Alternative Control of Tomato Wilt Using the Aqueous Extract of Calotropis procera
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Fusarium Pathogen from Tomato Plant
2.2. Pathogenicity Test
2.3. Determination of Disease Index
2.4. Molecular Identification of F. oxysporum
2.5. Preparation of Plant Extracts
2.6. Isolation of Phytochemical Compounds from C. procera Leaves
2.7. In Vitro Antifungal Activity
2.8. Effects of C. procera on Seeds Germination and Seedling Vigor In Vitro
2.9. Effects of C. procera on Disease Severity under Greenhouse Condition
2.10. Assay Total Phenol Contents
2.11. Total Flavonoid Content
2.12. Antioxidant Enzyme Activity Analysis
2.13. Statistical Analysis
3. Results
3.1. Isolation of the Causal Pathogen and Pathogenicity Tests
3.2. Identification of KENF7 by Using ITS Sequencing
3.3. In Vitro Antifungal Activity
3.4. Effect of Aqueous Extract on Seed Germination and Seedling Vigor Index
3.5. Effect of Aqueous Extract on Disease Reduction under Greenhouse
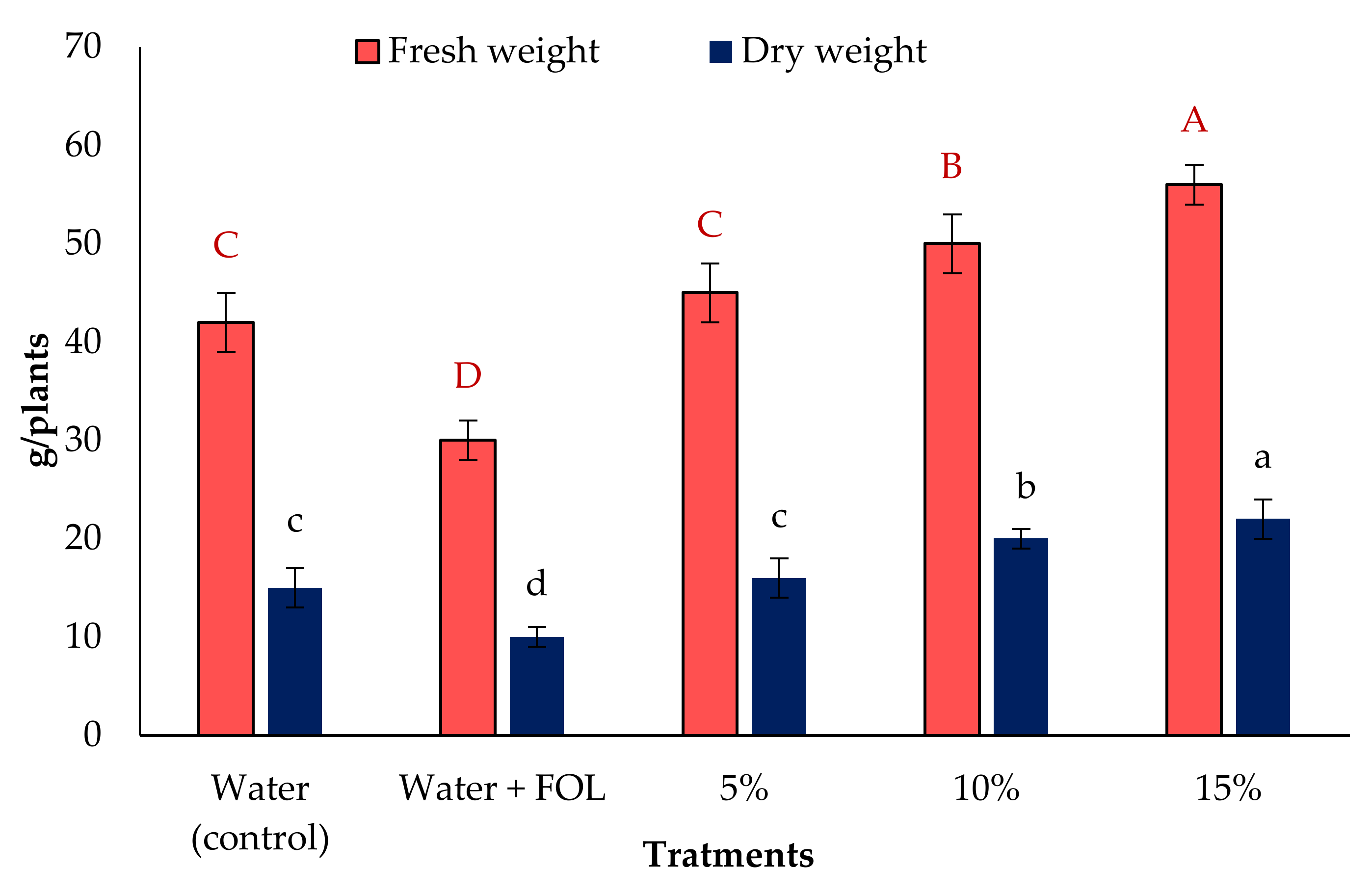
3.6. Vegetative Growth
3.7. Effect of Aqueous Extract on Enzymatic Activities
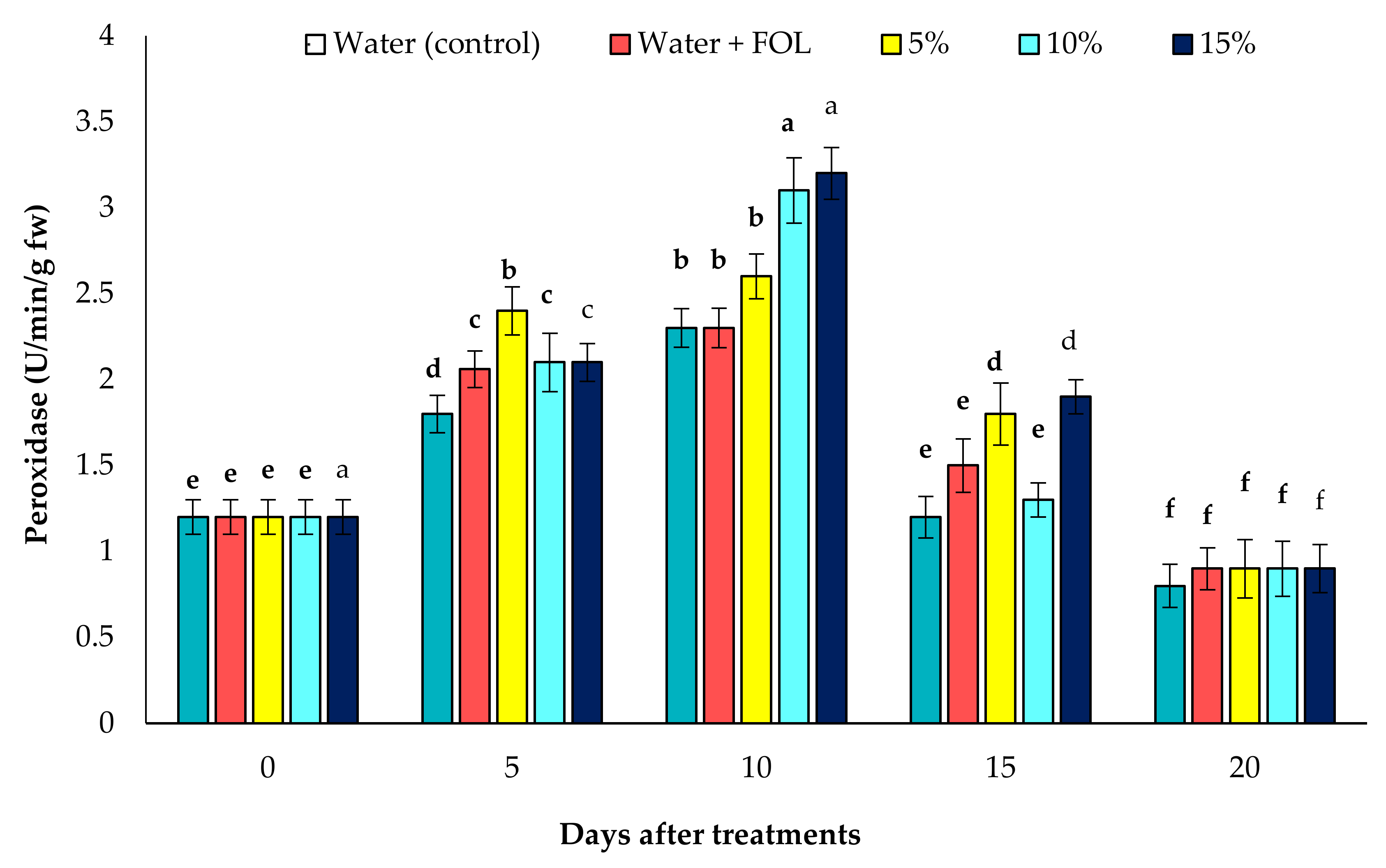
3.7.1. Activity of POD in Inoculated Plants
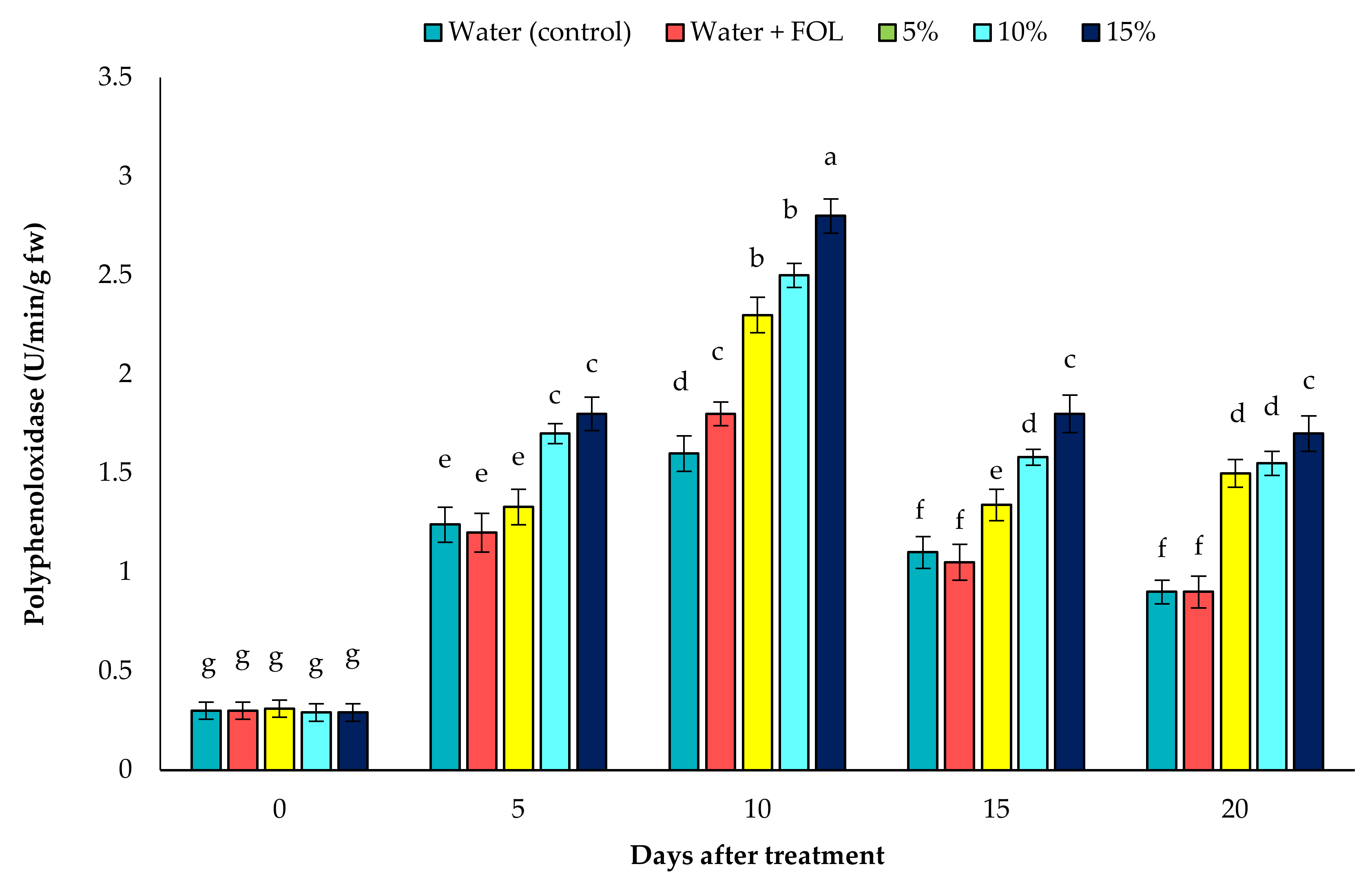
3.7.2. PPO Activity in Inoculated Plants
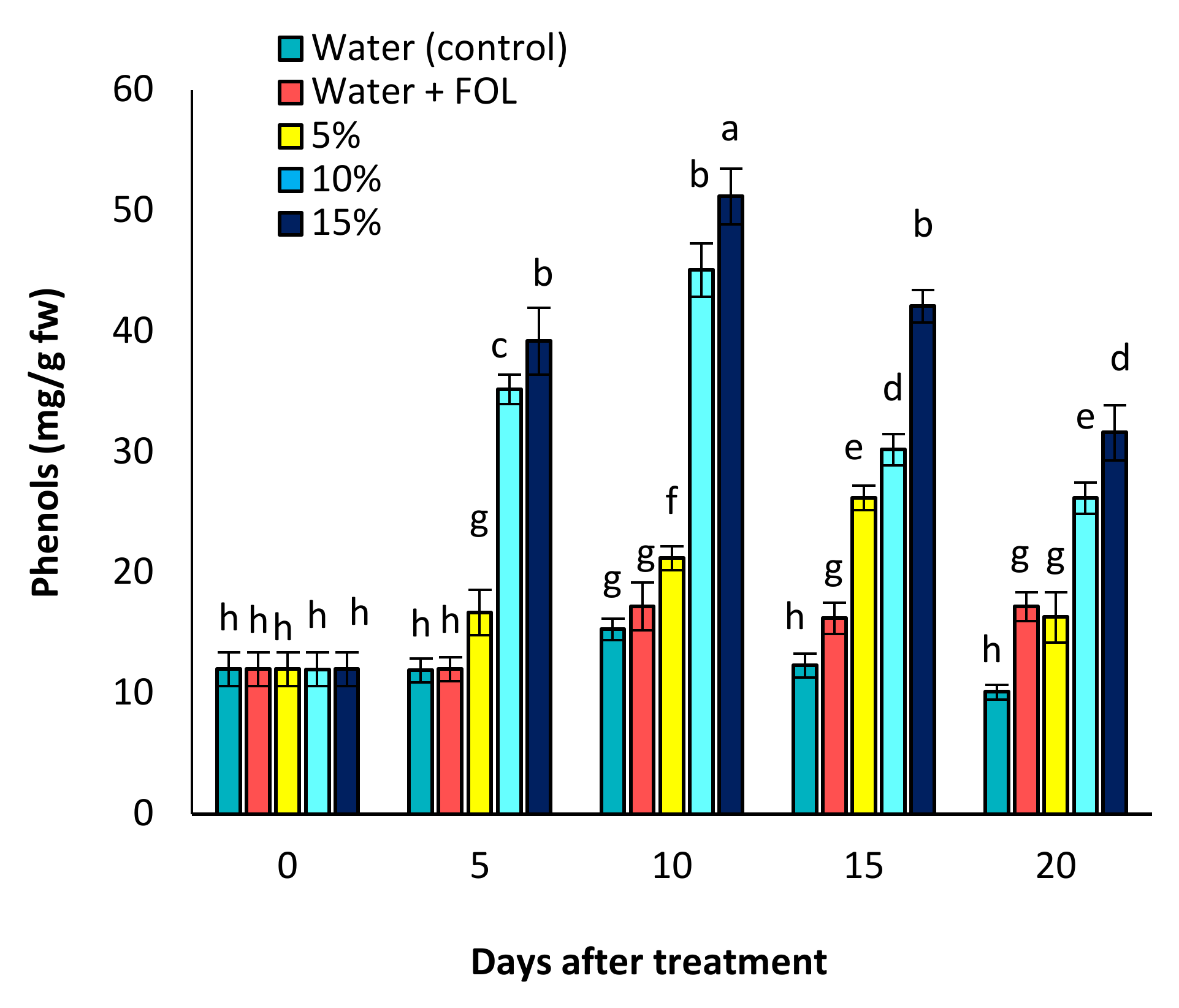
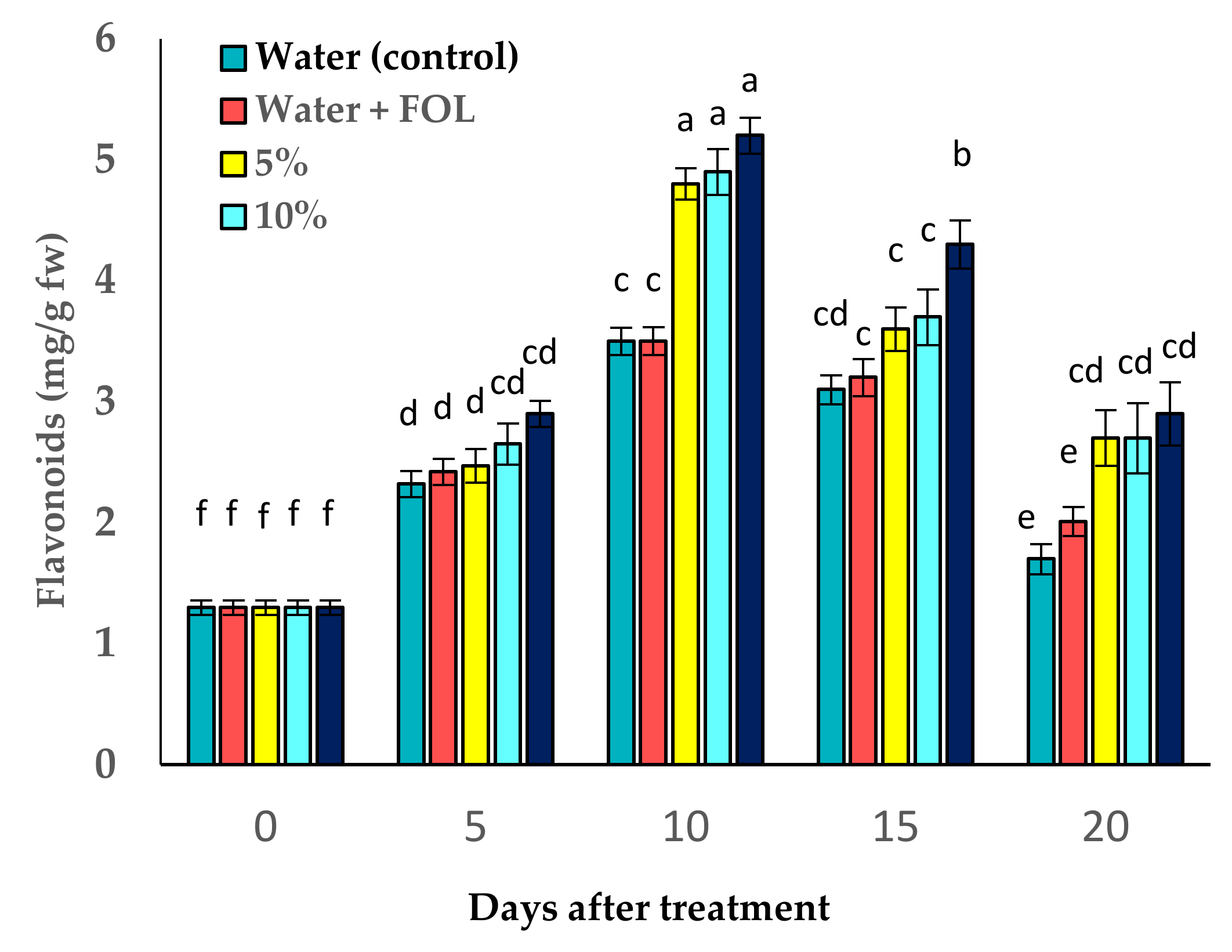
3.8. Effect of C. procera on Phenol and Flavonoid Contents
3.9. Identification of Phytochemical Components of C. procera AE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mousa, M.A.A.; Abo-Elyousr, K.A.M.; Abdel Aal, A.M.K.; Alshareef, N.O. Combination of Bacillus amyloliqefaciens and peppermint oil for control of Fusarium wilt disease of tomato. Agronomy 2021, 11, 2536. [Google Scholar] [CrossRef]
- Abo-Elyousr, K.A.M.; Mohammed, H. Biological Control of Fusarium Wilt in Tomato by Plant Growth-Promoting Yeasts and Rhizobacteria. Plant Pathol. J. 2009, 25, 99–204. [Google Scholar] [CrossRef]
- Dursun, A.; Melek, E.; Dönmez, M.F. Effects of foliar application of plant growth promoting bacterium on chemical contents, yield and growth of tomato (Lycopersicon esculentum L.) and cucumber (Cucumis sativus L.). Pak. J. Bot. 2020, 42, 3349–3356. [Google Scholar]
