iodide. Am. J. Anal. Chem. 2014, 05, 730–736. [Google Scholar] [CrossRef]
- Cho, Y.K.; Ahn, H.K. Purification and characterization of polyphenol oxidase from potato: II. Inhibition and catalytic mechanism. J. Food Biochem. 1999, 23, 593–605. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Kumar, A.; Dutt, S.; Bagler, G.; Ahuja, P.S.; Kumar, S. Engineering a thermo-stable superoxide dismutase functional at sub-zero to >50°C, which also tolerates autoclaving. Sci. Rep. 2012, 2, srep00387. [Google Scholar] [CrossRef]
- Angelini, R.; Manes, F.; Federico, R. Spatial and functional correlation between diamine-oxidase and peroxidase activities and their dependence upon de-etiolation and wounding in chick-pea stems. Planta 1990, 182, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14]Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Oxidants and Antioxidants Part A; Academic Press: San Diego, CA, USA, 1999; Volume 299, pp. 152–178. ISBN 0076-6879. [Google Scholar]
- Ghosh, S.; Derle, A.; Ahire, M.; More, P.; Jagtap, S.; Phadatare, S.D.; Patil, A.B.; Jabgunde, A.M.; Sharma, G.K.; Shinde, V.S.; et al. Phytochemical analysis and free radical scavenging activity of medicinal plants Gnidia glauca and Dioscorea bulbifera. PLoS ONE 2013, 8, e82529. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, A.; Ismail, I.A.I.A.; Dessoky, E.S.E.S.; El-Hallous, E.I.E.I.; Hafez, E. A tomato kinesin-like protein is associated with Tobacco Mosaic Virus infection. Biotechnol. Biotechnol. Equip. 2019, 33, 1424–1433. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Qari, S.H.S.H.; Hafez, E. Iris yellow spot virus–induced chloroplast malformation results in male sterility. J. Biosci. 2019, 44, 142. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, A.; Al-Askar, A.A.; Arishi, A.A.; Behiry, S.I. Trichoderma hamatum Strain Th23 Promotes Tomato Growth and Induces Systemic Resistance against Tobacco Mosaic Virus. J. Fungi 2022, 8, 228. [Google Scholar] [CrossRef]
- Hafez, E.E.; Abdelkhalek, A.A.; Abd El-Wahab, A.S.E.-D.; Galal, F.H. Altered gene expression: Induction/suppression in leek elicited by Iris Yellow Spot Virus infection (IYSV) Egyptian isolate. Biotechnol. Biotechnol. Equip. 2013, 27, 4061–4068. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Qari, S.H.; Abu-Saied, M.A.A.-R.; Khalil, A.M.; Younes, H.A.; Nehela, Y.; Behiry, S.I. Chitosan Nanoparticles Inactivate Alfalfa Mosaic Virus Replication and Boost Innate Immunity in Nicotiana glutinosa Plants. Plants 2021, 10, 2701. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Tabassum, B.; Abd_Allah, E.F. Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Blake, C.; Christensen, M.N.; Kovacs, A.T. Molecular aspects of plant growth promotion and protection by bacillus subtilis. Mol. Plant-Microbe Interact. 2021, 34, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, A.; Behiry, S.I.; Al-Askar, A.A. Bacillus velezensis PEA1 Inhibits Fusarium oxysporum Growth and Induces Systemic Resistance to Cucumber Mosaic Virus. Agronomy 2020, 10, 1312. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Ryu, C.-M.; Zhang, S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 2004, 94, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Yu, X.; Liang, X.; Liu, Y.; Borriss, R.; Liu, Y. Addition of plant-growth-promoting Bacillus subtilis PTS-394 on tomato rhizosphere has no durable impact on composition of root microbiome. BMC Microbiol. 2017, 17, 131. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, A.; Essack, M.; Lafi, F.F.; Andres-Barrao, C.; Jalal, R.; Alamoudi, S.; Razali, R.; Alzubaidy, H.; Shah, K.H.; Siddique, S.; et al. Bioprospecting desert plant Bacillus endophytic strains for their potential to enhance plant stress tolerance. Sci. Rep. 2019, 9, 18154. [Google Scholar] [CrossRef]
