Abstract
Unlike other studies that only determined the chemical composition of essential oils depending on their geographic origin, this research investigated the effect of weather conditions (temperature, precipitation, and insolation) on the chemical composition of Nepeta nuda L. essential oil. The collection of wild-growing N. nuda was carried out during three successive years, 2019, 2020, and 2021 at Rtanj Mountain (Serbia) on the same date (July 7th). Essential oil extraction from the plant was performed by hydro-distillation. After gas chromatographic-mass spectrometric analysis, a total of 102 volatile compounds were separated from N. nuda, during the observed period, 28 were unidentified, compromising between 5.0% and 8.7%, depending on the year. A multiple linear regression model was created, and statistical analyses were performed to provide knowledge about the prediction, feature profile, and the similarity in contents of active compounds of the N. nuda essential oil. The influence of temperature on the accumulation of the most abundant component, 1,8-cineole, was positive, while the impact of precipitation and insolation was negative. According to the cluster tree, there are four chemotypes of N. nuda essential oil: with nepetalactone, 1,8-cineole, mixed (nepetalactone+1,8-cineole+germacrene D), and nonspecific chemotypes. Bearing in mind that the biological activity of a raw material depends on the chemotype and environmental factors, this is a topic that deserves a more detailed approach. The N. nuda and its essential oil are promising materials with high biological potential, and these deserve further detailed investigation.
1. Introduction
Nepeta nuda L. (syn. N. pannonica L.) is a perennial forest-steppe plant widespread across Europe and Asia. In Serbia, it is found on dry meadows, rocky areas, and in light deciduous and evergreen woodland on hills and mountain ranges [1]. There are two other species from genus Nepeta that grow in Serbia, N. cataria L. (the most widespread plant from this genus around the world) and N. rtanjensis Diklić & Milojević (an endangered endemic plant that grows spontaneously exclusively on Mt. Rtanj) [2,3,4].
There are four registered subspecies of N. nuda that can be found in different growing areas. They differ from one another in morphological features. Endemic subspecies for Turkey and Greece are ssp. lydiae and ssp. glandulifera, while ssp. nuda and ssp. albiflora have a wide growing area through Europe and Asia, and the Balkans are their overlapping region [5]. N. nuda is a significant source of biologically active compounds. Apart from essential oil [2,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22], it is a good source of polyphenols [23], flavonoids [7], tannins, and coumarins [24,25].
However, due to a large growing area and number of subspecies, there are variations among chemical composition and usage. It is used in traditional medicine in Serbia, Bulgaria, and Russia, mainly as an herbal tea for treating many ailments, such as mental states (hysteria, melancholy, and asthenia), gastrointestinal and respiratory disorders, cystitis and prostate gland inflammation, dysmenorrhea, syphilis, and externally for wound healing and against mastitis [5]. Pharmacological studies show that it possesses antibacterial, antifungal and antiviral activities [7,12,16,26,27,28], as well as antioxidant [7,8,16,29,30,31,32], and anticancer properties [33].
Due to its pleasant aroma, which is similar to citrus and peppermint, it can be used in the food industry as an additive in juices [34], or in the pharmaceutical industry for mouthwash preparations [28]. In addition, this plant could be used to develop bio-herbicides due to its genotoxicity [10,35,36]. Nevertheless, investigations show that N. nuda essential oils have the great bioactive potential [8,10,12,14,15,16,17,33,35]; thus, in order to be able to standardize a recipe for specific preparation, it is necessary to examine the stability of the chemical composition of the raw material. Bearing in mind that all studies dealing with N. nuda essential oil only determine the chemical composition depending on the geographic origin, and wild populations are typically used, this research aimed to explore the influence of weather conditions (temperature, precipitation, and insolation) on the chemical composition of N. nuda from Rtanj Mt. during three successive years.
A multiple linear regression model was developed to predict the N. nuda essential oil active compound content by following weather condition data. In addition, Principal Component Analysis (PCA) was performed for a more thorough perception of the essential oil feature profile. Finally, the heat map and unrooted cluster tree were schemed to examine the similarity in active compound contents of the different samples visually.
2. Materials and Methods
2.1. Plant Material
The traditional collection of these wild medicinal plants is mostly connected to important dates, including saint days in the Serbian Orthodox Church and dates connected to important events in nature. The most crucial day for plant collecting in Serbia is the Nativity of Saint John the Baptist (7 July according to the Julian calendar and 24 June according to the Gregorian calendar), and this overlaps with the summer solstice.