- Jabnoun-Khiareddine, H.J.; Abdallah, R.A.B.; Remadi, M.D.; Nefzi, A.; Ayed, F. Grafting tomato cultivars for soil borne diseases suppression and plant growth and yield improvement. J. Plant Pathol. Microbiol. 2019, 10, 1–8. [Google Scholar]
- Oliver, B.B. Medicinal Plants in Tropical West Africa; Cambridge University Press: Cambridge, UK, 1986. [Google Scholar]
- Yossry, A.A.; Ali, S.M.A.; Imery, S.M. Fungitoxic properties of some plant extracts against growth of soil borne disease fungi. Ann. Agric. Sci. 1998, 3, 891–909. [Google Scholar]
- Nidhi, S.; Trivedi, P.C. Screening of leaf extracts of some plants for their nematicidal and fungicidal properties against Meloidogyne incognita and Fusarium oxysporum. Asian J. Exp. Sci. 2002, 16, 21–28. [Google Scholar]
- Baraka, M.A.; Omar, S.A.; Ebtehage, E.; Zian, A.H. Controlling seedling damping-off, root rot and wilt diseases of lupine (Lupinus albus L.). Agric. Res. J. Suez Canal Univ. 2006, 6, 57–68. [Google Scholar]
- Deepak, D. Phytochemistry of Indian Asclepiadaceae. In The Taxonomy and Phytochemistry of the Asclepidaceae in Tropical Asia; Kiew, R., Ed.; University Pertanian: Selangor, Malaysia, 1995; pp. 33–44. [Google Scholar]
- Arora, S.S. Calotropis procera (Ait) R. Br.-Ak: A new and free source of fibre and renewable hydrocarbons. Agric. Mech. Asia Afr. Lat. Am. 1982, 13, 71–75. [Google Scholar]
- Heneidak, S.; Grayer, R.J.; Kite, G.C.; Simmonds, M.S.J. Flavonoid glycosides from Egyptian species of the tribe Asclepiadeae (Apocynaceae, subfamily Asclepiadoideae). Biochem. Sys. Ecol. 2006, 34, 575–584. [Google Scholar] [CrossRef]
- Bhutani, K.K.; Gupta, D.K.; Kapil, R.S. Occurrence of D/E trans stereochemistry isomeric to ursane (cis) series in a new pentacyclic triterpene from Calotropis procera. Tetrahedron Lett. 1992, 33, 7593–7596. [Google Scholar] [CrossRef]
- Hanna, A.G.; Shalaby, N.M.M.; Morsy, N.A.M.; Andras, A.; Toth, G.; Malik, S.; Duddeck, H. Structure of a calotropagenin-derived artifact from Calotropis procera. Magn. Reson. Chem. 2002, 40, 599–602. [Google Scholar] [CrossRef]
- Chundattu, S.J.; Agrawal, V.K.; Ganesh, N. Phytochemical investigation of Calotropis procera. Arab. J. Chem. 2012, 9, S230–S234. [Google Scholar] [CrossRef] [Green Version]
