The Investigation of Phenylalanine, Glucosinolate, Benzylisothiocyanate (BITC) and Cyanogenic Glucoside of Papaya Fruits (Carica papaya L. cv. ‘Tainung No. 2’) under Different Development Stages between Seasons and Their Correlation with Bitter Taste
Abstract
1. Introduction
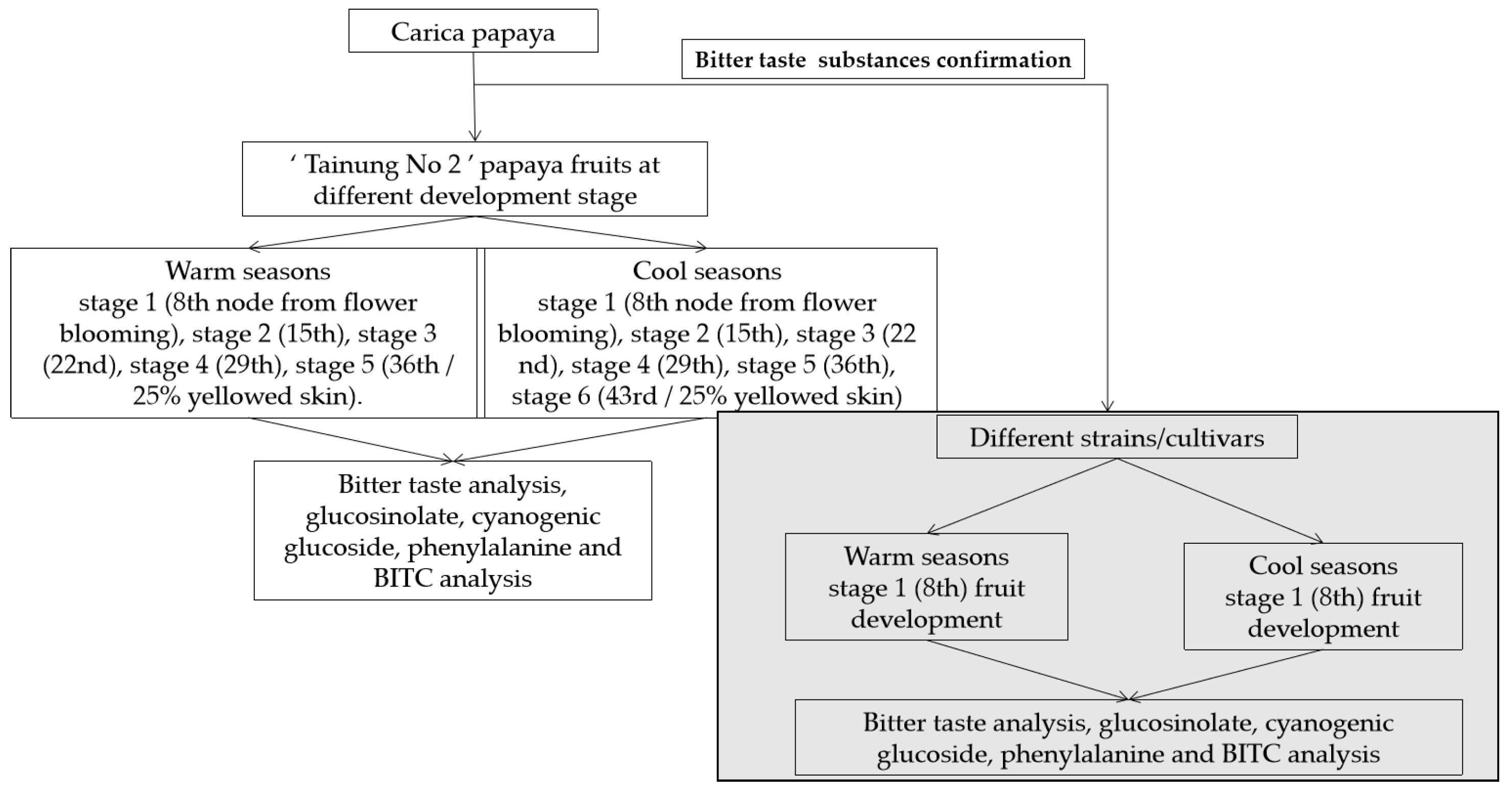
2. Materials and Methods
2.1. Different Development Stage ‘Tainung No. 2’ Papaya Fruits
2.2. Different Papaya Fruit Lines and Cultivars
2.3. Phenylalanine Analysis
2.4. Glucosinolate Analysis
2.5. Benzylisothiocyanate (BITC) Analysis
2.6. Cyanogenic Glucoside Analysis
2.7. Bitter Taste Analysis
2.8. Statistical Analysis
3. Results
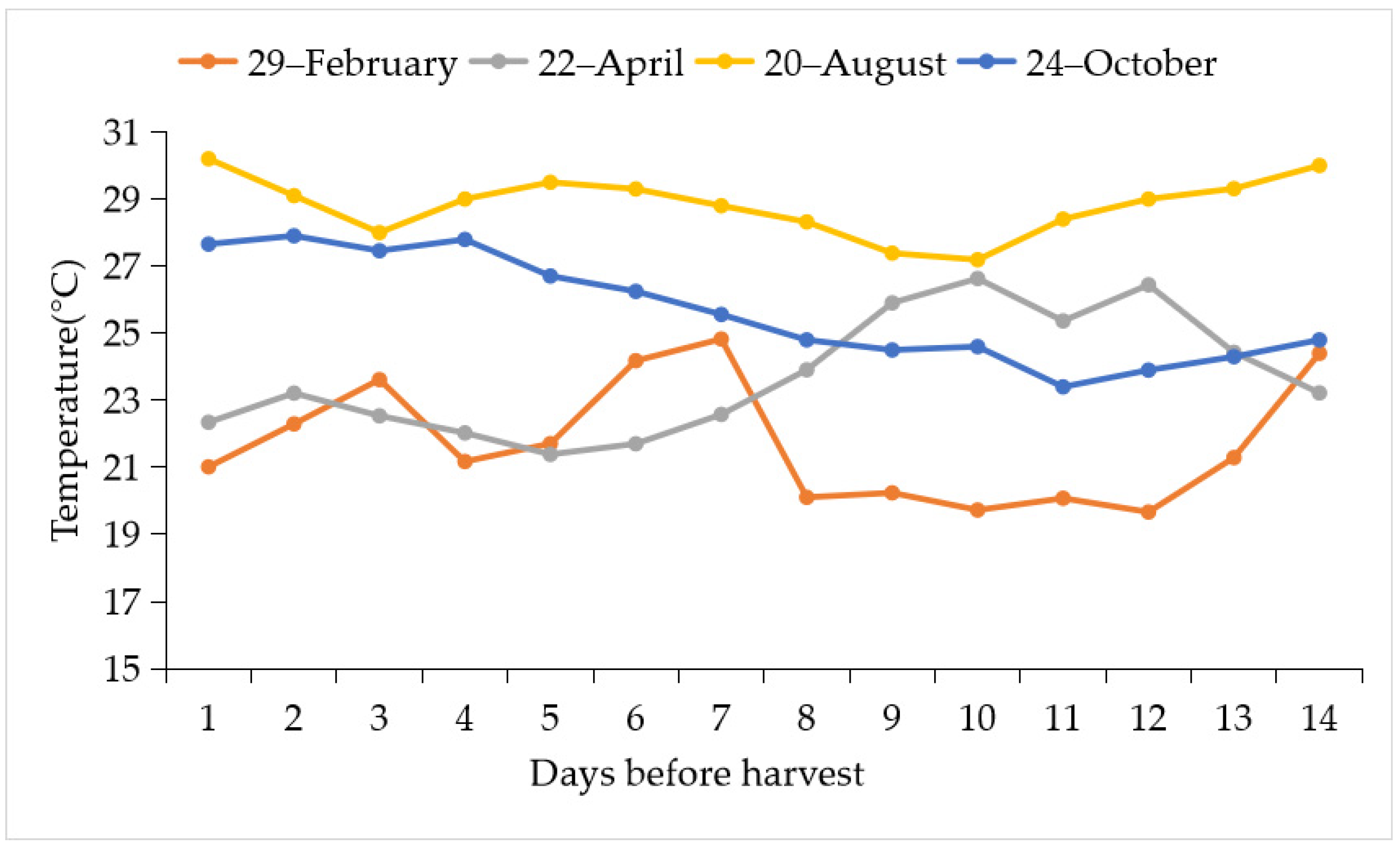
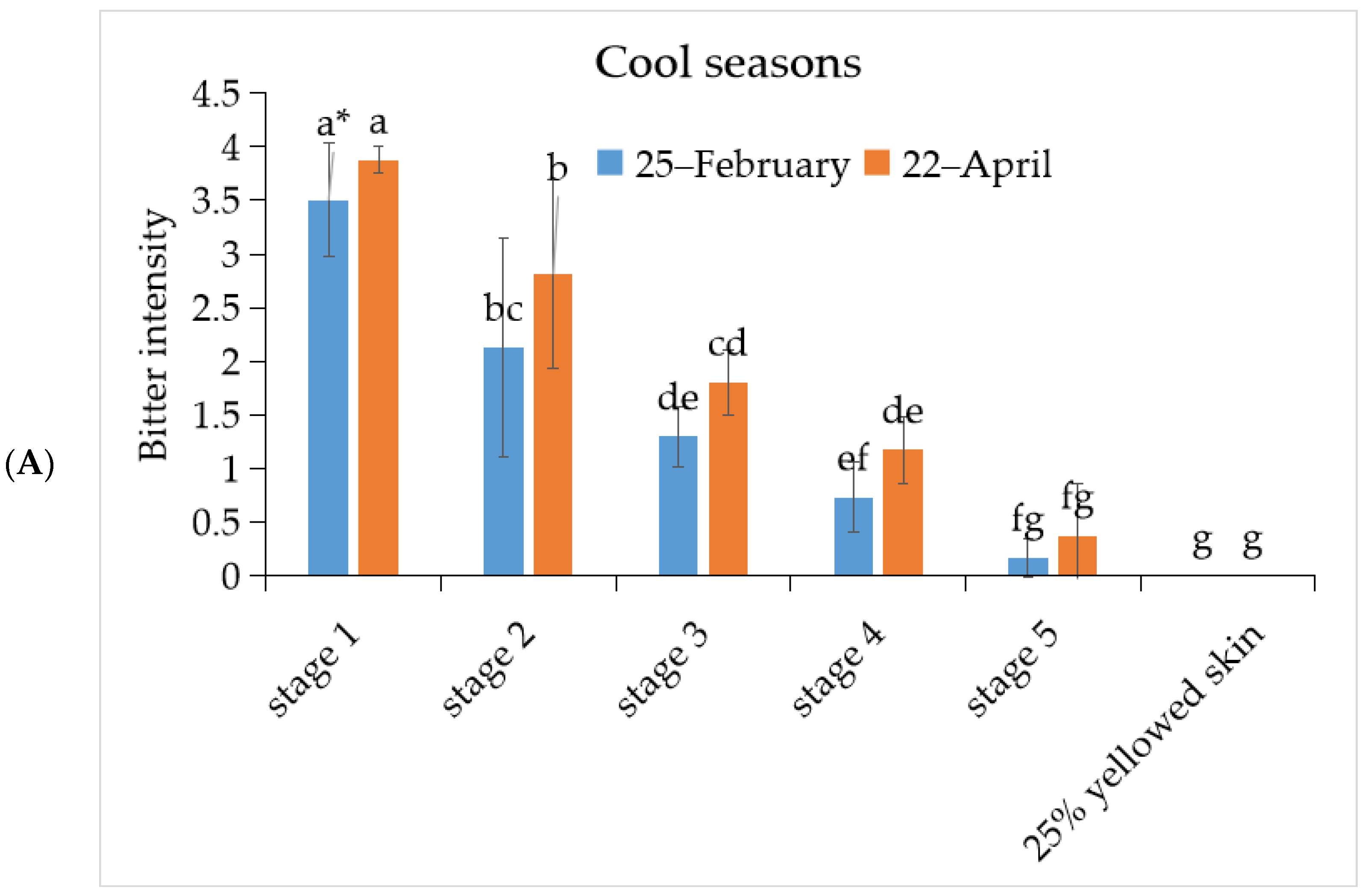
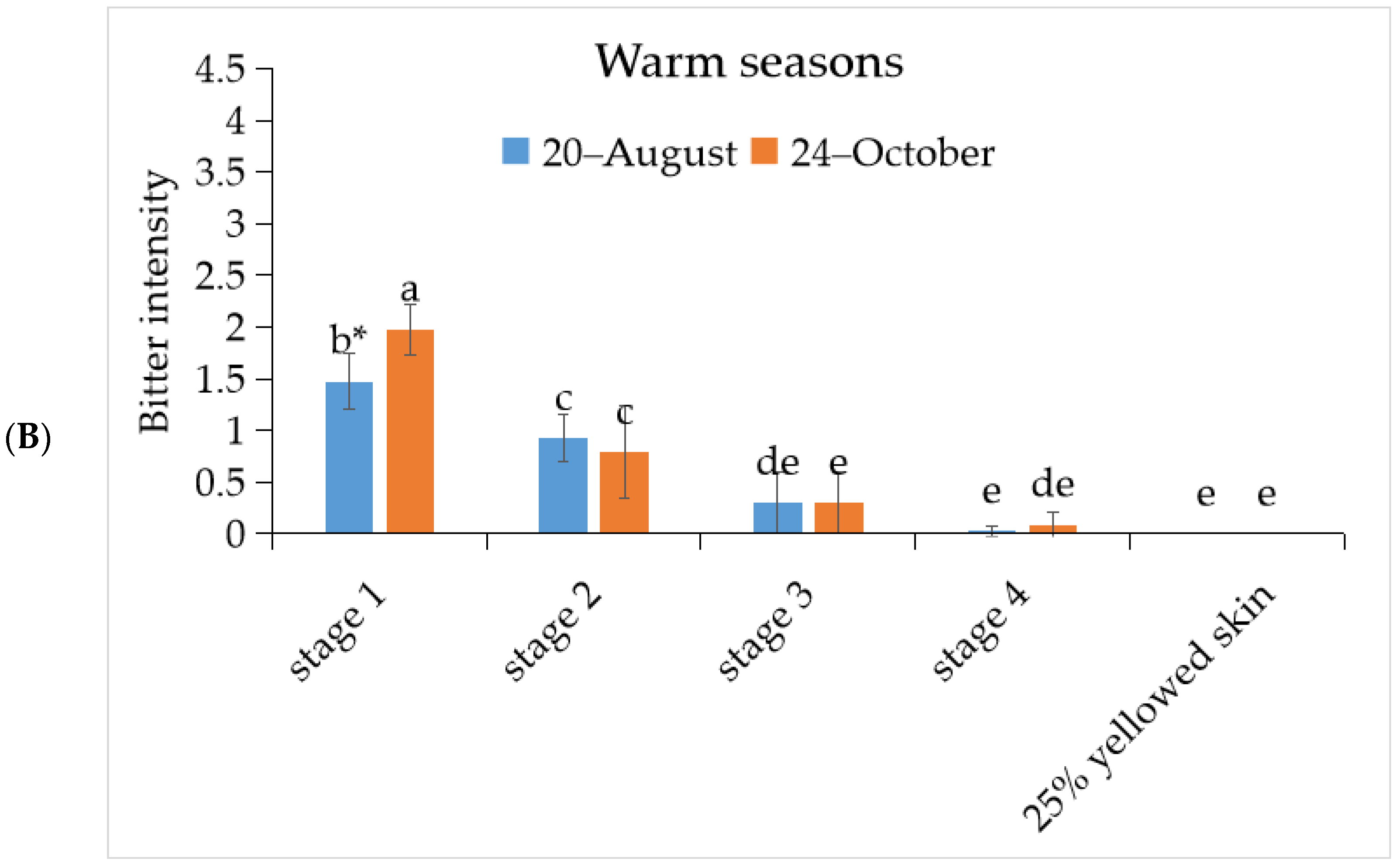
3.1. The Bitterness Intensity of ‘Tainung No. 2’ Papaya Fruit under Different Development Stage during Warm and Cool Seasons
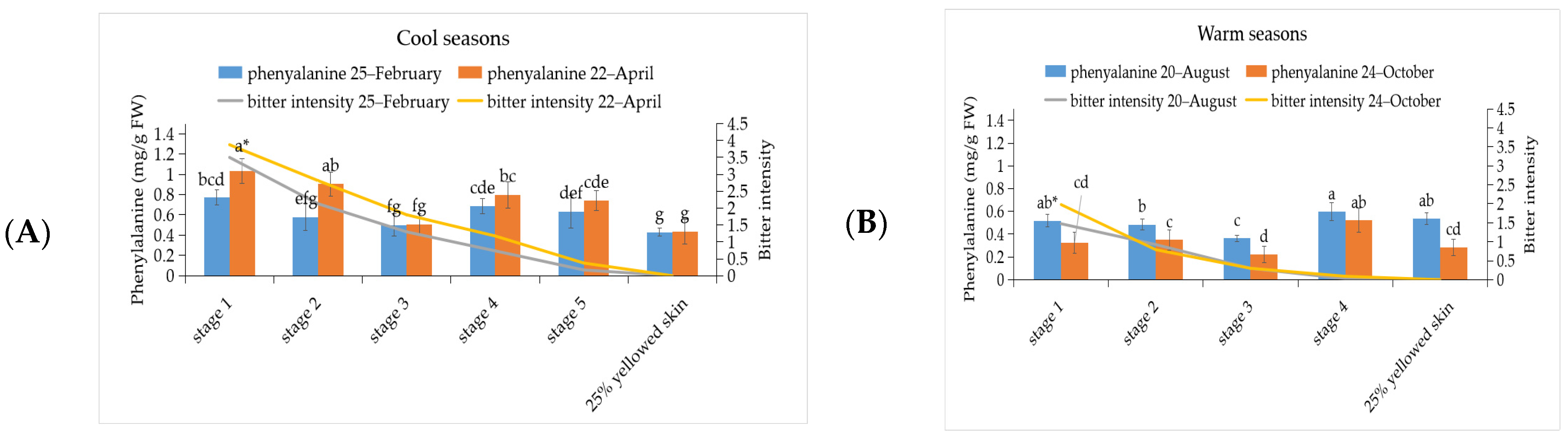
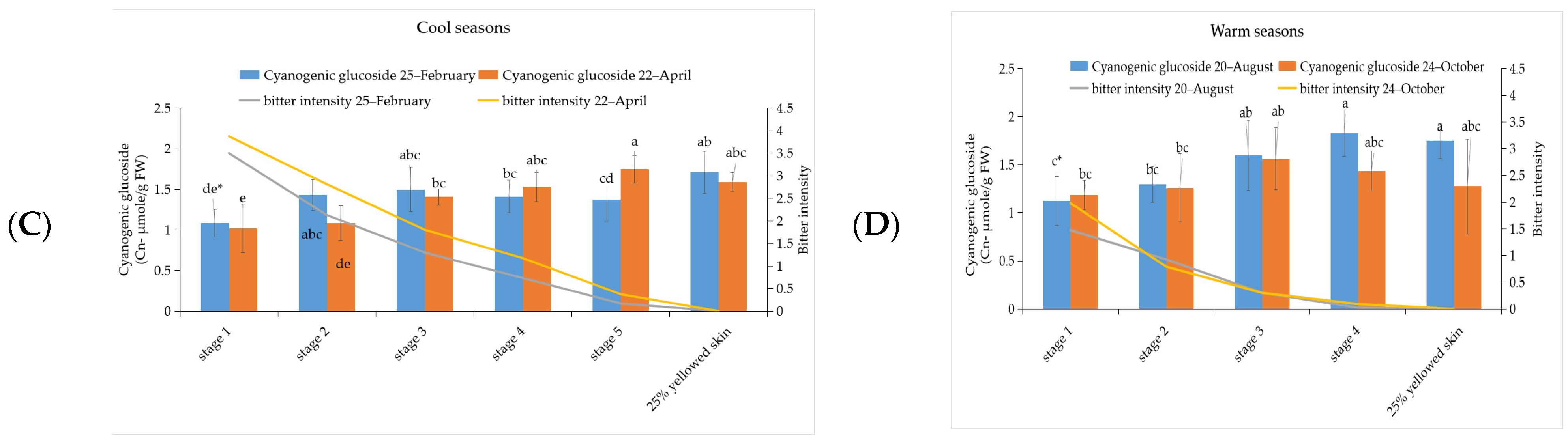
3.2. The Investigation of Phenylalanine and Cyanogenic Glucoside Content in ‘Tainung No. 2’ Papaya Fruit under Different Development Stage during Warm and Cool Seasons
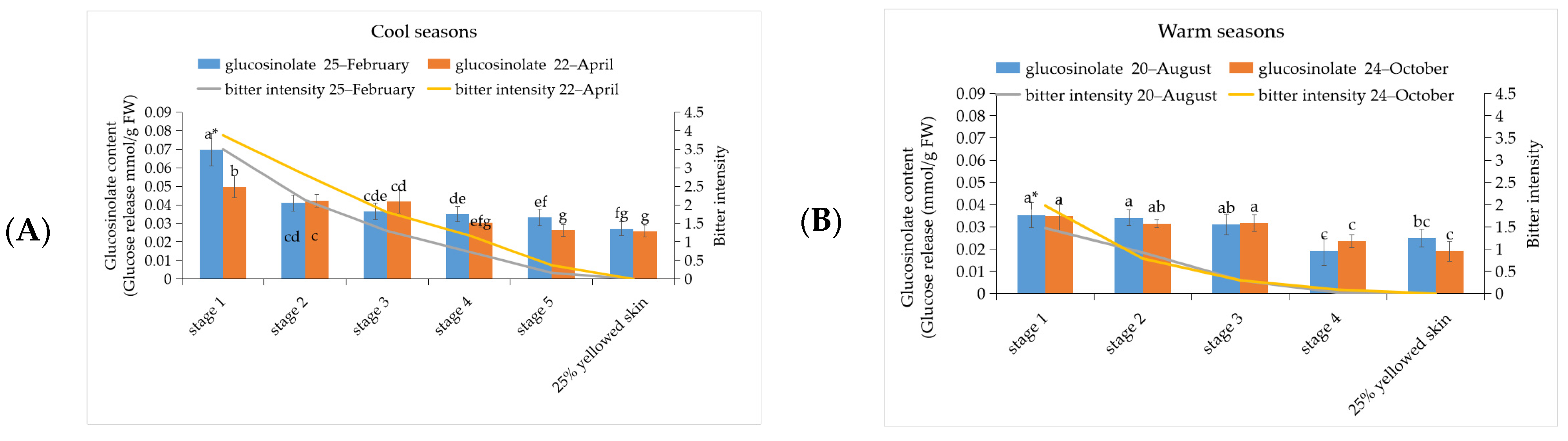
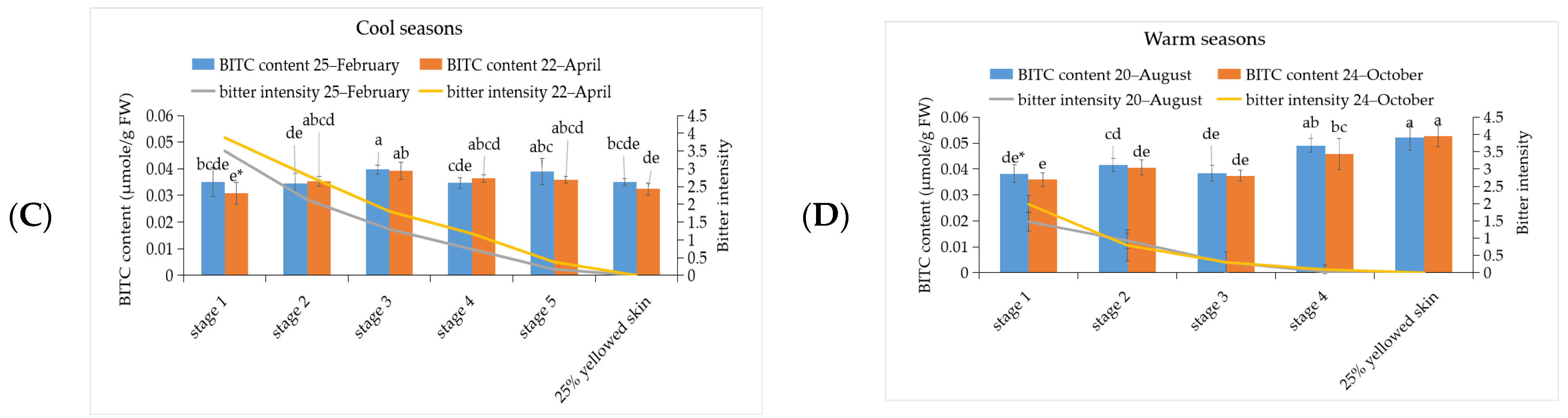
3.3. The Investigation of Glucosinolate and BITC Content in ‘Tainung No. 2’ Papaya Fruit under Different Development Stage during Warm and Cool Seasons
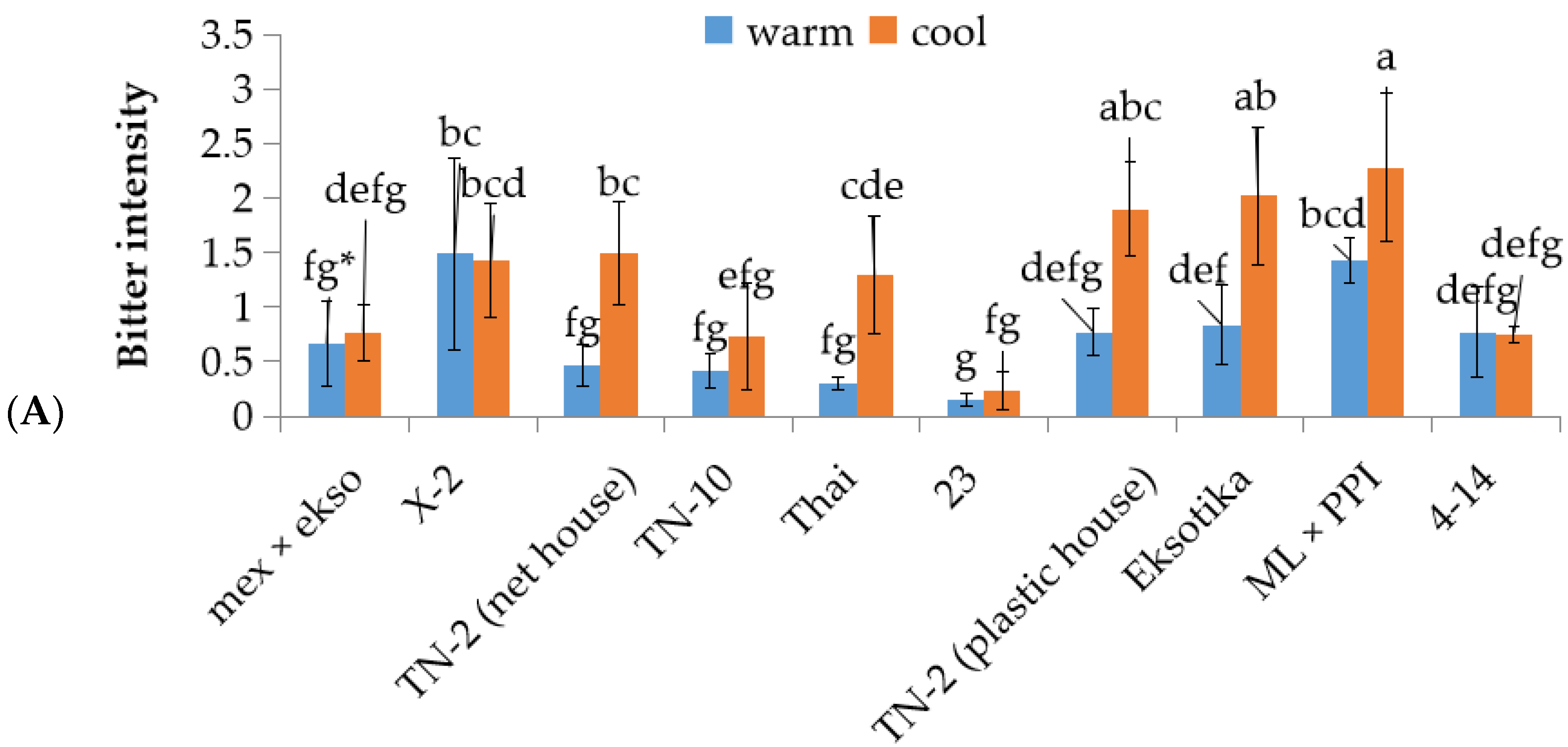
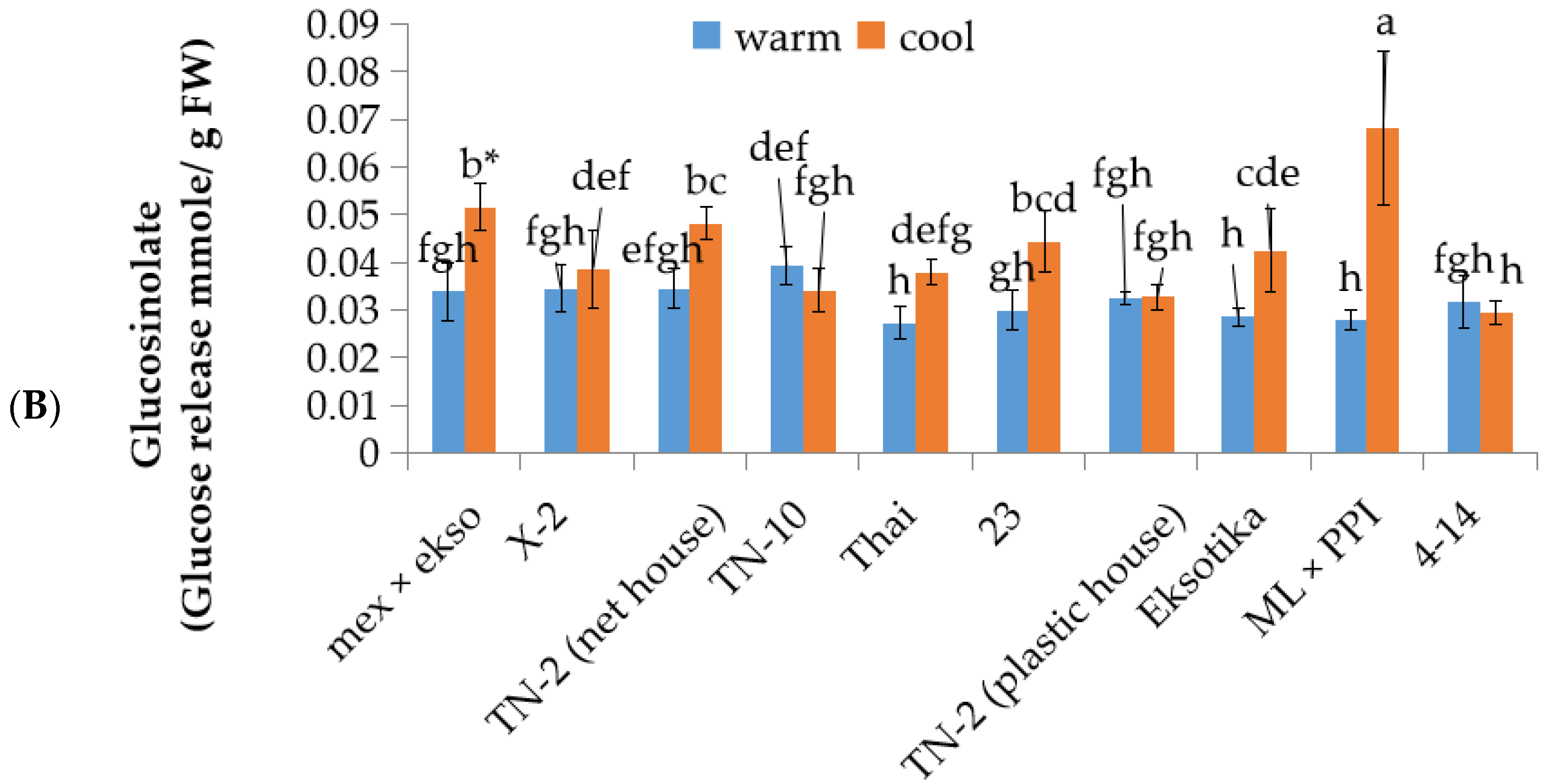
3.4. The Investigation of Bitterness Intensity and Glucosinolate Content in Different Lines/Cultivars of Papaya Fruit at First Stage Fruit Development in Warm and Cool Seasons
