Determination of Phenolic Compounds Using HPLC-UV Method in Wild Fruit Species
Abstract
:1. Introduction
2. Materials and Methods
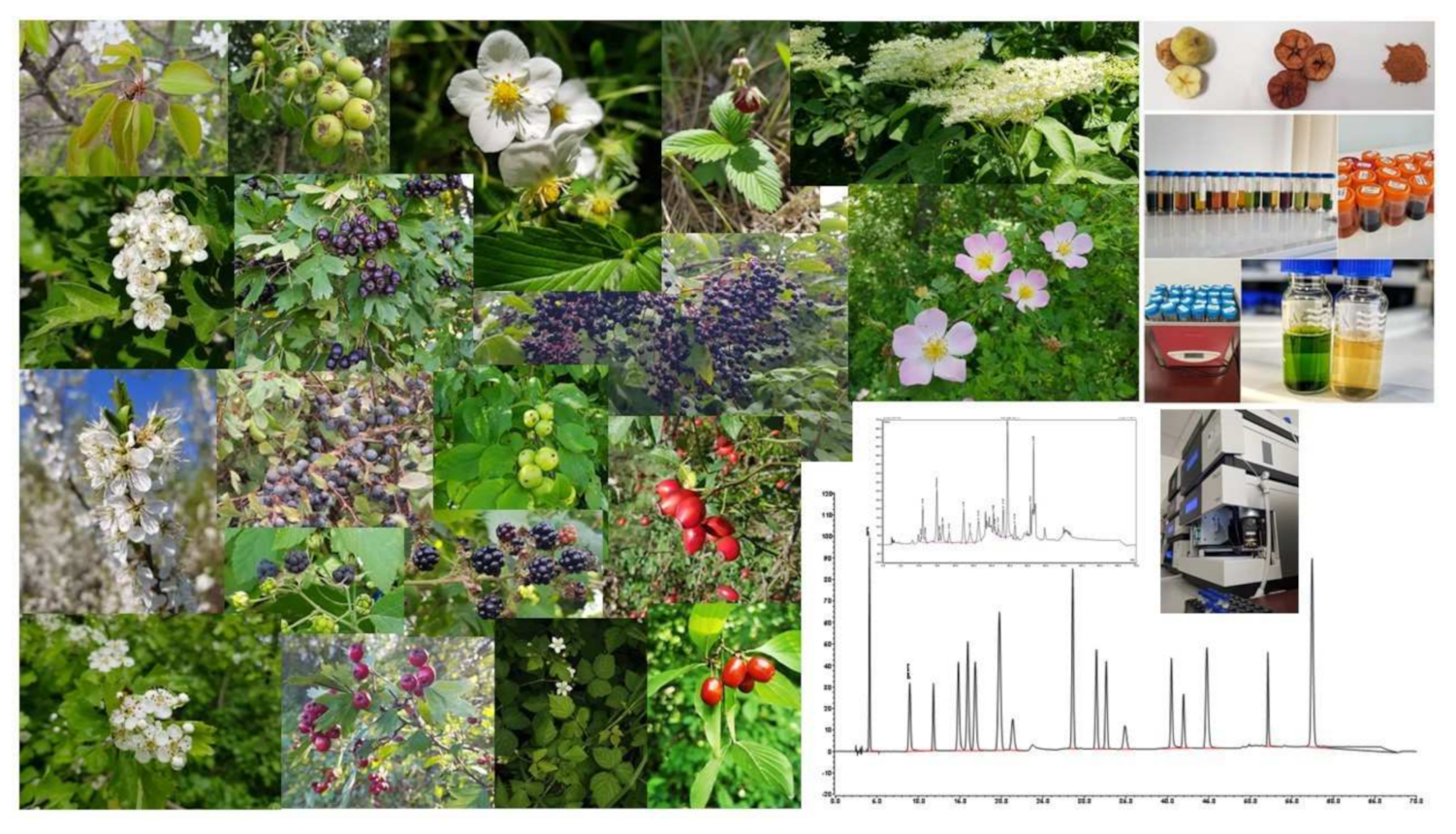
2.1. Wild Fruit Species Samples
2.2. Sample Preparation
2.3. HPLC Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.; Barros, L.; Ferreira, I.C. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Fernández-Ruiz, V.; Morales, P.; Ruiz-Rodríguez, B.M.; TorijaIsasa, E. Nutrients and bioactive compounds in wild fruits through different continents. In Wild Plants, Mushrooms and Nuts: Functional Food Properties and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 263–314. [Google Scholar]
- Pieroni, A.; Nedelcheva, A.; Dogan, Y. Local knowledge of medicinal plants and wild food plants among Tatars and Romanians in Dobruja (South-East Romania). Genet. Resour. Crop Evol. 2015, 62, 605–620. [Google Scholar] [CrossRef]
- Lattanzio, F.; Greco, E.; Carretta, D.; Cervellati, R.; Govoni, P.; Speroni, E. In vivo anti-inflammatory effect of Rosa canina L. extract. J. Ethnopharmacol. 2011, 137, 880–885. [Google Scholar] [CrossRef]
- Kirkeskov, B.; Christensen, R.; Bügel, S.; Bliddal, H.; Danneskiold-Samsøe, B.; Christensen, L.P.; Andersen, J.R. The effects of rose hip (Rosa canina) on plasma antioxidative activity and C-reactive protein in patients with rheumatoid arthritis and normal controls: A prospective cohort study. Phytomedicine 