Physiological Response to Short-Term Heat Stress in the Leaves of Traditional and Modern Plum (Prunus domestica L.) Cultivars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Chlorophyll a Fluorescence (ChlF)
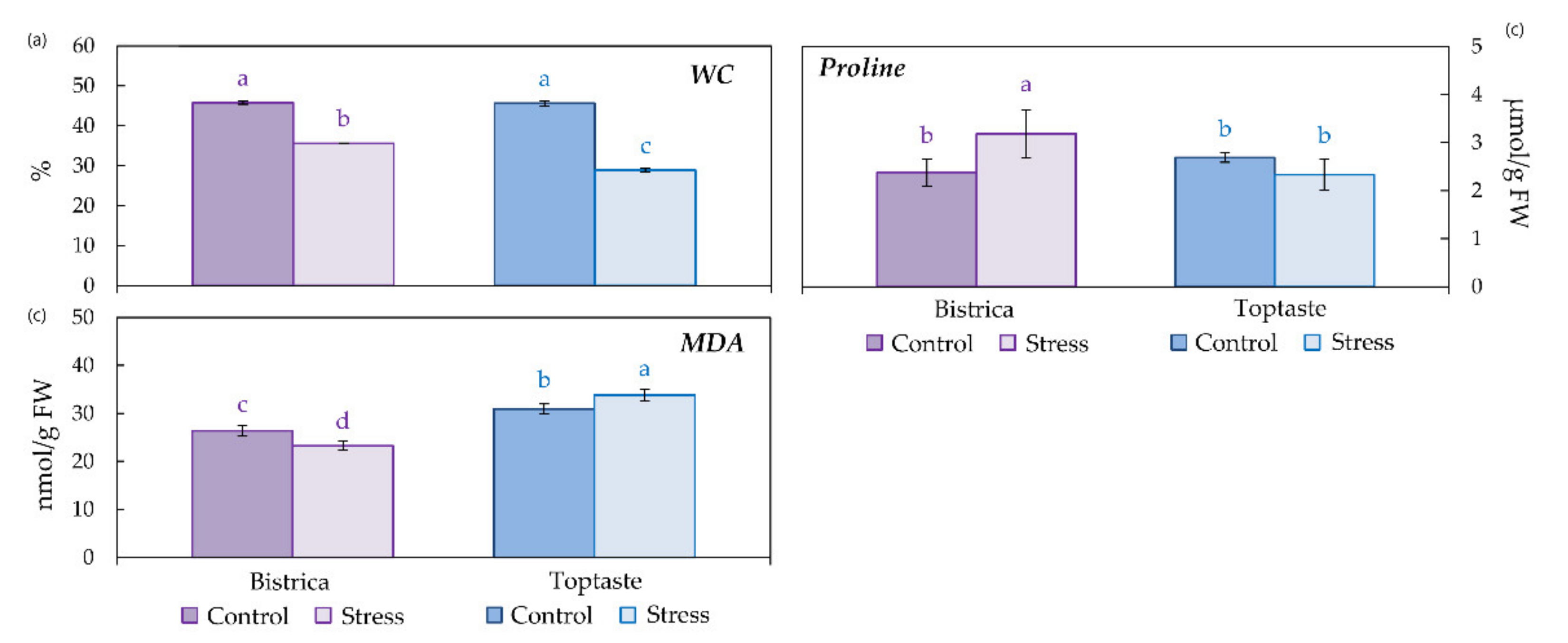
2.3. Water Content
2.4. Proline and Secondary Metabolites
2.4.1. Determination of Proline Content
2.4.2. Total Phenols Content
2.5. Lipid Peroxidation
2.6. Proteins and Enzyme Activities
2.6.1. Soluble Protein Concentration
2.6.2. Guaiacol Peroxidase Activity
2.6.3. Polyphenol Oxidase Activity
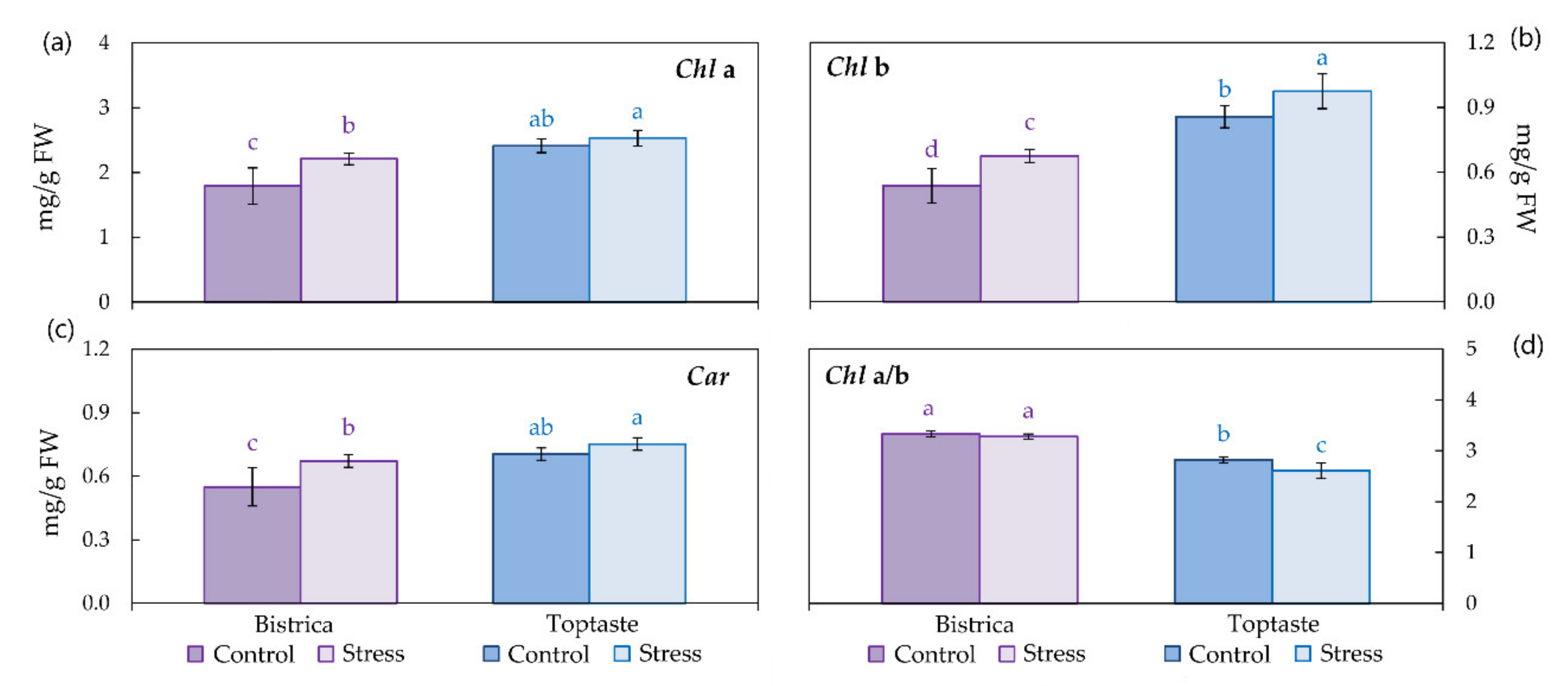
2.7. Photosynthetic Pigments
2.8. Data Analyses
3. Results
3.1. Determination of Stress Occurrence and Severity
3.2. Proteins, Phenols and Enzymatic Activity
3.3. Photosynthetic Pigments
3.4. Photosynthetic Efficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mihaljević, I.; Viljevac Vuletić, M.; Šimić, D.; Tomaš, V.; Horvat, D.; Josipović, M.; Zdunić, Z.; Dugalić, K.; Vuković, D. Comparative study of drought stress effects on traditional and modern apple cultivars. Plants 2021, 10, 561. [Google Scholar] [CrossRef]
- Mihaljević, I.; Viljevac Vuletić, M.; Tomaš, V.; Horvat, D.; Zdunić, Z.; Vuković, D. PSII photochemistry responses to drought stress in autochthonous and modern sweet cherry cultivars. Photosynthetica 2021, 59, 517–528. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R.; Mohanty, P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat Stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Wahid, A.; Close, T.J. Expression of dehydrins under heat stress and their relationship with water relations of sugarcane leaves. Biol. Plant. 2007, 51, 104–109. [Google Scholar] [CrossRef]
- Gulen, H.; Eris, A. Some physiological changes in strawberry (Fragaria × ananassa ‘Camarosa’) plants under heat stress. J. Hortic. Sci. Biotech. 2003, 78, 894–898. [Google Scholar] [CrossRef]
- Hao, H.-P.; Jiang, C.-D.; Zhang, S.-R.; Tang, Y.-D.; Shi, L. Enhanced thermal tolerance of photosystem II by elevating root zone temperature in Prunus mira Koehne seedlings. Plant Soil 2012, 353, 367–378. [Google Scholar] [CrossRef]