3.5. Correlation between Bitterness Intensity of Papaya Fruits with Phenylalanine, Cyanogenic Glucoside, Glucosinolate, and BITC Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Gonsalves, D. Transgenic papaya: Development, release, impact and challenges. Adv. Virus Res. 2006, 67, 317–354. [Google Scholar] [PubMed]
- Paull, R.E.; Nishijima, W.; Reyes, M.; Cavaletto, C. Postharvest handling and loss during marketing of papaya (Carica papaya L.). Postharvest Biol. Technol. 1997, 11, 165–179. [Google Scholar] [CrossRef]
- Wang, R.H. ‘Kaoshiung No 9’ papaya: A new cultivar for papaya production. Res. Bull. Kdares. 2014, 24, 2. [Google Scholar]
- Mendoza, E.M.T. Development of functional foods in the Philippines. Food Sci. Technol. Res. 2007, 13, 179–186. [Google Scholar] [CrossRef]
- Mano, R.; Ishida, A.; Ohya, Y.; Todoriki, H.; Takishita, S. Dietary intervention with Okinawan vegetables increased circulating endothelial progenitor cells in healthy young woman. Atherosclerosis 2009, 204, 544–548. [Google Scholar] [CrossRef]
- Sone, T.; Sakamoto, N.; Suga, K.; Imai, K.; Nakachi, K.; Sonlkin, P.; Sonklin, P.; Lipigorngoson, S.; Limtrakul, P.; Suttajit, M. Comparison of diets among elderly female residents in two suburban districts in Chiang Mai Provence, Thailand, in dry season-survey on high- and low-risk district of lung cancer incidence. Appl. Hum. Sci. 1998, 17, 49–56. [Google Scholar] [CrossRef][Green Version]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribuition of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Doorn, H.E.; Kruk, G.C.V.D.; Holst, G.J.V.; Riujs, N.C.M.E.R.; Postma, E.; Groeneweg, B.; Jongen, W.H.F. The glucosinolates sinigrin and progoitrin are important determinants for taste preference and bitterness of brussels sprouts. J. Sci. Food Agric. 1998, 78, 30–38. [Google Scholar] [CrossRef]