- Saxena, A.K.; Kumar, M.; Chakdar, H.; Anuroopa, N.; Bagyaraj, D.J. Bacillus species in soil as a natural resource for plant health and nutrition. J. Appl. Microbiol. 2020, 128, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Posada, L.F.; Ramírez, M.; Ochoa-Gómez, N.; Cuellar-Gaviria, T.Z.; Argel-Roldan, L.E.; Ramírez, C.A.; Villegas-Escobar, V. Bioprospecting of aerobic endospore-forming bacteria with biotechnological potential for growth promotion of banana plants. Sci. Hortic. 2016, 212, 81–90. [Google Scholar] [CrossRef]
- Arena, G.D.; Ramos-González, P.L.; Nunes, M.A.; Ribeiro-Alves, M.; Camargo, L.E.A.; Kitajima, E.W.; Machado, M.A.; Freitas-Astúa, J. Citrus leprosis virus C infection results in hypersensitive-like response, suppression of the JA/ET plant defense pathway and promotion of the colonization of its mite vector. Front. Plant Sci. 2016, 7, 1757. [Google Scholar] [CrossRef]
- Zhu, F.; Deng, X.-G.; Xu, F.; Jian, W.; Peng, X.-J.; Zhu, T.; Xi, D.-H.; Lin, H.-H. Mitochondrial alternative oxidase is involved in both compatible and incompatible host-virus combinations in Nicotiana benthamiana. Plant Sci. 2015, 239, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Zhang, Q.; Che, Y.; Zhu, P.; Zhang, Q.; Ji, Z. Glutathione contributes to resistance responses to TMV through a differential modulation of salicylic acid and reactive oxygen species. Mol. Plant Pathol. 2021, 22, 1668–1687. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, N.; Singh, M.; Dubey, R.S.; Venkataramanappa, V.; Datta, D. Phytochemicals and antioxidative enzymes defence mechanism on occurrence of yellow vein mosaic disease of pumpkin (Cucurbita moschata). Biotech 2013, 3, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Madhusudhan, K.N.; Srikanta, B.M.; Shylaja, M.D.; Prakash, H.S.; Shetty, H.S. Changes in antioxidant enzymes, hydrogen peroxide, salicylic acid and oxidative stress in compatible and incompatible host-tobamovirus interaction. J. Plant Interact. 2009, 4, 157–166. [Google Scholar] [CrossRef]
- Radwan, D.E.M.; Ismail, K.S. The impact of hydrogen peroxide against cucumber green mottle mosaic virus infection in watermelon plants. Polish J. Environ. Stud. 2020, 29, 3771–3782. [Google Scholar] [CrossRef]
- Rui, R.; Liu, S.; Karthikeyan, A.; Wang, T.; Niu, H.; Yin, J.; Yang, Y.; Wang, L.; Yang, Q.; Zhi, H. Fine-mapping and identification of a novel locus Rsc15 underlying soybean resistance to Soybean mosaic virus. Theor. Appl. Genet. 2017, 130, 2395–2410. [Google Scholar] [CrossRef] [PubMed]
- Yoda, H.; Yamaguchi, Y.; Sano, H. Induction of hypersensitive cell death by hydrogen peroxide produced through polyamine degradation in tobacco plants. Plant Physiol. 2003, 132, 1973–1981. [Google Scholar] [CrossRef]
- de Dios Alché, J. A concise appraisal of lipid oxidation and lipoxidation in higher plants. Redox Biol. 2019, 23, 101136. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.A.; Gullner, G.; Clemente-Moreno, M.J.; Künstler, A.; Juhász, C.; Díaz-Vivancos, P.; Király, L. Oxidative stress and antioxidative responses in plant–virus interactions. Physiol. Mol. Plant Pathol. 2016, 94, 134–148. [Google Scholar] [CrossRef]