- Gulzar, A.; Siddiqui, M.B.; Bi, S. Phenolic acid allelochemicals induced morphological, ultrastructural, and cytological modification on Cassia sophera L. and Allium cepa L. Protoplasma 2016, 253, 1211–1221. [Google Scholar] [CrossRef]
- Hussain, F.; Rasool, A.; Aziz, K.; Raisham, S.; Aziz, S.; Badshah, L.; Hussain, W. Allelopathic inhibition of germination, seedling growth and cell division of selected plant species by Calotropis procera (Ait.) Ait. Plant Sci. Today 2020, 7, 1–8. [Google Scholar] [CrossRef]
- Hayat, S.; Ahmad, H.; Ali, M.; Ren, K.; Cheng, Z. Aqueous garlic extract stimulates growth and antioxidant enzymes activity of tomato (Solanum lycopersicum). Sci. Hortic. 2018, 240, 139–146. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Younis, A.; Finnegan, P.M.; Ferrante, A. Comparison of Soaking Corms with Moringa Leaf Extract Alone or in Combination with Synthetic Plant Growth Regulators on the Growth, Physiology and Vase Life of Sword Lily. Plants 2020, 9, 1590. [Google Scholar] [CrossRef]
- Hayat, S.; Ahmad, H.; Nasir, M.; Khan, M.N.; Ali, M.; Hayat, K.; Khan, F.; Ma, Y.; Cheng, Z.; Khan, M.A.; et al. Some Physiological and Biochemical Mechanisms during Seed-to-Seedling Transition in Tomato as Influenced by Garlic Allelochemicals. Antioxidants 2020, 9, 235. [Google Scholar] [CrossRef] [Green Version]
- Hussein Mohamed, M.A.; Abo-Elyousr, K.A.M.; Hassan, M.A.H.; Hashem, M.; Hassan, E.A.; Alamri, S.A.M. Induction of defense mechanisms involved in disease resistance of onion blight disease caused by Botrytis allii. Egypt. J. Biol. Pest Control 2018, 28, 80. [Google Scholar] [CrossRef] [Green Version]
- Chuying, C.; Nan, C.; Jinyin, C.; Chunpeng, W. Clove Essential Oil as an Alternative Approach to Control Postharvest Blue Mold Caused by Penicillium italicum in Citrus Fruit. Biomolecules 2010, 9, 197. [Google Scholar] [CrossRef] [Green Version]
- Youssef, K.; Roberto, S.R.; Tiepo, A.N.; Constantino, L.V.; de Resende, J.T.V.; Abo-Elyousr, K.A.M. Salt solution treatments trigger antioxidant defense response against gray mold disease in table grapes. J. Fungi 2020, 6, 179. [Google Scholar] [CrossRef]