- Lazzeri, L.; Leoni, O.; Manici, L.M. Biocidal plant dried pellets for biofumigation. Ind. Crop Prodt. 2004, 20, 59–65. [Google Scholar] [CrossRef]
- Zhao, B.; Seow, A.; Lee, E.J.D.; Poh, W.T.; Teh, M.; Eng, P.; Wang, Y.T.; Tan, W.C.; Yu, M.C.; Lee, H.P. Dietary isothiocyanates, glutathione S-transferase-M1, -T1 polymorphisms and lung cancer risk among Chinese women in Singapore. Cancer Epidemiol. Biomarkers Prev. 2001, 10, 1063–1067. [Google Scholar]
- William, D.J.; Pun, S.; Chaliha, M.; Scheelings, P.; O’Hare, T. An unusual combination in papaya (Carica papaya): The good (glucosinolates) andthe bad (cyanogenic glycosides). J. Food Comp. Anal. 2003, 29, 82–86. [Google Scholar] [CrossRef]
- Engelen-Eigles, G.; Holden, G.; Cohen, J.D.; Gardner, G. The effect of temperature, photoperiod, and light quality on gluconasturtiin concentration in watercress (Nasturtium officinale R. Br.). J. Agric Food Chem. 2006, 54, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Charron, C.S.; Saxton, A.M.; Sams, C.E. Relationship of climate and genotype to seasonal variation in the glucosinolate-myrosinase system. I. Glucosinolate content in ten cultivars of Brassica oleracea grown in fall and spring seasons. J. Sci. Food Agric. 2005, 85, 671–681. [Google Scholar] [CrossRef]
- Rossetto, M.R.M.; Nascimento, J.R.O.D.; Purgatto, E.; Fabi, J.P.; Lajolo, F.M.; Cordenunsi, B.R. Benzylglucosinolate, benzylisothiocyanate, and myrosinase activity in papaya fruit during development and ripening. J. Agric. Food Chem. 2008, 56, 9592–9599. [Google Scholar] [CrossRef]