- Iqbal, N.; Fatma, M.; Khan, N.A.; Umar, S. Regulatory role of proline in heat stress tolerance: Modulation by salicylic acid. In Plant Signaling Molecules; Khan, M.I.R., Reddy, P.S., Ferrante, A., Khan, N.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 437–448. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Harsh, A.; Sharma, Y.K.; Joshi, U.; Rampuria, S.; Singh, G.; Kumar, S.; Sharma, R. Effect of short-term heat stress on total sugars, proline and some antioxidant enzymes in moth bean (Vigna aconitifolia). Ann. Agric. Sci. 2016, 61, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Fan, S.; Zhang, Q.; Wang, Y. Effect of heat stress on the MDA, proline and soluble sugar content in leaf lettuce seedlings. Agric. Sci. 2013, 4, 112–115. [Google Scholar] [CrossRef] [Green Version]
- Sairam, R.K.; Srivastava, G.C.; Saxena, D.C. Increased antioxidant activity under elevated temperatures: A mechanism of heat stress tolerance in wheat genotypes. Biol. Plant. 2000, 43, 245–251. [Google Scholar] [CrossRef]
- Jin, R.; Wang, Y.; Liu, R.; Gou, J.; Chan, Z. Physiological and metabolic changes of purslane (Portulaca oleracea L.) in response to drought, heat, and combined stresses. Front. Plant Sci. 2016, 6, 1123. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Yu, X.; Kjær, K.H.; Rosenqvist, E.; Ottosen, C.-O. Screening and validation of tomato genotypes under heat stress using Fv/Fm to reveal the physiological mechanism of heat tolerance. Exp. Environ. Bot. 2015, 118, 1–11. [Google Scholar] [CrossRef]
- Boeckx, T.; Webster, R.; Winters, A.L.; Webb, K.J.; Gay, A.; Kingston-Smith, A.H. Polyphenol oxidase-mediated protection against oxidative stress is not associated with enhanced photosynthetic efficiency. Ann. Bot. 2015, 116, 529–540. [Google Scholar] [CrossRef] [Green Version]
- Zushi, K.; Kajiwara, S.; Matsuzoe, N. Chlorophyll a fluorescence OJIP transient as a tool to characterize and evaluate response to heat and chilling stress in tomato leaf and fruit. Sci. Hortic. 2012, 148, 39–46. [Google Scholar] [CrossRef]
- Yan, K.; Chen, P.; Shao, H.; Shao, C.; Zhao, S.; Brestič, M. Dissection of photosynthetic electron transport process in sweet sorghum under heat stress. PLoS ONE 2013, 8, e62100. [Google Scholar] [CrossRef] [Green Version]