- Nelson, P.E.; Tousson, T.A.; Marasas, W.F.O. Fusarium Species: An Illustrated Manual for Identification; Pennsylvania State University Press: University Park, PA, USA, 1983. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phyloge-netics. In PCR Protocols. A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Lipman, D.J.; Pearson, W.R. Rapid and sensitive protein similarty searches. Science 1985, 227, 1435–1441. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Ruiz, J.C.; Peraza-Echeverría, L.; Soto-Hernández, R.M.; San MiguelChávez, R.; Pérez-Brito, D.; Tapia-Tussell, R.; Ortiz-Vázquez, E.; Rodríguez-García, C.M. Diospyros cuneata Inhibition of Fusarium oxysporum: Aqueous Extract and its Encapsulation by Ionic Gelation. J. Plant Pathol. Microbiol. 2016, 7, 332. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Reem, M.H.; Naveed, U.R.; Guangbiao, S.; Penghui, L.; Xiaochun, W.; Liang, G.; Jian, Z. De novo transcriptome sequencing and metabolite profiling analyses reveal the complex metabolic genes involved in the terpenoid biosynthesis in Blue Anise Sage (Salvia guaranitica L.). DNA Res. 2018, 25, 597–617. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Penghui, L.; Guangbiao, S.; Daopu, C.; Xiaochun, W.; Jian, Z. Transcriptome and metabolite analyses reveal the complex metabolic genes involved in volatile terpenoid biosynthesis in garden sage (Salvia officinalis). Sci. Rep. 2017, 7, 16074. [Google Scholar] [CrossRef] [Green Version]
- Alamri, S.A.M.; Hashem, M.; Moustafa, Y.S.; Nafady, N.A.; Abo-Elyousr, K.A.M. Biological control of root rot in lettuce caused by Exserohilum rostratum and Fusarium oxysporum via induction of the defense mechanism. Biol. Control 2019, 128, 76–84. [Google Scholar] [CrossRef]
- Abdul Baki, A.A.; Anderson, J.D. Vigour determination in soybean seed by multiple criteria. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Somda, I.; Leth, V.; Seeme, P. Evaluation of Lemongrass, Eucalyptus and Neem aqueous extracts for controlling seed-borne fungi of sorghum grown in Burkina Faso. World J. Agric. Sci. 2007, 3, 218–223. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Batra, G.; Kuhn, C. Polyphenoloxidase and peroxidase activities associated with acquired resistance and its inhibition by 2-thiouracil in virus-infected soybean. Physiol. Plant Pathol. 1975, 5, 239–248. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; Wiley: New York, NY, USA, 1984. [Google Scholar]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Enikuomehin, O.A.; Oyedeji, E.O. Fungitoxic effect of some plant extracts against tomato fruit rot pathogens. Arch. Phytopathol. Plant Prot. 2010, 43, 233–240. [Google Scholar] [CrossRef]