- Bennett, R.N.; Kiddle, G.; Wallsgrove, R.M. Biosynthesis of benzylglucosinolate, cyanogenic glucoside, and phenylpropanoids in Carica papaya. Phytochemistry 1997, 45, 59–66. [Google Scholar] [CrossRef]
- Kunisuke, I.; Amino, Y.; Kohmura, M.; Ueda, Y.; Kuroda, M. Human-interactions-taste. Compr. Nat. Prod. II 2010, 4, 631–671. [Google Scholar]
- Nafisi, M.; Sonderby, I.E.; Hansen, B.G.; Flores, F.G.; Eldin, H.H.N.; Norholm, M.H.H.; Jensen, N.B.; Li, J.; Halkier, B.A. Cytochromes P450 in the biosynthesis of glucosinolate and indole alkaloids. Phytochem. Rev. 2006, 5, 331–346. [Google Scholar] [CrossRef]
- Modhi, O.A.; Khamis, G.; AbdElgawad, H.; Mohammed, A.E.; Sheteiwy, M.S.; Elobeid, M.M.; Saleh, A.M. 2021-Lepidium sativum sprouts grown under elevated CO2 hyperaccumulate glucosinolates and antioxidants and exhibit enhanced biological and reduced antinutritional properties. Biomolecules 2021, 11, 1174. [Google Scholar]
- Gleadow, R.M.; Møller, B.L. Cyanogenic glycosides: Synthesis, physiology, and phenotypic plasticity. Annu. Rev. Plant Biol. 2014, 65, 155–185. [Google Scholar] [CrossRef]
- Dicenta, F.; Martinez-Gomez, P.; Grane, N.; Martin, M.L.; Leon, A.; Canovas, J.A.; Berenguer, V. Relationship between cyanogenic compounds in kernels, leaves, and roots of sweet and bitter kernelled almonds. J. Agric. Food Chem. 2002, 50, 2149–2152. [Google Scholar] [CrossRef]
- Linley, C.K.; Brimer, L.; Saka, J.D.K.; Mhone, A.R.; Mkumbira, J.; Johansson, L.; Bokanga, M.; Mahungu, N.M.; Rosling, H. Bitter taste in cassava roots correlates with cyanogenic glucoside levels. Sci. Food Agric. 2004, 84, 581–590. [Google Scholar]