Several customs are tied to that day; however, the most important is that people believe that plants should be collected on that day, which is defined as Biljober (biljo-ber, srb. biljka, noun-plant; brati, verb-picking). In Serbian agro-ecological conditions, N. nuda generally flowers between June and August. The flowering of one verticillaster started from bottom to top in approximately two to three weeks. For all three investigated years, we collected only verticillasters in the full flowering stage; therefore, the variations in the chemical composition for the ontogenetic stage of plants were minimal.
N. nuda was collected on 7 July 2019, 2020, and 2021, at an exact location (N43.7748737, E21.8890562), on the southern slopes of the mountain Rtanj to evaluate the impact of weather conditions on the chemical composition of a population over three years. Plants were grown in a region with the climate type: Cfb, a marine west coast climate, bordering on Dfb, a warm summer continental or hemiboreal climate according to the Köppen–Geiger classifications.
The plant materials were dried in the shade until reaching a constant weight, placed in paper bags, and stored under ambient conditions. Voucher specimens were determined as ssp. Nuda and then confirmed and deposited at the Herbarium of the Department of Biology and Ecology (BUNS Herbarium), Faculty of Natural Sciences, University of Novi Sad (2-1380, 2-1808, and 2-2140, respectively).
2.2. Weather Conditions
Mt. Rtanj is located in eastern Serbia and belongs to the Serbian Carpathians, an extension of the proper Carpathian Mountains across the Danube, connecting them with the Balkan Mountains in the southeast. Due to its geographic position, Mt. Rtanj has predominantly continental climatic features, which are characteristic of the entire Balkan Peninsula [37].
2.3. Essential Oil Extraction Andgc/ms Analysis
Aerial parts of N. nuda collected from three consecutive years (2019–2021) were dried, chopped, and subjected to hydro-distillation (ca. 40 g each plant material) using a Clevenger-type apparatus to extract essential oils over 3 h. Gas chromatographic-mass spectrometric analyses were performed according to Aćimović et al. [38] on a non-polar HP-5MS fused-silica capillary column (30 m × 0.25 mm × 0.25 μm) with helium as the carrier gas (a constant pressure was set to 19.6 psi to obtain linear velocity in Adams library of 1 mL min−1 at 210 °C).
The injection volume was 1 μL, and the split ratio was 100: 1 for all samples, under GC operating conditions: injector temperature 250 °C, MS source temperature 230 °C, and interface temperature 315 °C. The mass spectra were obtained in electron ionization mode at electron energy, 70 eV, with a mass scan range of m/z 40–600. The essential oil components were identified based on their linear retention index relative to C8-C32 n-alkanes, and compared to retention times obtained from the GC library (Adams ver. 4).
Furthermore, the mass spectral data were compared to the commercial MS data libraries Adams ver. 4 and NIST ver. 17 and with the data reported in the literature [35]. The relative mass percentage of the oil constituents was expressed as percentages by the FID peak area normalization. The method for essential oil analysis was previously validated and verified with quality control samples for a performance check daily and/or within the first 10 samples. A quality assurance manager checks the quality of these data every time and decide if analysis should procced or not.
2.4. Multiple Linear Regression Model
A multiple linear regression model was formed following the temperature, precipitation, and insulation data to predict the N. nuda essential oil active compound contents. In addition, the proper regression coefficients were determined. The regression coefficients used in multiple linear regression models were calculated by minimizing the sum of squared residuals for each model in Microsoft Excel using the Solver function.
2.5. Statistical Analysis
Principal Component Analysis (PCA) was performed to research the essential oil profile of N. nuda. The perspective trend for a more profound comprehension of the essential oil feature profile was realized by embracing the grouped samples’ PCA plot. The heat map was plotted to examine the similarity in the active compound contents of the different samples. A statistical study of the data was accomplished using the Statistica 10 software. The heat map and unrooted cluster tree were performed by the R software 4.0.3 (64-bit version) to visually investigate the likenesses among various samples.
3. Results
A total of 102 volatile compounds were separated from N. nuda during three investigated years, 28 were unidentified (NI), compromising between 5.0% and 8.7% depending on the year. The results show that 1,8-cineole with an average percent of 46.1%, germacrene D with 9.9%, caryophyllene oxide with 8.1%, and trans-caryophyllene with an average content of 5.0% were the most dominant compounds (Table 1).

Table 1.
The relative abundance (%) of the separated compounds from N. nuda during three investigated years.
Figure 1 depicted the average daily temperatures, the sum of precipitation, and the sum of insolation for 2018–19, 2019–20, and 2020–21 years, which was presented in this research. The yearly averages of these data were used to build a set of multiple linear regression models to predict the amount of specific compounds present in the plant material. The obtained multiple linear regression models showed the influence of the average daily temperatures, the sum of precipitation, and the sum of insolation on the concentration of each compound obtained by GC MS analysis. These are presented in Table 1.