- Abo-Elyousr, K.A.M.; Asran, M.R. Antibacterial activity of certain plant extracts against bacterial wilt of tomato. Arch. Phytopathol. Plant Prot. 2009, 42, 573–578. [Google Scholar] [CrossRef]
- Kareem, S.O.; Akpan, I.; Ojo, O.P. Antimicrobial Activities of Calotropis procera on Selected Pathogenic Microorganisms. Afr. J. Biomed. Res. 2008, 11, 105–110. [Google Scholar] [CrossRef]
- Nikam, P.S.; Jagtap, G.P.; Sontakke, P.L. Management of chickpea wilt caused by Fusarium oxysporium f. sp. ciceri. Afr. J. Agric. Res. 2007, 2, 692–697. [Google Scholar]
- Olufolaji, D.B.; Adeosun, B.O.; Onasanya, R.O. In vitro investigation on antifungal activity of some plant extracts against Pyricularia oryzae. Niger. J. Biotechnol. 2015, 29, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Doshi, H.; Satodia, H.; Thakur, H.C.; Parabia, F.; Khan, A. Phytochemical screening and biological activity of Calotropis procera (Ait) R. Br. (Asclepiadaceae) against selected Bacteria and Anopheles stephansi larvae. Int. Res. Plant Res. 2011, 1, 29–33. [Google Scholar] [CrossRef]
- Mohamed, N.H.; El-Hadidy, A.M. Studies of biologically active constituents of Verbascum eremobium Murb. and its inducing resistance against some diseases of cucumber. Egypt. J. Phytopathol. 2008, 36, 133–150. [Google Scholar]
- Wink, M.; Ashour, M.L.; El-Readi, M.Z. Secondary Metabolites from Plants Inhibiting ABC Transporters and Reversing Resistance of Cancer Cells and Microbes to Cytotoxic and Antimicrobial Agents. Front. Microbiol. 2012, 3, 130. [Google Scholar] [CrossRef] [Green Version]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.-M. Plant Secondary Metabolite Biosynthesis and Transcriptional Regulation in Response to Biotic and Abiotic Stress Conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Khan, M.I.; Kumar, R. Antifungal activity of leaf extract of Neem on seed mycoflora of wheat. Indian J. Seed Abs. 1992, 15, 299. [Google Scholar]
- Ahmed, M.; Mehbub, H.; Kamrul, H.; Dash, C.K. Efficacy of Different Plant Extract on Reducing Seed Borne Infection and Increasing Germination of Collected Rice Seed Sample. Univers. J. Plant Sci. 2013, 1, 66–73. [Google Scholar] [CrossRef]