- Balal, R.M.; Khan, M.M.; Shahid, M.A.; Mattson, N.S.; Abbas, T.; Ashfaq, M.; Garcia-Sanchez, F.; Ghazanfer, U.; Gimeno, V.; Iqbal, Z. Comparative studies on the physiobiochemical, enzymatic, and ionic modifications in salt-tolerant and salt-sensitive citrus rootstocks under NaCl stress. J. Am. Soc. Hortic. Sci. 2012, 137, 86–95. [Google Scholar] [CrossRef]
- Yanan, D.; Ran, C.; Rong, Z.; Jiang, W.; Xuesen, C.; Chengmiao, Y.; Zhiquan, M. Isolation, identification, and antibacterial mechanisms of Bacillus amyloliquefaciens QSB-6 and its effect on plant roots. Front. Microbiol. 2021, 12, 746799. [Google Scholar]
- Li, L.; Steffens, J.C. Overexpression of polyphenol oxidase in transgenic tomato plants results in enhanced bacterial disease resistance. Planta 2002, 215, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Zhao, P.; Wang, B.; Tariq, P.; Zhao, F.; Zhao, M.; Wang, Q.; Yang, T.; Fang, J. Overexpression of Polyphenol Oxidase Gene in Strawberry Fruit Delays the Fungus Infection Process. Plant Mol. Biol. Rep. 2016, 34, 592–606. [Google Scholar] [CrossRef]
- Mohammadi, M.; Kazemi, H. Changes in peroxidase and polyphenol oxidase activities in susceptible and resistant wheat heads inoculated with Fusarium graminearum and induced resistance. Plant Sci. 2002, 162, 491–498. [Google Scholar] [CrossRef]
- Khan, M.S.; Gao, J.; Chen, X.; Zhang, M.; Yang, F.; Du, Y.; Moe, T.S.; Munir, I.; Xue, J.; Zhang, X. Isolation and Characterization of Plant Growth-Promoting Endophytic Bacteria Paenibacillus polymyxa SK1 from Lilium lancifolium. Biomed Res. Int. 2020, 2020, 8650957. [Google Scholar] [CrossRef]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The Significance of Bacillus spp. in Disease Suppression and Growth Promotion of Field and Vegetable Crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef]
- Rais, A.; Jabeen, Z.; Shair, F.; Hafeez, F.Y.; Hassan, M.N. Bacillus spp., a bio-control agent enhances the activity of antioxidant defense enzymes in rice against Pyricularia oryzae. PLoS ONE 2017, 12, e0187412. [Google Scholar] [CrossRef] [PubMed]
- Akyol, H.; Riciputi, Y.; Capanoglu, E.; Caboni, M.; Verardo, V. Phenolic compounds in the potato and its byproducts: An overview. Int. J. Mol. Sci. 2016, 17, 835. [Google Scholar] [CrossRef] [PubMed]
- Nasr-Eldin, M.; Messiha, N.; Othman, B.; Megahed, A.; Elhalag, K. Induction of potato systemic resistance against the potato virus Y (PVY NTN), using crude filtrates of Streptomyces spp. under greenhouse conditions. Egypt. J. Biol. Pest Control 2019, 29, 62. [Google Scholar] [CrossRef]
- Lee, G.H.; Ryu, C.-M. Spraying of leaf-colonizing Bacillus amyloliquefaciens protects pepper from Cucumber mosaic virus. Plant Dis. 2016, 100, 2099–2105. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, A.; Al-Askar, A.A. Green Synthesized ZnO Nanoparticles Mediated by Mentha Spicata Extract Induce Plant Systemic Resistance against Tobacco Mosaic Virus. Appl. Sci. 2020, 10, 5054. [Google Scholar] [CrossRef]
- Guo, Q.; Li, Y.; Lou, Y.; Shi, M.; Jiang, Y.; Zhou, J.; Sun, Y.; Xue, Q.; Lai, H. Bacillus amyloliquefaciens Ba13 induces plant systemic resistance and improves rhizosphere microecology against tomato yellow leaf curl virus disease. Appl. Soil Ecol. 2019, 137, 154–166. [Google Scholar] [CrossRef]