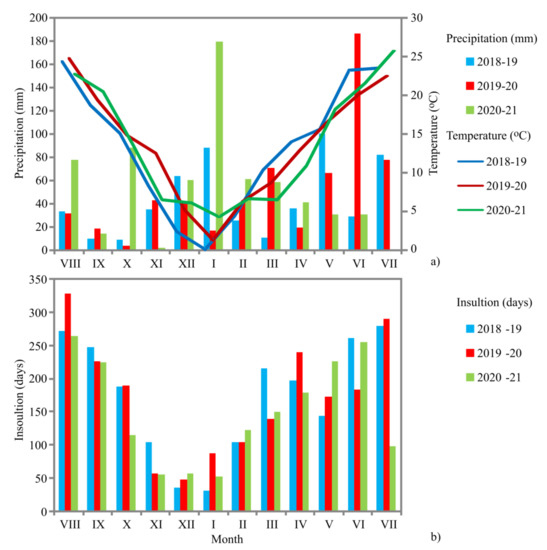
Figure 1.
The average daily temperatures, the sum of precipitation (a), and the sum of insolation (b) used in this research taken from the nearest meteorological station for Rtanj Mt.
The average daily temperatures, the sum of precipitation, and the sum of insolation used in this research are taken from the nearest meteorological station located in Niš (distance by air 48.6 km), Republic Hydro-meteorological Service of Serbia (Figure 1). In August–October, weather conditions were essential as N. nuda is a perennial plant, and the temperatures, precipitations, and insolation influence the accumulation of compounds necessary for the overwintering period. The dormancy period (November–April) is characterized by temperatures below 13.5 °C. The period from May to July, i.e., pre-flowering and flowering is very important for accumulating secondary metabolites.
The correlative heat map graphic was employed to analyze the relations in essential oil compounds of N. nuda samples from 2019, 2020, and 2021 are illustrated in Figure 2. It can be observed from the figure that the darker green area color, symbolizes the similarity in content for two active compounds, which presents a more significant correlation linking recognized active compounds. On the contrary, the lighter tone symbolizes a particular dissimilarity in the content of active compounds. Accordingly, the lighter color tone indicates a lower correlation.
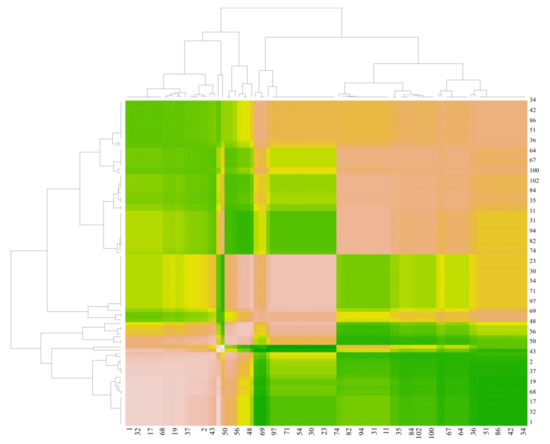
Figure 2.
Heat map of active compounds content in the essential oil of N. nuda samples from 2019, 2020, and 2021 (the compound codes are listed in Table 1).
Conversely, the red color signifies a negative correlation within active compounds. The correlation analysis data was used to test the relation between different compound content in the plant material, while the cluster analysis was employed to recognize the sequence of compound abundance obtained in plant material in samples from 2019, 2020, and 2021. The main idea was to map the investigated plant material presented with its inter-correlation features of the compound contents for the literature known cases.
Like the heat map statistic method, the PCA analysis was performed to test the correlations between the essential oil compounds of N. nuda samples from 2019, 2020, and 2021, and the results are presented in Figure 3. The points shown in the PCA graphics, which are geometrically close to each other, indicate the similarity of patterns representing these points.
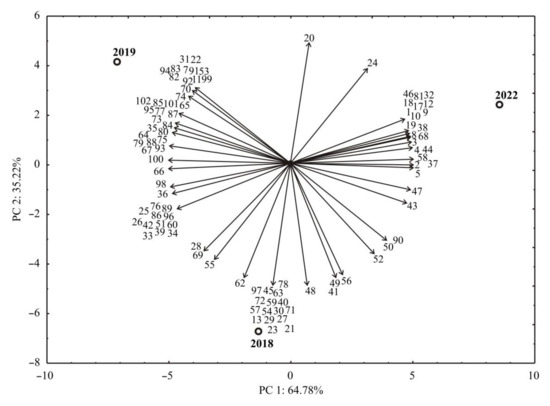
Figure 3.