- Ali, E.F.; Al-Yasi, H.M.; Issa, A.A.; Hessini, K.; Hassan, F.A.S. Ginger extract and fulvic acid foliar applications as novel practical approaches to improve the growth and productivity of Damask Rose. Plants 2022, 11, 412. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.; Bano, A.; Nosheen, A.; Yasmin, H.; Keyani, R.; Shah, S.T.A.; Anwar, Z.; Roberts, T.H. Induction of defense-related enzymes and enhanced disease resistance in maize against Fusarium verticillioides by seed treatment with Jacaranda mimosifolia formulations. Sci. Rep. 2021, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Gui, Y.; Li, Z.; Jiang, C.; Guo, J.; Niu, D. Induced Systemic Resistance for Improving Plant Immunity by Beneficial Microbes. Plants 2022, 11, 386. [Google Scholar] [CrossRef]
- Ramamoorthy, V.; Samiyappan, R. Induction of defence-related genes in Pseudomonas fluorescens—treated chilli plants in response to infection by Colletotrichum capsici. J. Mycol. Plant Pathol. 2001, 31, 146–155. [Google Scholar]
- Saravanakumar, D.; Vijayakumar, C.; Kumar, N. PGPR-induced defense responses in the tea plant against blister blight disease. Crop Prot. 2007, 26, 556–565. [Google Scholar] [CrossRef]
- Lanubile, A.; Ferrarini, A.; Maschietto, V.; Delledonne, M.; Marocco, A.; Bellin, D. Functional genomic analysis of constitutive and inducible defense responses to Fusarium verticillioides infection in maize genotypes with contrasting ear rot resistance. BMC Genom. 2014, 15, 710. [Google Scholar] [CrossRef] [Green Version]
- Vanitha, S.C.; Umesha, S. Pseudomonas fluorescens mediated systemic resistance in tomato is driven through an elevated synthesis of defense enzymes. Biol. Plant. 2011, 55, 317–322. [Google Scholar] [CrossRef]
- Shabana, Y.M.; Mohamed, E.; Shahin, A.A.A.; El Sawy, M.M.; Draz, I.S.; Youssif, A.W. Efficacy of plant extracts in controlling wheat leaf rust disease caused by Puccinia triticina. Egypt. J. Basic Appl. Sci. 2017, 4, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Odhav, M.B.; Mohanlall, V. Antifungal activity of stigmasterol, sitosterol and ergosterol from Bulbine natalensis Baker (Asphodelaceae) B. J. Med. Plants Res. 2012, 6, 5135–5141. [Google Scholar] [CrossRef]