- Feng, B.; Liu, P.; Li, G.; Dong, S.T.; Wang, F.H.; Kong, L.A.; Zhang, W. Effect of heat stress on the photosynthetic characteristics in flag leaves at the grain-filling stage of different heat-resistant winter wheat varieties. J. Agron. Crop Sci. 2014, 200, 134–155. [Google Scholar] [CrossRef]
- Haworth, M.; Marino, G.; Brunetti, C.; Killi, D.; de Carlo, A.; Centritto, M. The impact of heat stress and water deficit on the photosynthetic and stomatal physiology of olive (Olea europaea L.)–A case study of the 2017 heat wave. Plants 2018, 7, 76. [Google Scholar] [CrossRef] [Green Version]
- Force, L.; Critchley, C.; van Rensen, J.S. New fluorescence parameters for monitoring photosynthesis in plants. Photosynth. Res. 2003, 78, 17–23. [Google Scholar] [CrossRef]
- Biško, A.; Jelačić, T.; Miloloža, D.; Savić, Z.; Brus, K. The plum industry in the Republic of Croatia. Acta Hortic. 2019, 1260, 215–220. [Google Scholar] [CrossRef]
- Ivković, F. Šljiva Bistrica—Nacionalni ponos hrvatskih voćara. Glas. Zaštite Bilja 2010, 5, 32–33. [Google Scholar]
- Družić, J.; Voća, S.; Čmelik, Z.; Dobričević, N.; Duralija, B.; Skendrović Babojelić, M. Fruit quality of plum cultivars Elena and Bistrica. Agric. Conspec. Sci. 2007, 72, 307–310. [Google Scholar]
- Molnár, A.M.; Ladányi, M.; Kovácz, S. Evaluation of the production traits and fruit quality of German plum cultivars. Acta Univ. Agric. Silvic. Mendelianae Brun. 2016, 64, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanism, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor and Francis: London, UK, 2000; pp. 445–483. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Kumar, D.; Al Hassan, M.; Naranjo, M.A.; Agrawal, V.; Boscaiu, M.; Vicente, O. Effects of salinity and drought on growth, ionic relations, compatible solutes and activation of antioxidant systems in oleander Nerium oleander (L.). PLoS ONE 2017, 12, e0185017. [Google Scholar] [CrossRef]