The PCA biplot diagram describing the relations between essential oil compounds of N. nuda samples from 2019, 2020, and 2021 (the compound codes and samples codes are noted in Table 1).
The vector’s orientation describing the variable in factor space indicates an increasing trend of these variables, and the length of the vector is proportional to the square of the correlation values between the fitting value for the variable and the variable itself. The angles between corresponding variables indicate the degree of their correlations (small angles corresponding to high correlations). Therefore, the correlation between different compounds content and also the content of the obtained compounds can be easily accessed visually from Figure 3.
Comprehensively explaining the structure of the experimental data would provide a more profound description of connections between different samples of N. nuda from 2019, 2020, and 2021. PCA was used, and the obtained results are displayed in Figure 3. The first two PCs demonstrated 100% of the total variance in the laboratory data. The first PC explained 64.78%, and the second 35.22% of the total variance between the experimental data. The parting within samples could be seen from the PCA figure, where the samples from N. nuda essential oil composition during 2019 are grouped on the bottom, 2020 on the left, and 2021 on the right side of the graphic.
According to the literature and average values from this study (Table 2), the unrooted cluster tree with 33 samples of N. nuda essential oil was plotted (Figure 4), showing that there are four chemotypes: (1) nepetalactone chemotype, which could be divided into two subgroups: with a high nepetalactone content (62.7–71.0%) and with a very high nepetalactone content (74.3–84.8%); (2) 1,8-cineole chemotype (21.0–63.8%); (3) mixed chemotype—nepetalactone+1,8-cineole+germacrene D (28.4–39.1%, 22.9–24.6%, and 13.5%, respectively); and (4) nonspecific chemotypes, with two subgroups: single dominant compound—geijerene [8] and β-elemene [6], and mixed compounds [6,12,15,16,18]. Table 2 shows 32 accessions of N. nuda essential oil from the literature and the results from this study with the 12 most abundant compounds (on average more than 1.0%).

Table 2.
N. nuda essential oil composition from this study and literature (32 accessions).
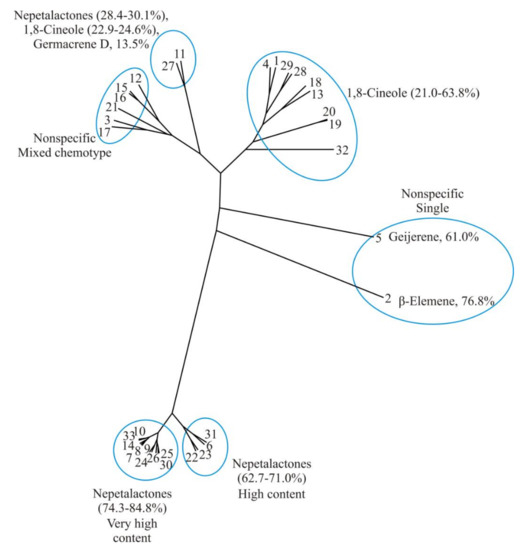
Figure 4.
Unrooted cluster tree for the chemical composition of essential oil compounds of N. nuda according to the literature and this study (the samples are coded according to Table 2).
As stated in the unrooted cluster tree (Figure 4), N. nuda essential oil nepetalactone dominated in nearly all samples (in various proportions, the highest content was 84.8%, [9]. In contrast, in samples 19 and 20, 1,8-cineole reached 62.2% and 63.8%, respectively [2], and, in two samples, non-specific single compounds were geijerene, 61.0% [8], and β-elemene, 76.8% [6].
4. Discussion
The main volatile compounds obtained by GC-MS technique from N. nuda during all three years were 1,8-cineole with 46.1% on average (ranging from 44.2% to 49.1%), germacrene D with 9.9% on average (ranging from 5.8% to 12.5%), caryophyllene oxide with 8.1% (ranging from 5.1% to 13.8%), and trans-caryophyllene with 5.0% (ranging from 3.1% to 6.6%) (Table 1). The sesquiterpenes, trans-caryophyllene, and germacrene D, were significantly lower in the second year of investigations (3.1% and 5.8%, respectively).
On the other hand, by comparing the first and the second investigation year (5.4% (2019) and 6.6% (2020) for trans-caryophyllene, and 12.5% (2019) and 11.4% (2020) for germacrene D), while oxygenated sesquiterpene caryophyllene oxide was present at a significantly higher concentration (13.8%) in 2020 in comparison to 2019 (5.4%) and 2021 (5.1%). The transformation of sesquiterpenes could influence this to oxygenated forms under favorable weather conditions (lower temperatures and high precipitation). However, weather conditions during the vegetative period influenced the accumulation of essential oil compounds, especially during the pre-flowering and flowering periods (June).