- Dempsey, D.M.A.; Vlot, A.C.; Wildermuth, M.C.; Klessig, D.F. Salicylic acid biosynthesis and metabolism. Arab. Book/Am. Soc. Plant Biol. 2011, 9, e0156. [Google Scholar] [CrossRef]
- Breen, S.; Williams, S.J.; Outram, M.; Kobe, B.; Solomon, P.S. Emerging Insights into the Functions of Pathogenesis-Related Protein 1. Trends Plant Sci. 2017, 22, 871–879. [Google Scholar] [CrossRef]
- Iglesias, V.A.; Meins, F.; Meins, F., Jr. Movement of plant viruses is delayed in a β-1, 3-glucanase-deficient mutant showing a reduced plasmodesmatal size exclusion limit and enhanced callose deposition. Plant J. 2000, 21, 157–166. [Google Scholar] [CrossRef]
- Otulak-Kozieł, K.; Kozieł, E.; Lockhart, B. Plant cell wall dynamics in compatible and incompatible potato response to infection caused by Potato virus Y (PVYNTN). Int. J. Mol. Sci. 2018, 19, 862. [Google Scholar] [CrossRef]
- Bucher, G.L.; Tarina, C.; Heinlein, M.; Di Serio, F.; Meins, F., Jr.; Iglesias, V.A. Local expression of enzymatically active class I β-1, 3-glucanase enhances symptoms of TMV infection in tobacco. Plant J. 2001, 28, 361–369. [Google Scholar] [CrossRef]
- Dobnik, D.; Baebler, Š.; Kogovšek, P.; Pompe-Novak, M.; Štebih, D.; Panter, G.; Janež, N.; Morisset, D.; Žel, J.; Gruden, K. β-1, 3-glucanase class III promotes spread of PVY NTN and improves in planta protein production. Plant Biotechnol. Rep. 2013, 7, 547–555. [Google Scholar] [CrossRef]
- Beckman, C.H. Phenolic-storing cells: Keys to programmed cell death and periderm formation in wilt disease resistance and in general defence responses in plants? Physiol. Mol. Plant Pathol. 2000, 57, 101–110. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Dessoky, E.S.; Hafez, E. Polyphenolic genes expression pattern and their role in viral resistance in tomato plant infected with Tobacco mosaic virus. Biosci. Res. 2018, 15, 3349–3356. [Google Scholar]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q.; Chen, Z. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Song, S.; Yan, X.; Fang, L.; Zeng, B.; Zhu, Y. Endogenous salicylic acid shows different correlation with baicalin and baicalein in the medicinal plant Scutellaria baicalensis Georgi subjected to stress and exogenous salicylic acid. PLoS ONE 2018, 13, e0192114. [Google Scholar] [CrossRef]
- Kang, J.-H.; McRoberts, J.; Shi, F.; Moreno, J.E.; Jones, A.D.; Howe, G.A. The flavonoid biosynthetic enzyme chalcone isomerase modulates terpenoid production in glandular trichomes of tomato. Plant Physiol. 2014, 164, 1161–1174. [Google Scholar] [CrossRef]
- Sonnante, G.; D’Amore, R.; Blanco, E.; Pierri, C.L.; De Palma, M.; Luo, J.; Tucci, M.; Martin, C. Novel hydroxycinnamoyl-coenzyme A quinate transferase genes from artichoke are involved in the synthesis of chlorogenic acid. Plant Physiol. 2010, 153, 1224–1238. [Google Scholar] [CrossRef]
- Moglia, A.; Lanteri, S.; Comino, C.; Hill, L.; Knevitt, D.; Cagliero, C.; Rubiolo, P.; Bornemann, S.; Martin, C. Dual catalytic activity of hydroxycinnamoyl-coenzyme A quinate transferase from tomato allows it to moonlight in the synthesis of both mono- and dicaffeoylquinic acids. Plant Physiol. 2014, 166, 1777–1787. [Google Scholar] [CrossRef]
- Niggeweg, R.; Michael, A.J.; Martin, C. Engineering plants with increased levels of the antioxidant chlorogenic acid. Nat. Biotechnol. 2004, 22, 746. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A.; Hafez, E. Differential induction and suppression of the potato innate immune system in response to Alfalfa mosaic virus infection. Physiol. Mol. Plant Pathol. 2020, 110, 101485. [Google Scholar] [CrossRef]