- Woodrow, P.; Ciarmiello, L.F.; Annunziata, M.G.; Pacifico, S.; Iannuzzi, F.; Mirto, A.; D’Amelia, L.; Dell’Aversana, E.; Piccolella, S.; Fuggi, A.; et al. Durum wheat seedling responses to simultaneous high light and salinity involve a fine reconfiguration of amino acids and carbohydrate metabolism. Physiol. Plant. 2017, 159, 290–312. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Verma, S.; Dubey, R.S. Leads toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 2003, 164, 645–655. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Siegel, B.Z.; Galston, W. The isoperoxidases of Pisum sativum. Plant Physiol. 1967, 42, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Raymond, J.; Rakariyatham, N.; Azanza, J. Purification and some properties of polyphenoloxidase from sunflower seeds. Phytochemistry 1993, 34, 927–931. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Meth. Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathur, S.; Jajoo, A.; Mehta, P.; Bharti, S. Analysis of elevated temperature-induced inhibition of photosystem II using chlorophyll a fluorescence induction kinetics in wheat leaves (Triticum aestivum). Plant Biol. 2011, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gosavi, G.U.; Jadhav, A.S.; Kale, A.A.; Gadakh, S.R.; Pawar, B.D.; Chimote, V.P. Effect of heat stress on proline, chlorophyll content, heat shock proteins and antioxidant enzyme activity in sorghum (Sorghum bicolor) at seedlings stage. Indian J. Biotechnol. 2014, 13, 356–363. [Google Scholar]
- Gür, A.; Demirel, U.; Özden, M.; Kahraman, A.; Çopur, O. Diurnal gradual heat stress affects antioxidant enzymes, proline accumulation and some physiological components in cotton (Gossypium hirsutum L.). Afr. J. Biotechnol. 2010, 9, 1008–1015. [Google Scholar]
- Tommasino, E.; Griffa, S.; Grunberg, K.; Ribotta, A.; López Colomba, E.; Carloni, E.; Quiroga, M.; Luna, C.M. Malondialdehyde content as a potential biochemical indicator of tolerant Cenchrus ciliaris L. genotypes under heat stress treatment. Grass Forage Sci. 2012, 67, 456–459. [Google Scholar] [CrossRef]
- Gulen, H.; Eris, A. Effect of heat stress on peroxidase activity and total protein content in strawberry plants. Plant Sci. 2004, 166, 739–744. [Google Scholar] [CrossRef]
- Chaitanya, K.V.; Sundar, D.; Masilamani, S.; Reddy, A.R. Variation in heat stress-induced antioxidant enzyme activities among three mulberry cultivars. Plant Growth Regul. 2002, 36, 175–180. [Google Scholar] [CrossRef]