On the one hand, 1,8-cineole was the amplest component (46.1% on average for three years). The influence of temperatures was positive (the regression coefficient was 9.711), while the impacts of precipitation and insolation were negative (−0.624 and 0.327, respectively) on the accumulation. The same trend was noticed for trans-caryophyllene and germacrene, the positive influence of the temperature with regression coefficient 5.567 and 11.992, accordingly, while the impacts of precipitation (−0.603 and −1.511, respectively) and insolation (−0.245 and −0.463, respectively) were negative. On the other hand, the influence of increased temperatures was negative on the accumulation of caryophyllene oxide (the regression coefficient was −14.497), while the impacts of precipitation and insolation were positive (1.881 and 0.652, respectively).
Correlative analysis (using a correlation heat map) was employed to test the relations between different essential oil compounds content in N. nuda ssp. nuda samples from 2019, 2020, and 2021 (Figure 2). The results indicated statistically significant (p < 0.05), high correlation coefficients between the contents of δ-terpineol, α-terpineol, and δ-elemene and the contents of α-copaene, β-bourbonene, α-calacorene, palustrol, cadalene, heptacosane, and untriacontane. High correlation coefficients (p < 0.005) were noticed between the contents of α-pinene, δ-terpineol, α-terpineol, δ-elemene, and α-ylangene, and the contents of β-bourbonene, γ-muurolene, and cadalene content. The PCA analysis also explained the complex relations between different compound contents.
According to the literature references, the unrooted cluster tree unveiled the four chemotypes of N. nuda essential oil: the nepetalactone chemotype group (with two groups with high nepetalactone content and very high nepetalactone content); 1,8-cineole chemotype group; mixed chemotype group (high content of nepetalactone+1,8-cineole+germacrene D); and nonspecific chemotypes group (with a single dominant compound—geijerene and β-elemene, and the group of mixed compounds.
Based on the results of this study, the samples investigated here belong in the second chemotype group (high 1,8-cineole content). Even though there are several classifications of N. nuda chemotypes with monoterpene and sesquiterpene dominant or nepetalactones-rich and nepetalactones-poor groups, this is the first study including all data available via online scientific databases applied for constructing an unrooted cluster tree (Figure 3), to demonstrate the differences between groups. This model was previously successfully applied to Hyssopus officinalis [39], Helichrysum italicum [40] and Marrubium vulgare [41].
5. Conclusions
Analysis of N. nuda essential oil during three successive years showed that the most dominant compounds were 1,8-cineole (44.2–49.1%), germacrene D (5.8–12.5%), caryophyllene oxide (5.1–13.8%), and trans-caryophyllene (3.1–6.6%). Nevertheless, the weather conditions slightly influenced the accumulation ratio of essential oil compounds. The impact of temperature on the accumulation of the most abundant component of N. nuda essential oil, 1,8-cineole, was positive, while the influence of precipitation and insolation was negative.
The same trend was noticed for trans-caryophyllene and germacrene, while the effect on caryophyllene oxide accumulation was the opposite. We created a multiple linear regression model and performed statistical analyses to provide knowledge about the prediction, feature profile, and the similarity in active compound contents of the N. nuda essential oil. Based on the results of the cluster tree, there were four chemotypes of N. nuda: nepetalactone, 1,8-cineole, mixed (nepetalactone+1,8-cineole+germacrene D), and nonspecific chemotypes.
The N. nuda and its essential oil are promising materials with high biological potential that deserve additional thorough studies. The superb quality of N. nuda essential oil indicates the possibility of producing this species in plantations in Serbia. According to the results, the weather conditions in Rtanj Mountain (Serbia) are favorable for growing this species. Weather conditions in the period of August–October were essential for the accumulation of compounds necessary for the overwintering period. In the future, global climate warming could contribute to the quality of this plant essential oil. Due to its position Mt. Rtanj can become a significant growing region for N. nuda as a high-quality raw material. Future research should focus on optimizing the technology of growing this plant.