2011, 18, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Bedreag, C.F.G.; Trifan, A.; Bucur, L.A.; Arcus, M.; Tebrencu, C.; Miron, A.; Costache, I.I. Chemical and antioxidant studies on Crataegus pentagyna leaves and flowers. Rom. Biotechnol. Lett. 2014, 19, 9859. [Google Scholar]
- Chang, Q.; Zuo, Z.; Harrison, F.; Chow, M.S.S. Hawthorn. J. Clin. Pharmacol. 2002, 42, 605–612. [Google Scholar] [CrossRef]
- Sikora, E.; Bieniek, M.I.; Borczak, B. Composition and antioxidant properties of fresh and frozen stored blackthorn fruits (Prunus spinosa L.). Acta Sci. Pol. Technol. Aliment. 2013, 12, 365–372. [Google Scholar]
- Guarrera, P.M. Food medicine and minor nourishment in the folk traditions of Central Italy (Marche, Abruzzo and Latium). Fitoterapia 2003, 74, 515–544. [Google Scholar] [CrossRef]
- Mihailović, N.R.; Mihailović, V.B.; Kreft, S.; Ćirić, A.R.; Joksović, L.G.; Đurđević, P.T. Analysis of phenolics in the peel and pulp of wild apples (Malus sylvestris (L.) Mill.). J. Food Compost. Anal. 2018, 67, 1–9. [Google Scholar] [CrossRef]
- Savić, A.; Alimpić Aradski, A.; Duletić Laušević, S.; Marin, P. Antioxidant activity, phenolic and flavonoid contents of methanol extract of Pyrus pyraster fruit. In Proceedings of the 6th Balkan Botanical Congress, Rijeka, Croatia, 14–18 September 2015; Available online: http://hdl.handle.net/123456789/2133 (accessed on 10 October 2021).