- Rivero, R.M.; Ruiz, J.M.; García, P.C.; López-Lefebre, L.R.; Sánchez, E.; Romero, L. Resistance to cold and heat stress: Accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci. 2001, 160, 315–321. [Google Scholar] [CrossRef]
- Ancillotti, C.; Bogani, P.; Biricolti, S.; Calistri, E.; Checchini, L.; Ciofi, L.; Gonnelli, C.; Del Bubba, M. Changes in polyphenol and sugar concentrations in wild type and genetically modified Nicotiana langsdorffii Weinmann in response to water and heat stress. Plant Physiol. Biochem. 2015, 97, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Siddika, M.R.; Rakib, M.A.; Abu Zubair, M.; Islam, M.M.; Haque, M.S.; Al-Khayri, J.M. Regulatory mechanism of enhancing polyphenol oxidase activity in leaf of Basella alba induced by high temperature stress. Emir. J. Food Agric. 2015, 27, 82–93. [Google Scholar] [CrossRef] [Green Version]
- Shahid, M.; Saleem, M.F.; Anjum, S.A.; Shahid, M.; Afzal, I. Effect of terminal heat stress on proline, secondary metabolites and yield components of wheat (Triticum aestivum L.) genotypes. Philipp. Agric. Sci. 2017, 100, 278–286. [Google Scholar]
- Wang, J.; Yuan, B.; Huang, B. Differential heat-induced changes in phenolic acids associated with genotypic variations in heat tolerance for hard fescue. Crop Sci. 2019, 59, 667–674. [Google Scholar] [CrossRef]
- Martinez, V.; Mestre, T.C.; Rubio, F.; Girones-Vilaplana, A.; Moreno, D.A.; Mittler, R.; Rivero, R.M. Accumulation of flavonols over hydroxycinnamic acids favors oxidative damage protection under abiotic stress. Front. Plant Sci. 2016, 7, 838. [Google Scholar] [CrossRef] [PubMed]
- Tzortzakis, N.; Chrysargyris, A.; Aziz, A. Adaptive response of a native Mediterranean grapevine cultivar upon short-term exposure to drought and heat stress in the context of climate change. Agronomy 2020, 10, 249. [Google Scholar] [CrossRef] [Green Version]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Martinazzo, E.G.; Ramm, A.; Bacarin, M.A. The chlorophyll a fluorescence as an indicatior of the temperature stress in the leaves of Prunus persica. Braz. J. Plant Physiol. 2012, 24, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Markulj Kulundžić, A.; Viljevac Vuletić, M.; Matoša Kočar, M.; Mijić, A.; Varga, I.; Sudarić, A.; Cesar, V.; Lepeduš, H. The combination of increased temperatures and high irradiation causes changes in photosynthetic efficiency. Plants 2021, 10, 2076. [Google Scholar] [CrossRef]