- Gao, Q.; Jiang, H.; Tang, F.; Cao, H.Q.; Wu, X.W.; Qi, F.F.; Sun, J.; Yang, J. Evaluation of the bitter components of bamboo shoots using a metabolomics approach. Food Funct. 2019, 10, 90–98. [Google Scholar] [CrossRef]
- Anna, S.; Oleszek, W. Changes of cyanogenic glucosides in white clover (Trifolium repens L.) during the growing season. J. Agric. Food Chem. 1997, 45, 4333–4336. [Google Scholar]
- Mateja, S.; Stampar, F.; Veberic, R.; Petkovsek, M.M. Cyanogenic glycosides and phenolics in apple seeds and their changes during long term storage. Sci. Hortic. 2019, 255, 30–36. [Google Scholar]
- Ping, D.; Cui, B.; Zhu, H.; Phommakoun, B.; Zhang, D.; Li, Y.; Zhao, F.; Zhao, Z. Accumulation pattern of amygdalin and prunasin and its correlation with fruit and kernel agronomic characteristics during apricot (Prunus armeniaca L.) kernel development. Foods 2021, 10, 397. [Google Scholar]
- Seigler, D.S.; Pauli, G.F.; Nahrstedt, A.; Leen, R. Cyanogenic allosides and glucosides from Passiflora edulis and Carica papaya. Phytochemistry 2002, 60, 873–882. [Google Scholar] [CrossRef]
- Elin, S.O.; Jorgensen, L.B.; Jaroszewski, J.W. Cyanogenesis in glucosinolate producing plants: Carica papaya and Carica quercifolia. Phytochemistry 2002, 60, 269–273. [Google Scholar]
- Ambrose, J.A. A shortened method for the fluorometric determination of phenylalanine. Clin. Chemi. 1969, 15, 15–23. [Google Scholar] [CrossRef]
- Doorn, H.E.V.; Kruk, G.C.V.D.; Holst, G.J.V. Large scale determination of glucosinolates in brussels sprouts samples after degradation of endogenous glucose. J. Agric. Food Chem. 1999, 47, 1029–1034. [Google Scholar] [CrossRef]