- Egea, T.; Signorini, M.A.; Bruschi, P.; Rivera, D.; Obón, C.; Alcaraz, F.; Palazón, J.A. Spirits and liqueurs in European traditional medicine: Their history and ethnobotany in Tuscany and Bologna (Italy). J. Ethnopharmacol. 2015, 175, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Güler, B.; Manav, E.; Uğurlu, E. Medicinal plants used by traditional healers in Bozüyük (Bilecik–Turkey). J. Ethnopharmacol. 2015, 173, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Leporatti, M.L.; Ivancheva, S. Preliminary comparative analysis of medicinal plants used in the traditional medicine of Bulgaria and Italy. J. Ethnopharmacol. 2003, 87, 123–142. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Cornescu, F. Variability in physical and chemical characteristics of Cornelian cherry fruits (Cornus mas L.) from Romanian Oltenia region’s spontaneous flora and role of the climatic conditions. Rev. Bras. Bot. 2020, 43, 677–682. [Google Scholar] [CrossRef]
- Cosmulescu, S.N.; Trandafir, I.; Cornescu, F. Antioxidant capacity, total phenols, total flavonoids and colour component of cornelian cherry (Cornus mas L.) wild genotypes. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 390–394. [Google Scholar] [CrossRef] [Green Version]
- Dinda, B.; Kyriakopoulos, A.M.; Dinda, S.; Zoumpourlis, V.; Thomaidis, N.S.; Velegraki, A.; Dinda, M. Cornus mas L. (cornelian cherry), an important European and Asian traditional food and medicine: Ethnomedicine, phytochemistry and pharmacology for its commercial utilization in drug industry. J. Ethnopharmacol. 2016, 193, 670–690. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Wasilewski, T.; Bujak, T.; Gaweł-Bęben, K.; Osika, P.; Czerwonka, D. Cornus mas L. extract as a multifunctional material for manufacturing cosmetic emulsions. Chin. J. Nat. Med. 2018, 16, 284–292. [Google Scholar] [CrossRef]
- Mechchate, H.; Es-Safi, I.; Haddad, H.; Bekkari, H.; Grafov, A.; Bousta, D. Combination of catechin, epicatechin, and rutin: Optimization of a novel complete antidiabetic formulation using a mixture design approach. J. Nutr. Biochem. 2021, 88, 108520. [Google Scholar] [CrossRef] [PubMed]
- Binang, K.; Takuwa, D.T. Development of reverse phase-high performance liquid chromatography (RP-HPLC) method for determination of selected antihypertensive active flavonoids (rutin, myricetin, quercetin, and kaempferol) in medicinal plants found in Botswana. Phys. Sci. Rev. 2021. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Trandafir, I.; Nour, V. Phenolic acids and flavonoids profiles of extracts from edible wild fruits and their antioxidant properties. Int. J. Food Prop. 2017, 20, 3124–3134. [Google Scholar] [CrossRef] [Green Version]
- Shebeko, S.K.; Zupanets, I.A.; Popov, O.S.; Tarasenko, O.O.; Shalamay, A.S. Effects of quercetin and its combinations on health. In Polyphenols: Mechanisms of Action in Human Health and Disease; Academic Press: Cambridge, MA, USA, 2018; pp. 373–394. [Google Scholar]