- Mihaljević, I.; Lepeduš, H.; Šimić, D.; Viljevac Vuletić, M.; Tomaš, V.; Vuković, D.; Dugalić, K.; Teklić, T.; Skendrović Babojelić, M.; Zdunić, Z. Photochemical efficiency of photosystem II in two apple cultivars affected by elevated temperature and excess light in vivo. S. Afr. J. Bot. 2020, 130, 316–326. [Google Scholar] [CrossRef]
- Viljevac, M.; Dugalić, K.; Mihaljević, I.; Šimić, D.; Sudar, R.; Jurković, Z.; Lepeduš, H. Chlorophyll content, photosynthetic efficiency and genetic markers in two sour cherry (Prunus cerasus L.) genotypes under drought stress. Acta Bot. Croat. 2013, 72, 221–235. [Google Scholar] [CrossRef] [Green Version]

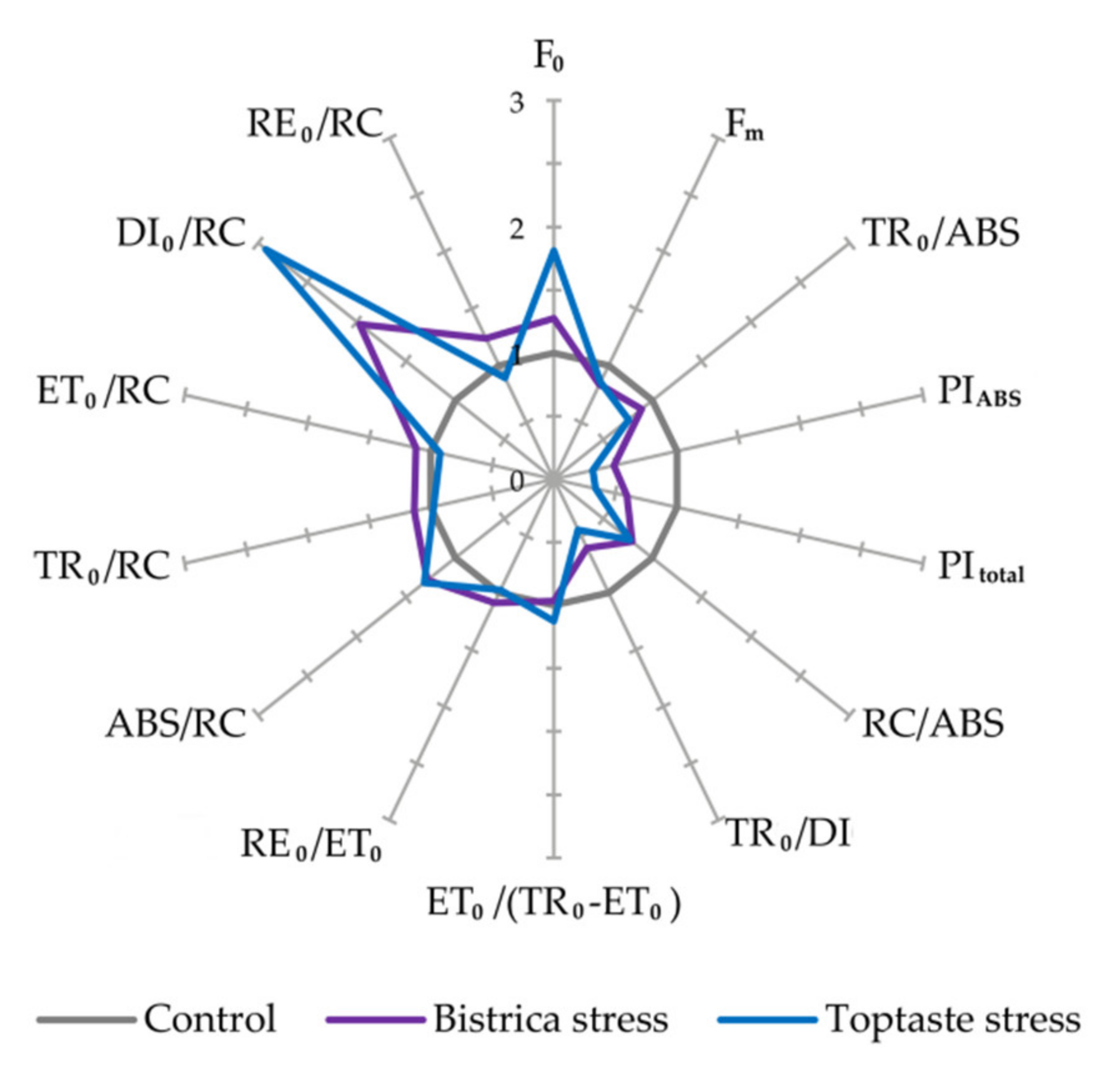
| F0—Minimal fluorescence intensity (20 µs) |
| Fm—Maximal fluorescence intensity |
| TR0/ABS, i.e., Fv/Fm—Maximum quantum yield of PSII |
| PIABS—Performance index per absorption basis |
| PItotal—Performance index for energy conservation from exciton to the reduction of PSI end acceptors |
| RC/ABS—Density of reaction centers on chlorophyll basis |
| TR0/DI0—Flux ratio trapping per dissipation |
| ET0/(TR0–ET0)—Efficiency of the conversion of excitation energy to electron transport |
| RE0/ET0—Efficiency with which an electron from the intersystem electron carriers moves to reduce end electron acceptors at the PSI acceptor side |
| RC/CS0—Density of reaction centres (QA−reducing PSII reaction) at t = 0 |
| ABS/RC—Absorption per active RC |
| TR0/RC—Trapping per active RC |
| ET0/RC—Electron transport per active RC |
| DI0/RC—Dissipation per active RC |
| RE0/RC—Electron flux reducing end electron acceptors at PSI acceptor side per RC |
| All fluorescence parameters are in relative units, RC—reaction center |
| Bistrica | Toptaste | |||
|---|---|---|---|---|
| Parameter | Control | Stress | Control | Stress |
| F0 | 450.17 ± 18.22 b | 573.42 ± 95.85 b | 474.5 ± 15.93 b | 858.42 ± 347.18 a |
| Fm | 2672.8 ± 117.9 a | 2227.5 ± 238.4 c | 2797.3 ± 126.2 a | 2385.2 ± 212.6 b |