- Zhang, Y.; Cho, C.C.; Posner, G.H.; Talalay, P. Spectroscopic quantitation of organic isothiocyanates by cyclocondensation with vicinal dithiols. Anal. Biochem. 1992, 205, 100–107. [Google Scholar] [CrossRef]
- Bradbury, J.H.; Bradbury, M.G.; Egan, S.V. Comparison of methods of analysis of cyanogens in cassava. Acta Hort. 1994, 375, 87–96. [Google Scholar] [CrossRef]
- Scharbert, S.; Hofmann, T. Molecular definition of black tea taste by means of quantitative studies, taste reconstitution, and omission experiments. J. Agric. Food Chem. 2005, 53, 5377–5384. [Google Scholar] [CrossRef]
- Akihiro, I.; Fierro, F.; Chaney, M.E.; Lauterbur, M.E.; Hayakawa, T.; Tosi, A.T.; Niv, M.Y.; Imai, H. Lowered sensitivity of bitter taste receptors to β-glucosidase in bamboo lemurs: An instance of parallel and adaptive functional decline in TAS2R1. Proc. R. Soc. B 2021, 288, 20210346. [Google Scholar]
- Hodges, D.M.; Munro, K.D.; Forney, C.F.; McRae, K.B. Glucosinolate and free sugar content in cauliflower (Brassica oleracea var. botrytis cv. Freemont) during controlled-atmosphere storage. Postharvest Biol. Technol. 2006, 40, 123–132. [Google Scholar] [CrossRef]
- Bell, L.; Lignou, S.; Wagstaff, C. High glucosinolate content in rocket leaves (Diplotaxis tenuifolia and Eruca sativa) after multiple harvests is associated with increased bitterness, pungency, and reduced consumer liking. Foods 2020, 9, 1799. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Moller, P.; Sorensen, H.; de Trejo, M.C. Glucosinolates in broccoli stored under controlled atmosphere. J. Amer. Soc. Hort. Sci. 1995, 120, 1069–1074. [Google Scholar] [CrossRef]