Author Contributions
Conceptualization, M.A. and M.R.; methodology, B.L.; software, L.P.; validation, J.S.J., M.C. and M.P.; formal analysis, B.L.; investigation, M.A.; resources, M.R.; data curation, M.P.; writing—original draft preparation, M.A.; writing—review and editing, B.L.; visualization, L.P.; supervision, J.S.J. and M.C.; project administration, L.P.; funding acquisition, M.A., B.L. and M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education, Science and Technological Development of the Republic of Serbia, Grant Numbers 451-03-9/2021-14/200051, 451-03-9/2021-14/200012, 451-03-9/2021-14/200032, 451-03-9/2021-14/200134, 451-03-68/2021-14/200125 and 451-03-9/2021-14/200026.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors gratefully acknowledge the administrative and technical support of Adonis Sokobanja Ltd.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Josifović, M. (Ed.) Flora SR Srbije; Srpska Akademija Nauke i Umetnosti: Belgrade, Serbia, 1975; Volume 6. (In Serbian) [Google Scholar]
- Chalchat, J.-C.; Petrovic, S.D.; Gorunovic, M.S. Quantity and Composition of Essential Oil of the Wild Plant Nepeta nuda L. from Yugoslavia. J. Essent. Oil Res. 1998, 10, 423–425. [Google Scholar] [CrossRef]
- Grbic-Ljaljevic, M.; Stupar, M.; Vukojevic, J.; Sokovic, M.; Misic, D.; Grubisic, D.; Ristic, M. Antifungal activity of Nepeta rtanjensis essential oil. J. Serb. Chem. Soc. 2008, 73, 961–965. [Google Scholar] [CrossRef]
- Aćimović, M.; Zeremski, T.; Kiprovski, B.; Brdar-Jokanović, M.; Popović, V.; Koren, A.; Sikora, V. Nepeta cataria–Cultivation, Chemical Composition and Biological Activity. J. Agron. Technol. Eng. Manag. 2021, 4, 620–634. Available online: https://www.fimek.edu.rs/downloads/casopisi/jatem/issue/v4_4/3._Acimovic_et_al_2021_4(4)_620-634.pdf (accessed on 17 November 2021).
- Aćimović, M.; Stanković Jeremić, J.; Cvetković, M. Phyto-pharmacological aspects of Nepeta nuda L.: A systematic review. Lek. Sirov. 2020, 40, 75–83. [Google Scholar] [CrossRef]
- Carikci, S. Characterization of Nepeta viscida, N. nuda subsp. nuda and the putative hybrid N. × tmolea essential oils. Rec. Nat. Prod. 2021, 15, 388–395. [Google Scholar] [CrossRef]
- Ðorđević, S.M.; Stanisavljević, D.M.; Milenković, M.T.; Karabegović, I.T.; Lazić, M.L.; Nikolova, M.T.; Veličković, D. Formulation of refreshing nonalcoholic beverage with extracts of medicinal plants. Prog. Nutr. 2019, 21, 620–630. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Ceylan, O.; Targan, S.; Zeljković, S.Ć. Chemical composition and biological activities of the essential oils of two endemic Nepeta species. Ind. Crop. Prod. 2018, 125, 5–8. [Google Scholar] [CrossRef]
- Narimani, R.; Moghaddam, M.; Pirbalouti, A.G.; Mojarab, S. Essential oil composition of seven populations belonging to two Nepeta species from Northwestern Iran. Int. J. Food Prop. 2017, 20, 2272–2279. [Google Scholar] [CrossRef][Green Version]
- Bozok, F.; Cenet, M.; Sezer, G.; Ulukanli, Z. Essential oil and bioherbicidal potential of the aerial parts of Nepeta nuda subsp. albiflora (Lamiaceae). J. Essent. Oil-Bear. Plants 2017, 20, 148–154. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; Sharopov, F.S.; Satyal, P.; Azimova, S.S.; Wink, M. Chemical composition of the essential oils of some Central Asian Nepeta species (Lamiaceae) by GLC-MS. Nat. Prod. Commun. 2016, 11, 1891–1893. [Google Scholar] [CrossRef]
- Gormez, A.; Bozari, S.; Yanmis, D.; Gulluce, M.; Agar, G.; Sahin, F. Antibacterial activity and chemical composition of essential oil obtained from Nepeta nuda against phytopathogenic bacteria. J. Essent. Oil Res. 2013, 25, 149–153. [Google Scholar] [CrossRef]
- Kilic, O.; Hayta, S.; BagcI, E. Chemical Composition of Essential Oil of Nepeta nuda L. subsp. nuda (Lamiaceae) from Turkey. Chem. Asian J. 2011, 23, 2788–2790. Available online: https://asianjournalofchemistry.co.in/User/ViewFreeArticle.aspx?ArticleID=23_6_98 (accessed on 17 November 2021).