| TR0/ABS i.e., Fv/Fm | 0.83 ± 0.01 a | 0.74 ± 0.06 b | 0.83 ± 0.01 a | 0.63 ± 0.18 c |
| PIABS | 3.13 ± 0.7 a | 1.53 ± 0.82 b | 2.81 ± 0.38 a | 0.89 ± 0.5 c |
| PItotal | 2.57 ± 0.62 a | 1.52 ± 0.83 b | 1.79 ± 0.31 a | 0.61 ± 0.44 c |
| RC/ABS | 0.53 ± 0.04 a | 0.42 ± 0.05 c | 0.48 ± 0.04 b | 0.37 ± 0.04 d |
| TR0/DI0 | 4.95 ± 0.34 a | 2.99 ± 0.80 b | 4.9 ± 0.21 a | 2.17 ± 1.10 c |
| ET0/(TR0 − ET0) | 1.18 ± 0.18 a | 1.14 ± 0.31 a | 1.19 ± 0.10 a | 1.34 ± 0.85 a |
| RE0/ET0 | 0.45 ± 0.02 a | 0.49 ± 0.07 a | 0.39 ± 0.03 b | 0.38 ± 0.10 b |
| ABS/RC | 1.88 ± 0.14 d | 2.41 ± 0.28 b | 2.09 ± 0.18 c | 2.75 ± 0.32 a |
| TR0/RC | 1.57 ± 0.12 b | 1.78 ± 0.24 a | 1.74 ± 0.15 ab | 1.7 ± 0.42 ab |
| ET0/RC | 0.84 ± 0.08 a | 0.94 ± 0.22 a | 0.94 ± 0.07 a | 0.87 ± 0.14 a |
| DI0/RC | 0.32 ± 0.03 c | 0.63 ± 0.16 b | 0.36 ± 0.03 c | 1.05 ± 0.59 a |
| RE0/RC | 0.38 ± 0.04 b | 0.47 ± 0.16 a | 0.37 ± 0.04 b | 0.33 ± 0.10 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viljevac Vuletić, M.; Mihaljević, I.; Tomaš, V.; Horvat, D.; Zdunić, Z.; Vuković, D. Physiological Response to Short-Term Heat Stress in the Leaves of Traditional and Modern Plum (Prunus domestica L.) Cultivars. Horticulturae 2022, 8, 72. https://doi.org/10.3390/horticulturae8010072
Viljevac Vuletić M, Mihaljević I, Tomaš V, Horvat D, Zdunić Z, Vuković D. Physiological Response to Short-Term Heat Stress in the Leaves of Traditional and Modern Plum (Prunus domestica L.) Cultivars. Horticulturae. 2022; 8(1):72. https://doi.org/10.3390/horticulturae8010072
Chicago/Turabian StyleViljevac Vuletić, Marija, Ines Mihaljević, Vesna Tomaš, Daniela Horvat, Zvonimir Zdunić, and Dominik Vuković. 2022. "Physiological Response to Short-Term Heat Stress in the Leaves of Traditional and Modern Plum (Prunus domestica L.) Cultivars" Horticulturae 8, no. 1: 72. https://doi.org/10.3390/horticulturae8010072
APA StyleViljevac Vuletić, M., Mihaljević, I., Tomaš, V., Horvat, D., Zdunić, Z., & Vuković, D. (2022). Physiological Response to Short-Term Heat Stress in the Leaves of Traditional and Modern Plum (Prunus domestica L.) Cultivars. Horticulturae, 8(1), 72. https://doi.org/10.3390/horticulturae8010072