- Chowdhury, M.; Kiraga, S.; Islam, N.; Ali, M.; Reza, N.; Lee, W.H.; Chung, S.O. Effects of temperature, relative humidity, and carbon dioxide concentration on growth and glucosinolate content of kale grown in a plant factory. Foods 2021, 10, 1524. [Google Scholar] [CrossRef] [PubMed]
- Melissa, M.R.; Nair, V.; Benavides, J.; Zevallos, L.C.; Velazquez, D.A.J. UVA, UVB light doses and harvesting time differentially tailor glucosinolate and phenolic profiles in broccoli sprouts. Molecules 2017, 22, 1065. [Google Scholar]
- Park, J.E.; Kim, J.; Purevdorj, E.; Son, Y.J.; Nho, C.W.; Yoo, G. Effects of long light exposure and drought stress on plant growth and glucosinolate production in pak choi (Brassica rapa subsp. chinensis). Food Chem. 2021, 340, 128167. [Google Scholar] [CrossRef]
- Justen, V.L.; Fritz, V.A. Temperature-induced glucosinolate accumulation is associated with expression of BrMYB transcription factors. HortScience 2013, 48, 47–52. [Google Scholar] [CrossRef]
- Pereira, F.M.V.; Rosa, E.; Fahey, F.W.; Stephenson, K.K.; Carvalho, R.; Aires, A. Influence of temperature and ontogeny on the levels of glucosinolates in broccoli (Brassica oleracea var. italica) sprouts and their effect on the induction of mammalian phase 2 enzymes. J. Agric. Food Chem. 2002, 50, 6239–6244. [Google Scholar] [CrossRef] [PubMed]
- Kosson, R.; Horbowicz, M. Effect of long term storage on some nutritive component and isothiocyanate content in roots of two horseradish types. Veg. Crops Res. Bull. 2008, 69, 155–164. [Google Scholar] [CrossRef][Green Version]
| Bitter Substances | Bitterness Intensity (r) |
|---|---|
| Phenylalanine | 0.35 *** |
| Glucosinolate | 0.805 *** |
| Cyanogenic glucoside | −0.54 *** |