- Abdulkhaleq, L.A.; Assi, M.A.; Noor, M.H.M.; Abdullah, R.; Saad, M.Z.; Taufiq-Yap, Y.H. Therapeutic uses of epicatechin in diabetes and cancer. Vet. World 2017, 10, 869–872. [Google Scholar] [CrossRef]
- Veličković, J.M.; Kostić, D.A.; Stojanović, G.S.; Mitić, S.S.; Mitić, M.N.; Ranđelović, S.S.; Đorđević, A.S. Phenolic composition, antioxidant and antimicrobial activity of the extracts from Prunus spinosa L. fruit. Hem. Ind. 2014, 68, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Viapiana, A.; Wesolowski, M. The phenolic contents and antioxidant activities of infusions of Sambucus nigra L. Plant Foods Hum. Nutr. 2017, 72, 82–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawidowicz, A.L.; Wianowska, D.; Baraniak, B. The antioxidant properties of alcoholic extracts from Sambucus nigra L. (antioxidant properties of extracts). Food Sci. Technol. 2006, 39, 308–315. [Google Scholar] [CrossRef]
- Contardi, M.; Lenzuni, M.; Fiorentini, F.; Summa, M.; Bertorelli, R.; Suarato, G.; Athanassiou, A. Hydroxycinnamic acids and derivatives formulations for skin damages and disorders: A review. Pharmaceutics 2021, 13, 999. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Bishayee, A. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran J. Basic Med. Sci. 2019, 22, 225. [Google Scholar] [PubMed]
- Oszmiański, J.; Wojdyło, A.; Nowicka, P.; Teleszko, M.; Cebulak, T.; Wolanin, M. Determination of phenolic compounds and antioxidant activity in leaves from wild Rubus L. species. Molecules 2015, 20, 4951–4966. [Google Scholar] [CrossRef] [Green Version]
- Andreotti, C.; Costa, G.; Treutter, D. Composition of phenolic compounds in pear leaves as affected by genetics, ontogenesis and the environment. Sci. Hortic. 2006, 109, 130–137. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Zhou, B.; Gao, W.; Cao, J.; Huang, L. Chemical composition and antioxidant and anti-inflammatory potential of peels and flesh from 10 different pear varieties (Pyrus spp.). Food Chem. 2014, 152, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Demir, N.; Yildiz, O.; Alpaslan, M.; Hayaloglu, A.A. Evaluation of volatiles, phenolic compounds and antioxidant activities of rose hip (Rosa L.) fruits in Turkey. LWT Food Sci. Technol. 2014, 57, 126–133. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Vasilieva, A.G.; Chirikova, N.K. Fragaria viridis fruit metabolites: Variation of LC-MS profile and antioxidant potential during ripening and storage. Pharmaceuticals 2020, 13, 262. [Google Scholar] [CrossRef] [PubMed]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Kumar, C.S. Syringic acid (SA)—A review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Nićiforović, N.; Abramovič, H. Sinapic acid and its derivatives: Natural sources and bioactivity. Compr. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef] [PubMed]
| Species/Part | Flavonoids mg/100 g DW * | ||||||
|---|---|---|---|---|---|---|---|
| C ** | EC | RUT | MYR | QCT | KP | ||
| Malus sylvestris | Fr | 144.62 ± 21.36 | n.d. | n.d. | 46.22 ± 2.64 | n.d. | n.d. |
| Lv | 19.42 ± 1.14 | n.d. | 351.15 ± 19.15 | 232.36 ± 14.25 | n.d. | 14.52 ± 0.10 | |