- Bazzini, A.A.; Manacorda, C.A.; Tohge, T.; Conti, G.; Rodriguez, M.C.; Nunes-Nesi, A.; Villanueva, S.; Fernie, A.R.; Carrari, F.; Asurmendi, S. Metabolic and miRNA profiling of TMV infected plants reveals biphasic temporal changes. PLoS ONE 2011, 6, e28466. [Google Scholar] [CrossRef]
- Ullah, A.; Bano, A.; Janjua, H.T. Microbial Secondary Metabolites and Defense of Plant Stress. Microb. Serv. Restor. Ecol. 2020, 11, 37–46. [Google Scholar] [CrossRef]
- Nas, F.; Aissaoui, N.; Mahjoubi, M.; Mosbah, A.; Arab, M.; Abdelwahed, S.; Khrouf, R.; Masmoudi, A.-S.; Cherif, A.; Klouche-Khelil, N. A comparative GC-MS analysis of bioactive secondary metabolites produced by halotolerant Bacillus spp. isolated from the Great Sebkha of Oran. Int. Microbiol. Off. J. Spanish Soc. Microbiol. 2021, 24, 455–470. [Google Scholar] [CrossRef] [PubMed]
- María Teresa, R.-C.; Rosaura, V.-G.; Elda, C.-M.; Ernesto, G.-P. The avocado defense compound phenol-2,4-bis (1,1-dimethylethyl) is induced by arachidonic acid and acts via the inhibition of hydrogen peroxide production by pathogens. Physiol. Mol. Plant Pathol. 2014, 87, 32–41. [Google Scholar] [CrossRef]
- Abdullah, A.S.H.; Mirghani, M.E.S.; Jamal, P. Antibacterial activity of Malaysian mango kernel. African J. Biotechnol. 2011, 10, 18739–18748. [Google Scholar] [CrossRef]
- Ahsan, T.; Chen, J.; Zhao, X.; Irfan, M.; Wu, Y. Extraction and identification of bioactive compounds (eicosane and dibutyl phthalate) produced by Streptomyces strain KX852460 for the biological control of Rhizoctonia solani AG-3 strain KX852461 to control target spot disease in tobacco leaf. AMB Express 2017, 7, 54. [Google Scholar] [CrossRef]
- Octarya, Z.; Novianty, R.; Suraya, N. Saryono Antimicrobial activity and GC-MS analysis of bioactive constituents of Aspergillus fumigatus 269 isolated from Sungai Pinang hot spring, Riau, Indonesia. Biodiversitas 2021, 22, 1839–1845. [Google Scholar] [CrossRef]
- Naeim, H.; El-Hawiet, A.; Abdel Rahman, R.A.; Hussein, A.; El Demellawy, M.A.; Embaby, A.M. Antibacterial activity of Centaurea pumilio L. Root and aerial part extracts against some multidrug resistant bacteria. BMC Complement. Med. Ther. 2020, 20, 79. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Gumber, D.; Abbot, V.; Dhiman, S.; Sharma, P. Pyrrole: A resourceful small molecule in key medicinal hetero-aromatics. RSC Adv. 2015, 5, 15233–15266. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, D.; Singh, G.; Monga, V.; Kumar, B. Recent advancements in the development of heterocyclic anti-inflammatory agents. Eur. J. Med. Chem. 2020, 200, 112438. [Google Scholar] [CrossRef]
- Kumari, N.; Menghani, E.; Mithal, R. GCMS analysis of compounds extracted from actinomycetes AIA6 isolates and study of its antimicrobial efficacy. Indian J. Chem. Technol. 2019, 26, 362–370. [Google Scholar]
- Ser, H.-L.; Palanisamy, U.D.; Yin, W.-F.; Abd Malek, S.N.; Chan, K.-G.; Goh, B.-H.; Lee, L.-H. Presence of antioxidative agent, Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- in newly isolated Streptomyces mangrovisoli sp. nov. Front. Microbiol. 2015, 6, 854. [Google Scholar] [CrossRef] [PubMed]
- Pooja, S.; Aditi, T.; Naine, S.J.; Devi, C.S. Bioactive compounds from marine Streptomyces sp. VITPSA as therapeutics. Front. Biol. 2017, 12, 280–289. [Google Scholar] [CrossRef]