- Abo-Elyousr, K.A.M.; Ibrahim, Y.E.; Balabel, N.M. Induction of disease defensive enzymes in response to treatment with acibenzolar-S-methyl (ASM) and Pseudomonas fluorescens Pf2 and inoculated with Ralstonia solanacearum race 3, biovar2 (phylotype II). J. Phytopathol. 2012, 160, 382–389. [Google Scholar] [CrossRef]
- Khaledi, N.; Taheri, P.; Tarighi, S. Antifungal activity of various essential oils against Rhizoctonia solani and Macrophomina phaseolina as major bean pathogens. J. Appl. Microbiol. 2015, 118, 704–717. [Google Scholar] [CrossRef]
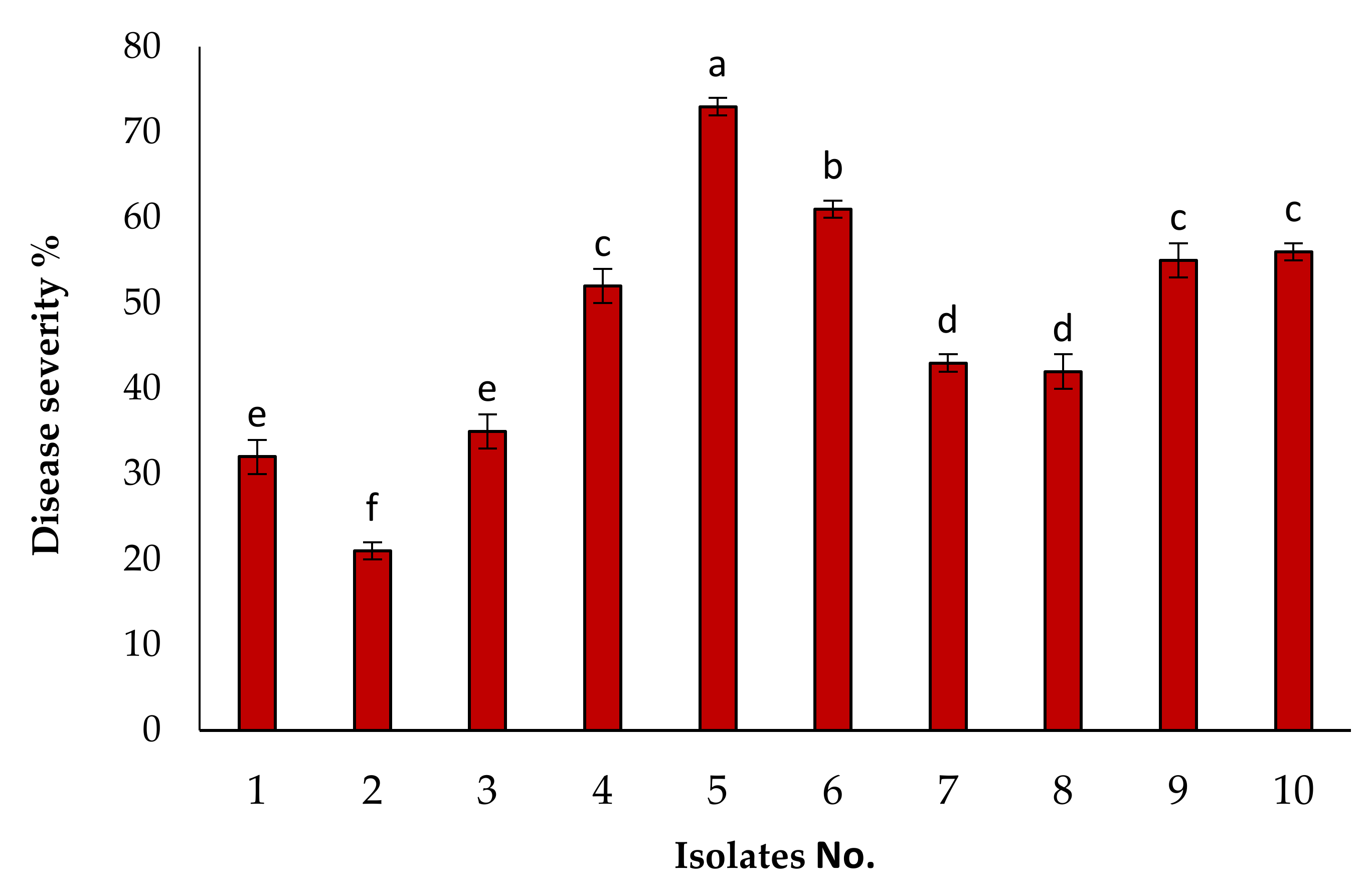
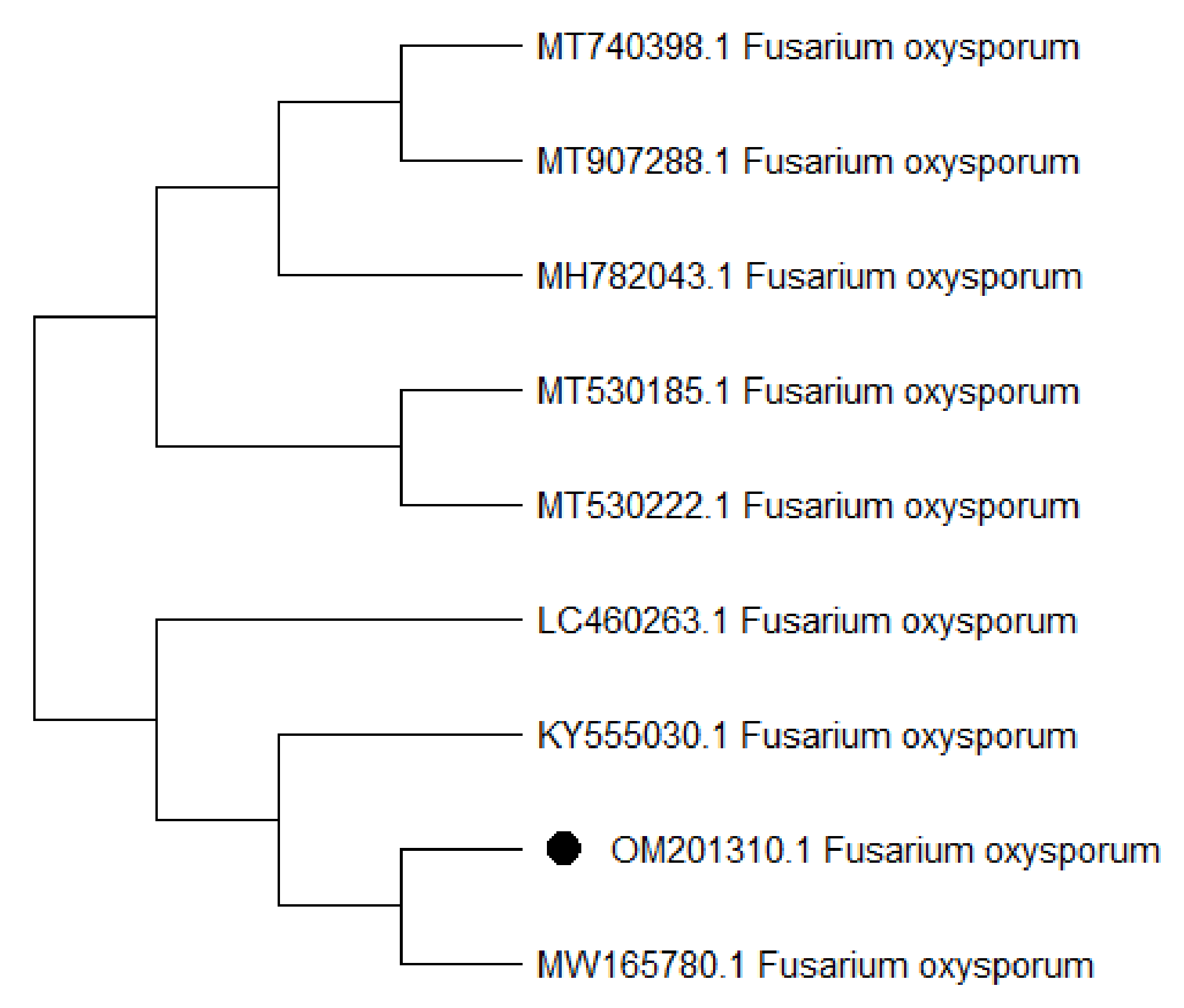
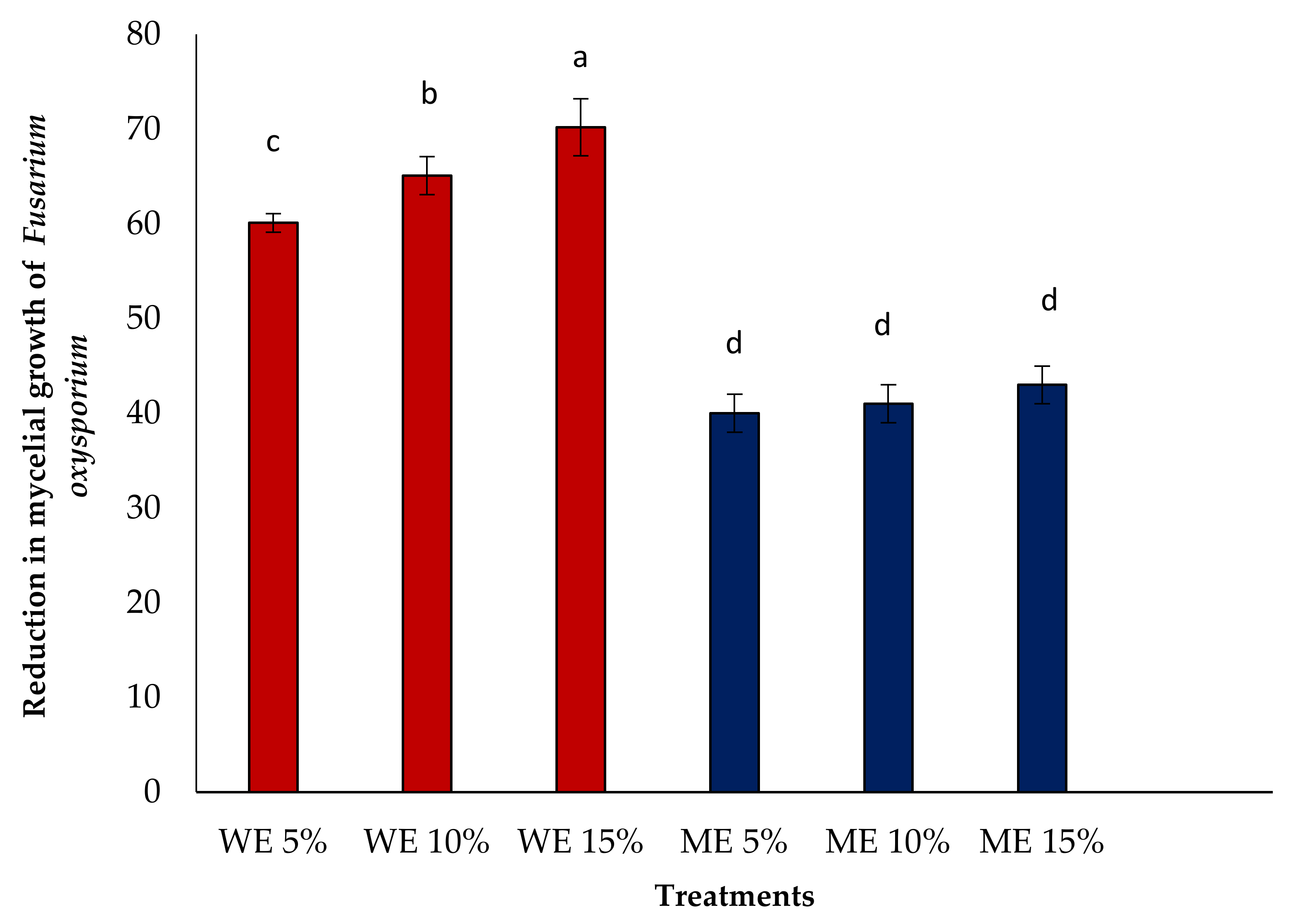
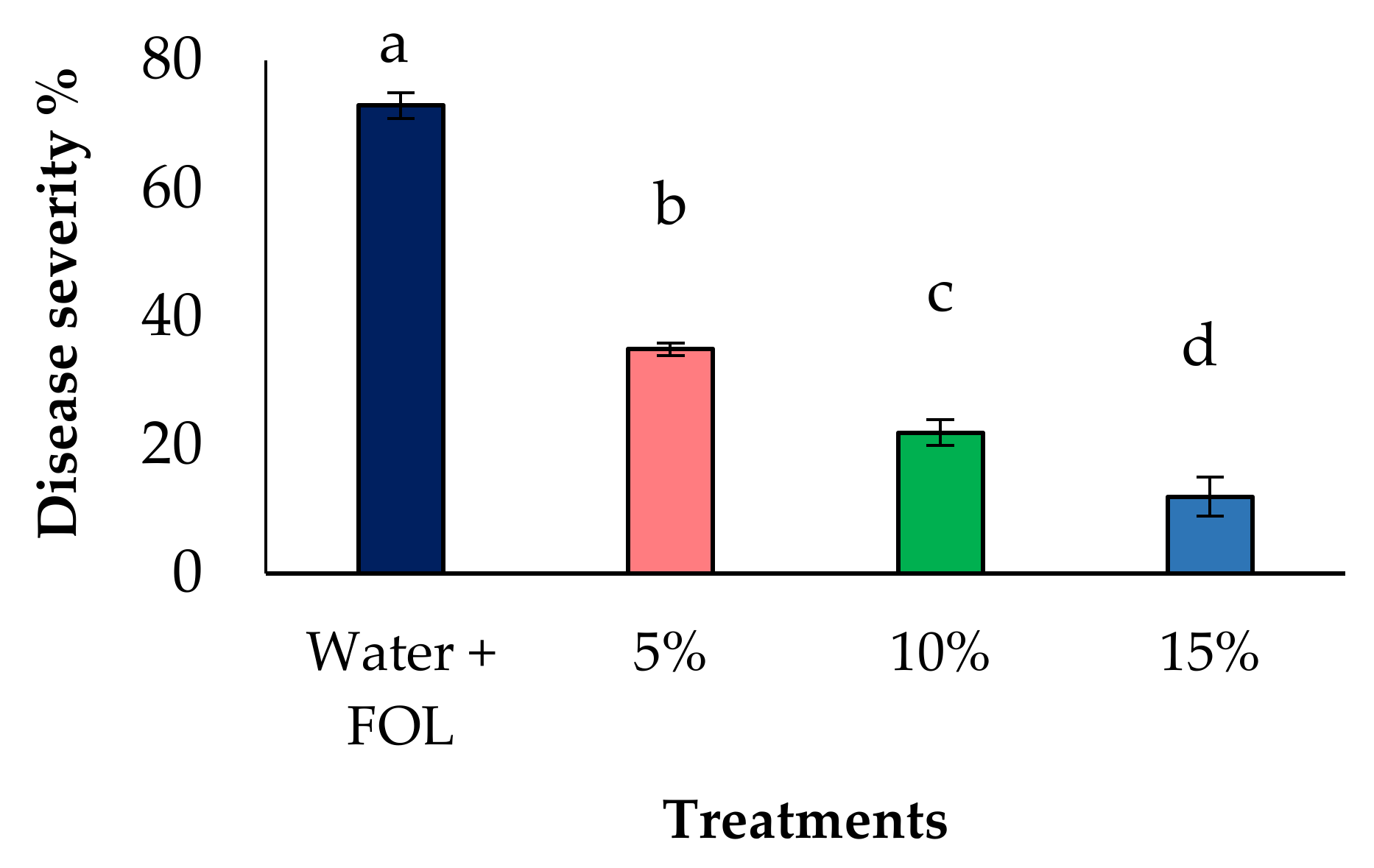

| Concentration | Germination (%) | MSL (cm) | MRL (cm) | Vigor Index (VI %) |
|---|---|---|---|---|
| C. procera 5% | 75 b (+10.3) | 1.2 (±0.4) b | 4.5 (±0.9) b | 427.5 |
| C. procera 10% | 75 b (+10.3%) | 1.3 (±0.2) b | 5.2 (±0.7) a | 487.5 |
| C. procera 15% | 85 a (+25%) | 1.5 (±0.1) a | 5.2 (±0.5) a | 569.5 |
| Infected control | 68 c (-) | 1.1 (±0.2) b | 3.1(±0.7) c | 285.6 |
| Healthy control | 85 a (+25%) | 1.5 (±0.1) c | 5.1 (±0.4) a | 561.0 |
| No. | Compound Name | R.T | Formula | M.W | Peak Area (%) |
|---|---|---|---|---|---|
| 1. | 1,3-Dipalmitin, TMS derivative | 4.27 | C38H76O5Si | 640 | 5.98 |
| 2. | Palmitoyl glycerol | 4.62 | C19H38O4 | 330 | 1.36 |
| 3. | Palmitic acid | 5.23 | C16H32O2 | 256 | 6.87 |
| 4. | Ethyl iso-allocholate | 6.14 | C26H44O5 | 436 | 2.65 |
| 5. | Oxiraneundecanoic acid, 3-pentyl-, methyl ester, cis | 8.53 | C19H36O3 | 312 | 1.12 |
| 6. | Astaxanthin | 9.18 | C40H52O4 | 596 | 1.32 |
| 7. | α-D-mannopyranoside | 9.96 | C15H28B2O6 | 326 | 1.84 |
| 8. | Biocytin | 11.79 | C16H28N4O4S | 372 | 9.98 |
| 9. | Ascaridole | 13.08 | C10H16O2 | 168 | 2.57 |
| 10. | (+)-delta-Cadinene | 13.89 | C15H24 | 204 | 12.24 |
| 11. | N-Benzylideneisopropylamine | 14.57 | C10H13N | 147 | 16.53 |
| 12. | 2-(3,4-dimethoxyphenyl)-3,5-dihydroxy-7-methoxy-4H-1- Benzopyran-4-one | 15.69 | C18H16O7 | 344 | 1.24 |
| 13. | 5,8,11-Eicosatrienoic acid, (Z)-, TMS derivative | 16.84 | C23H42O2Si | 378 | 1.67 |
| 14. | All-trans-beta-Carotene | 18.24 | C40H56 | 536.4 | 1.27 |
| 15. | 1-Monooleoylglycerol, 2TMS derivative | 18.69 | C27H56O4Si2 | 500 | 1.64 |