| BITC | −0.46 *** |
| Glucosinolate in different lines and cultivars | 0.44 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jioe, I.P.J.; Lin, H.-L.; Shiesh, C.-C. The Investigation of Phenylalanine, Glucosinolate, Benzylisothiocyanate (BITC) and Cyanogenic Glucoside of Papaya Fruits (Carica papaya L. cv. ‘Tainung No. 2’) under Different Development Stages between Seasons and Their Correlation with Bitter Taste. Horticulturae 2022, 8, 198. https://doi.org/10.3390/horticulturae8030198
Jioe IPJ, Lin H-L, Shiesh C-C. The Investigation of Phenylalanine, Glucosinolate, Benzylisothiocyanate (BITC) and Cyanogenic Glucoside of Papaya Fruits (Carica papaya L. cv. ‘Tainung No. 2’) under Different Development Stages between Seasons and Their Correlation with Bitter Taste. Horticulturae. 2022; 8(3):198. https://doi.org/10.3390/horticulturae8030198
Chicago/Turabian StyleJioe, Irvan Prawira Julius, Huey-Ling Lin, and Ching-Chang Shiesh. 2022. "The Investigation of Phenylalanine, Glucosinolate, Benzylisothiocyanate (BITC) and Cyanogenic Glucoside of Papaya Fruits (Carica papaya L. cv. ‘Tainung No. 2’) under Different Development Stages between Seasons and Their Correlation with Bitter Taste" Horticulturae 8, no. 3: 198. https://doi.org/10.3390/horticulturae8030198
APA StyleJioe, I. P. J., Lin, H.-L., & Shiesh, C.-C. (2022). The Investigation of Phenylalanine, Glucosinolate, Benzylisothiocyanate (BITC) and Cyanogenic Glucoside of Papaya Fruits (Carica papaya L. cv. ‘Tainung No. 2’) under Different Development Stages between Seasons and Their Correlation with Bitter Taste. Horticulturae, 8(3), 198. https://doi.org/10.3390/horticulturae8030198