| Primer Name | Abbreviation | Nucleotide Sequence | References |
|---|---|---|---|
| 16S ribosomal RNA | 16S rRNA | Forward: AGAGTGATCCTGGCTCAG | [26] |
| Reverse: GGTTACCTTGTTACGACTT | |||
| Tobacco mosaic virus-coat protein | TMV-CP | Forward: ACGACTGCCGAAACGTTAGA | [27] |
| Reverse: CAAGTTGCAGGACCAGAGGT | |||
| Pathogenesis related protein-1 | PR-1 | Forward: GTTCCTCCTTGCCACCTTC | [28] |
| Reverse: TATGCACCCCCAGCATAGTT | |||
| Endoglucanase | PR-2 | Forward: TATAGCCGTTGGAAACGAAG | [28] |
| Reverse: CAACTTGCCATCACATTCTG | |||
| Phenylalanine Ammonia-Lyase | PAL | Forward: ACGGGTTGCCATCTAATCTGACA | [29] |
| Reverse: CGAGCAATAAGAAGCCATCGCAAT | |||
| Chalcone Synthase | CHS | Forward: CACCGTGGAGGAGTATCGTAAGGC | [29] |
| Reverse: TGATCAACACAGTTGGAAGGCG | |||
| Hydroxycinnamoyl Co A: quinate (break)hydroxycinnamoyl transferase | HQT | Forward: CCCAATGGCTGGAAGATTAGCTA | [29] |
| Reverse: CATGAATCACTTTCAGCCTCAACAA | |||
| β-actin | β-actin | Forward: TGGCATACAAAGACAGGACAGCCT | [30] |
| Reverse: ACTCAATCCCAAGGCCAACAGAGA |
| Treatment * | Fresh Weight g/Plant | Dry Weight g/Plant | Shoot Length cm/Plant | Root Length cm/Plant |
|---|---|---|---|---|
| NT | 7.15 ± 0.37 bc | 2.24 ± 0.03 ab | 27.67 ± 3.30 | 14.17 ± 1.55 ab |
| TMV | 6.28 ± 0.61 c | 1.89 ± 0.07 c | 26.17 ± 0.24 | 9.33 ± 2.63 b |
| T1 | 9.36 ± 2.20 ab | 2.27 ± 0.11 ab | 28.33 ± 4.50 | 14 ± 4.55 ab |
| T2 | 6.47 ± 0.87 bc | 2.14 ± 0.15 bc | 27.33 ± 2.49 | 11.33 ± 1.25 ab |
| T3 | 10.35 ± 1.19 a | 2.43 ± 0.16 a | 29.67 ± 2.06 | 16.33 ± 2.87 a |
| Peak | R. Time (min.) | Area | Name | Chemical Formula | Molecular Structure |
|---|---|---|---|---|---|
| 2 | 11.960 | 560.11 | Nonane, 5-(2-methylpropyl)- | C13H28 |  |
| 3 | 12.187 | 1.592.35 | Phenol, 2,4-bis(1,1-dimethylethyl)- | C14H22O |  |
| 6 | 13.689 | 475.26 | Eicosane | C20H42 |  |
| 12 | 15.435 | 535.02 | Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)- | C10H16N2O2 | 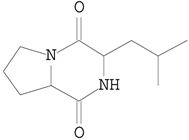 |
| 13 | 15.481 | 549.58 | Pentadecanoic acid | C15H30O2 |  |
| 14 | 15.542 | 835.29 | L-Proline, N-valeryl-, heptadecyl ester | C26H49NO3 |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Gendi, H.; Al-Askar, A.A.; Király, L.; Samy, M.A.; Moawad, H.; Abdelkhalek, A. Foliar Applications of Bacillus subtilis HA1 Culture Filtrate Enhance Tomato Growth and Induce Systemic Resistance against Tobacco mosaic virus Infection. Horticulturae 2022, 8, 301. https://doi.org/10.3390/horticulturae8040301
El-Gendi H, Al-Askar AA, Király L, Samy MA, Moawad H, Abdelkhalek A. Foliar Applications of Bacillus subtilis HA1 Culture Filtrate Enhance Tomato Growth and Induce Systemic Resistance against Tobacco mosaic virus Infection. Horticulturae. 2022; 8(4):301. https://doi.org/10.3390/horticulturae8040301
Chicago/Turabian StyleEl-Gendi, Hamada, Abdulaziz A. Al-Askar, Lóránt Király, Marwa A. Samy, Hassan Moawad, and Ahmed Abdelkhalek. 2022. "Foliar Applications of Bacillus subtilis HA1 Culture Filtrate Enhance Tomato Growth and Induce Systemic Resistance against Tobacco mosaic virus Infection" Horticulturae 8, no. 4: 301. https://doi.org/10.3390/horticulturae8040301
APA StyleEl-Gendi, H., Al-Askar, A. A., Király, L., Samy, M. A., Moawad, H., & Abdelkhalek, A. (2022). Foliar Applications of Bacillus subtilis HA1 Culture Filtrate Enhance Tomato Growth and Induce Systemic Resistance against Tobacco mosaic virus Infection. Horticulturae, 8(4), 301. https://doi.org/10.3390/horticulturae8040301