| 16. | Tristrimethylsilyl ether derivative of 1,25-dihydroxy vitamin D2 | 18.89 | C37H68O3Si3 | 644 | 0.69 |
| 17. | 5,8,11-Eicosatriynoic acid,tert-butyldimethylsilyl ester | 19.36 | C26H42O2Si | 414 | 2.26 |
| 18. | Octadecanoic acid,9,10-epoxy-18-(trimethylsiloxy)-, methyl ester, cis- | 19.92 | C22H44O4Si | 400 | 0.59 |
| 19. | 10,12,14-Nonacosatriynoic acid | 20.34 | C29H46O2 | 426 | 0.42 |
| 20. | 9,12-Octadecadienoic acid(Z,Z)-, 2,3-bis[(trimethylsilyl)oxy]propyl ester | 20.67 | C27H54O4Si2 | 498 | 2.18 |
| 21. | Rhodopin | 21.13 | C40H58O | 554 | 1.45 |
| 22. | Palmitic acid, methyl ester | 21.76 | C17H34O2 | 270 | 0.82 |
| 23. | Methyl 2-O,3-O-bis(trimethylsilyl)-4-O,6-O- (methylboranediyl)-β-D-glucopyranoside | 22.79 | C14H31BO6Si2 | 362 | 3.69 |
| 24. | Methyl 2-O,3-O-bis(trimethylsilyl)-4-O,6-O- (methylboranediyl)-α-D-glucopyranoside | 23.68 | C14H31BO6Si2 | 362 | 2.73 |
| 25. | Glycodeoxycholic acid | 24.07 | C26H43NO5 | 449 | 0.54 |
| 26. | à-D-Glucofuranose, 6-O-(trimethylsilyl)-, cyclic 1,2:3,5-bis(butylboronate) | 24.25 | C17H34B2O6Si | 384 | 0.56 |
| 27. | 1,25-Dihydroxyvitamin D3, TMS derivative | 24.58 | C30H52O3Si | 488 | 0.56 |
| 28. | Oleic acid, methyl ester | 24.79 | C19H36O2 | 296 | 3.94 |
| 29. | Glyceryl 2-linoleate | 25.18 | C27H52O4Si2 | 496 | 1.14 |
| 30. | Stigmasterol | 26.18 | C32H56OSi | 484 | 1.09 |
| 31. | Trilinolein | 31.64 | C57H98O6 | 878 | 1.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abo-Elyousr, K.A.M.; Ali, E.F.; Sallam, N.M.A. Alternative Control of Tomato Wilt Using the Aqueous Extract of Calotropis procera. Horticulturae 2022, 8, 197. https://doi.org/10.3390/horticulturae8030197
Abo-Elyousr KAM, Ali EF, Sallam NMA. Alternative Control of Tomato Wilt Using the Aqueous Extract of Calotropis procera. Horticulturae. 2022; 8(3):197. https://doi.org/10.3390/horticulturae8030197
Chicago/Turabian StyleAbo-Elyousr, Kamal A. M., Esmat F. Ali, and Nashwa M. A. Sallam. 2022. "Alternative Control of Tomato Wilt Using the Aqueous Extract of Calotropis procera" Horticulturae 8, no. 3: 197. https://doi.org/10.3390/horticulturae8030197
APA StyleAbo-Elyousr, K. A. M., Ali, E. F., & Sallam, N. M. A. (2022). Alternative Control of Tomato Wilt Using the Aqueous Extract of Calotropis procera. Horticulturae, 8(3), 197. https://doi.org/10.3390/horticulturae8030197