- Gkinis, G.; Bozin, B.; Mimica-Dukic, N.; Tzakou, O. Antioxidant Activity of Nepeta nuda L. ssp. nuda Essential Oil Rich in Nepetalactones from Greece. J. Med. Food 2010, 13, 1176–1181. [Google Scholar] [CrossRef]
- Mancini, E.; Apostolides Arnold, N.; De Feo, V.; Formisano, C.; Rigano, D.; Piozzi, F.; Senatore, F. Phytotoxic effects of essential oils of Nepeta curviflora Boiss. and Nepeta nuda L. subsp. albiflora growing wild in Lebanon. J. Plant Interact. 2009, 4, 253–259. [Google Scholar] [CrossRef]
- Alim, A.; Goze, I.; Cetin, A.; Atas, A.; Cetinus, S.; Vural, N. Chemical Composition and In Vitro Antimicrobial and Antioxidant Activities of the Essential Oil of Nepeta nuda L. subsp. albiflora (boiss.) Gams. Afr. J. Microbiol. Res. 2009, 3, 463–467. Available online: https://www.semanticscholar.org/paper/Chemical-composition-and-in-vitro-antimicrobial-and-Alim-Goze/b7661af754881eca241981a4cd2449658ad30de3 (accessed on 17 November 2021).
- Kobaisy, M.; Tellez, M.R.; Dayan, F.E.; Mamonov, L.K.; Mukanova, G.S.; Stipaeva, G.T.; Gemejieva, N.G. Composition and phytotoxic activity of Nepeta pannonica L. essential oil. J. Essent. Oil Res. 2005, 17, 704–707. [Google Scholar] [CrossRef]
- Kokdil, G.; Kurucu, S.; Yildiz, A. Essential oil composition of Nepeta nuda L. ssp. Nuda. Flavour Fragr. J. 1998, 13, 233–234. [Google Scholar] [CrossRef]
- De Pooter, H.L.; Nicolai, B.; De Buyck, L.F.; Goetghebeur, P.; Schamp, N.M. The essential oil of Nepeta nuda. Identification of a new nepetalactone diastereoisomer. Phytochemistry 1987, 26, 2311–2314. [Google Scholar] [CrossRef]
- Handjieva, N.V.; Popov, S.S.; Evstatieva, L.N. Constituents of Essential Oils from Nepeta cataria L., N. grandiflora M.B. and N. nuda L. J. Essent. Oil Res. 1996, 8, 639–643. [Google Scholar] [CrossRef]
- Sarer, E.; Konuklugil, B. Composition of the Essential Oil from Nepeta nuda ssp. albiflora (Boiss.) Gams. J. Essent. Oil Res. 1996, 8, 687–688. [Google Scholar] [CrossRef]
- Kokdil, G.; Kurucu, S.; Topçu, G. Composition of the Essential Oil of Nepeta nuda L. ssp. albiflora (Boiss.) Gams. Flavour Fragr. J. 1996, 11, 167–169. [Google Scholar] [CrossRef]
- Wieteska, A.; Jadczak, D.; Wesołowska, A. Comparison of the biological value of selected catnip plant (Nepeta sp.). Asian J. Med. Plants 2018, 6, 191–195. [Google Scholar] [CrossRef]
- Boikova, O.; Grishkina, K. Content is of tannins of the Nepeta pannonica growing in Tula region. Probl. Sci. Thought 2019, 7, 12–14. (In Russian) [Google Scholar]
- Boikova, O.; Grishkina, K. Content of kumarins in Nepeta pannonica growing in the Tula region. Probl. Sci. Thought 2019, 7, 9–11. (In Russian) [Google Scholar]
- Todorov, D.; Shishkova, K.; Dragolova, D.; Hinkov, A.; Kapchina-Toteva, V.; Shishkov, S. Antiviral activity of medicinal plant Nepeta nuda. Biotechnol. Biotechnol. Equip. 2015, 29, S39–S43. [Google Scholar] [CrossRef]
- Angelova, P.; Hinkov, A.; Tsvetkov, V.; Shishkova, K.; Todorov, D.; Shishkov, S. Inhibition of human herpes virus type 2 replication by water extract from Nepeta nuda L. Acta Microbiol. Bulg. 2016, 32, 148–149. [Google Scholar]
- Smiljković, M.; Dias, M.I.; Stojković, D.; Barros, L.; Bukvički, D.; Ferreira, I.C.F.R.; Soković, M. Characterization of phenolic compounds in tincture of edible Nepeta nuda: Development of antimicrobial mouthwash. Food Funct. 2018, 9, 5417–5425. [Google Scholar] [CrossRef]
- Cvetković, J.; Milutinović, M.; Boljević, J.; Aničić, N.; Živković, N.; Živković, S.; Mišić, D. Paraquat-Mediated Oxidative Stress in Nepeta pannonica L. Bot. Serb. 2015, 39, 121–128. Available online: https://botanicaserbica.bio.bg.ac.rs/arhiva/pdf/2015_39_2_634_full.pdf (accessed on 17 November 2021).
- Aras, A.; Dogru, M.; Bursal, E. Determination of antioxidant potential of Nepeta nuda subsp. lydiae. Anal. Chem. Lett. 2016, 6, 758–765. [Google Scholar] [CrossRef]
- Aras, A.; Bursal, E.; Dogru, M. UHPLC-ESIMS/MS analyses for quantification of phenolic compounds of Nepeta nuda subsp. Lydiae. J. App. Pharm. Sci. 2016, 6, 9–13. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Eskici, M.; Karanfil, A.; Tepe, B. Phenolic profile, enzyme inhibitory and antioxidant activities of two endemic Nepeta species: Nepeta nuda subsp. glandulifera and N. cadmea. S. Afr. J. Bot. 2019, 120, 298–301. [Google Scholar] [CrossRef]
- Kabalay, B.; Mutlu, D.; Arslan, S.; Semiz, G.; Kocabıyık, K. Chemical Composition and Cytotoxicity of Nepeta nuda subsp. lydiae p. h. Davis Essential Oil towards Colon Carcinoma. In Proceedings of the 4th International Symposium EuroAsian Diversity, Kiev, Ukraine, 3–6 July 2018; p. 154. Available online: https://www.researchgate.net/publication/326462977_Chemical_composition_and_Cytotoxicity_of_Nepeta_nuda_subsp_lydiae_P_H_Davis_Essential_Oil_Towards_Colon_Carcinoma (accessed on 17 November 2021).
- Gašić, U.; Stojković, D.; Ivanov, M.; Miletić, M.; Mišić, D.; Veljić, M.; Soković, M. Water soluble biomolecules from Nepeta nuda regulate microbial growth: A case study of apple juice preservation. Lek. Sirov. 2021, 41, 35–42. Available online: https://www.lekovitesirovine.rs/ojs/index.php/lekovite/article/view/155 (accessed on 17 November 2021).
- Bozari, S.; Agar, G.; Aksakal, O.; Erturk, F.A.; Yanmis, D. Determination of chemical composition and genotoxic effects of essential oil obtained from Nepeta nuda on Zea mays seedlings. Toxicol. Ind. Health 2013, 29, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Dragoeva, A.; Stoyanova, Z.; Koleva, V.; Dragolova, D. Allelopathic activity of Nepeta nuda L. subsp. nuda water extracts. Acta Sci. Nat. 2017, 4, 46–51. [Google Scholar] [CrossRef]
- Jakšić, P.; Grozdanović, A. Contribution to knowledge of the butterflies of Mt. Rtanj Serbia, (Lepidoptera: Hesperioidae and Papilonoidae). Acta Entomol. Serbica 2007, 12, 63–72. Available online: http://www.eds.org.rs/AES/vol12-2/AES%2012_2_%2007.pdf (accessed on 17 November 2021).
- Milica, A.; Mirjana, C.; Jovana, S.J.; Lato, P.; Ana, V.; Ivana, Č.; Biljana, K. Biological activity and profiling of Salvia sclarea essential oil obtained by steam and hydrodistillation extraction methods via chemometrics tools. Flavour Fragr. J. 2022, 37, 20–32. [Google Scholar] [CrossRef]
- Aćimović, M.; Pezo, L.; Zeremski, T.; Lončar, B.; MarjanovićJeromela, A.; Stanković Jeremić, J.; Cvetković, M.; Sikora, V.; Ignjatov, M. Weather conditions influence on Hyssop essential oil quality. Processes 2021, 9, 1152. [Google Scholar] [CrossRef]
- Aćimović, M.; Ljujić, J.; Vulić, J.; Zheljazkov, V.D.; Pezo, L.; Varga, A.; Tumbas Šaponjac, V. Helichrysum italicum (Roth) G. Don essential oil from Serbia: Chemical composition, classification and biological activity—May it be a suitable new crop for Serbia? Agronomy 2021, 11, 1282. [Google Scholar] [CrossRef]
- Aćimović, M.; Ivanović, S.; Simić, K.; Pezo, L.; Zeremski, T.; Ovuka, J.; Sikora, V. Chemical characterization of Marrubium vulgare volatiles from Serbia. Plants 2021, 10, 600. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).