| Pyrus pyraster | Fr | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Lv | 44.93 ± 3.25 | 8.64 ± 0.56 | 231.88 ± 10.24 | 13.29 ± 1.10 | n.d. | n.d. | |
| Rosa canina | Fr | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Lv | 545.16 ± 15.47 | n.d. | 565.29 ± 26.10 | 88.90 ± 4.30 | n.d. | n.d. | |
| Fl | 158.29 ± 11.36 | 48.26 ± 2.65 | 420.09 ± 16.28 | 57.01 ± 2.48 | n.d. | 4.68 ± 0.05 | |
| Crataegus pentagyna | Fr | 13.63 ± 1.20 | 37.28 ± 1.25 | 34.56 ± 1.10 | 26.25 ± 1.25 | n.d. | n.d. |
| Lv | 8.59 ± 0.50 | n.d. | 233.65 ± 10.47 | 53.02 ± 1.70 | n.d. | n.d. | |
| Fl | 225.10 ± 21.42 | n.d. | 140.28 ± 14.84 | 247.69 ± 14.56 | n.d. | n.d. | |
| Crataegus monogyna | Fr | 31.18 ± 4.09 | 143.25 ± 10.64 | 43.50 ± 2.25 | 6.43 ± 0.50 | n.d. | 5.12 ± 0.05 |
| Lv | 62.40 ± 6.49 | n.d. | 153.19 ± 4.56 | 17.21 ± 0.80 | n.d. | n.d. | |
| Fl | 69.38 ± 6.23 | n.d. | 49.27 ± 2.31 | 35.34 ± 2.84 | n.d. | n.d. | |
| Sambucus nigra | Fr | n.d. | n.d. | 270.29 ± 15.70 | 10.92 ± 0.64 | 8.11 ± 0.10 | n.d. |
| Lv | 238.25 ± 17.45 | 5.49 ± 0.01 | 131.82 ± 8.45 | 10.98 ± 0.24 | n.d. | n.d. | |
| Fl | 87.76 ± 4.58 | n.d. | 769.10 ± 21.84 | 147.91 ± 12.35 | n.d. | n.d. | |
| Rubus caesius | Fr | n.d. | 26.07 ± 2.63 | 3.04 ± 0.10 | n.d. | n.d. | n.d. |
| Lv | 246.99 ± 10.20 | 16.07 ± 0.45 | 2.79 ± 0.01 | 50.57 ± 1.35 | n.d. | n.d. | |
| Fl | 34.36 ± 4.26 | 16.15 ± 1.84 | n.d. | 30.30 ± 1.28 | 18.68 ± 1.49 | n.d. | |
| Rubus fruticosus | Fr | n.d. | 5.80 ± 0.54 | n.d. | n.d. | n.d. | n.d. |
| Lv | 50.06 ± 2.15 | 14.48 ± 0.21 | 64.29 ± 2.31 | 12.47 ± 0.70 | n.d. | n.d. | |
| Fl | 221.27 ± 19.87 | 88.79 ± 1.26 | 32.37 ± 1.64 | 51.32 ± 2.84 | n.d. | n.d. | |
| Fragaria viridis | Fr | 1.27 ± 0.25 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Lv | 311.76 ± 21.35 | 778.11 ± 15.15 | 242.36 ± 14.20 | 66.98 ± 2.15 | n.d. | n.d. | |
| Fl | 25.85 ± 2.54 | 120.03 ± 10.64 | n.d. | 7.50 ± 0.03 | n.d. | n.d. | |
| Cornus mas | Fr | 268.16 ± 16.97 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Lv | 105.57 ± 9.10 | 13.98 ± 0.24 | 191.81 ± 21.10 | 27.28 ± 1.15 | n.d. | n.d. | |
| Prunus spinosa | Fr | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Lv | 205.93 ± 11.48 | n.d. | 114.52 ± 7.15 | 127.62 ± 9.46 | n.d. | n.d. | |
| Fl | 494.74 ± 42.15 | n.d. | 14.50 ± 0.67 | 6.15 ± 0.10 | 32.56 ± 1.65 | 5.64 ± 0.08 | |
| Bd | n.d. | 18.26 ± 14.23 | n.d. | 56.70 ± 1.23 | 99.19 ± 4.37 | n.d. | |
| Species/Part | Phenolic Acids mg/100 g DW * | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GA ** | NCHA | CHA | VA | CFA | SYA | pCA | FA | SA | SAA | EA | ||
| Malus sylvestris | Fr | 91.59 ± 1.20 | n.d. | 249.78 ± 32.51 | n.d. | n.d. | n.d. | 0.63 ± 0.01 | n.d. | n.d. | n.d. | n.d. |
| Lv | n.d. | n.d. | 10.58 ± 0.50 | 4.88 ± 0.01 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 2537.69 ± 40.2 | |
| Pyrus pyraster | Fr | 23.91 ± 1.14 | n.d. | 50.70 ± 4.15 | n.d. | n.d. | n.d. | 0.22 ± 0.01 | n.d. | n.d. | n.d. | 4.78 ± 0.23 |
| Lv | 87.55 ± 7.20 | 3.07 ± 0.01 | 656.78 ± 25.40 | 13.69 ± 0.61 | 4.35 ± 0.01 | n.d. | 5.33 ± 0.01 | n.d. | 135.48 ± 4.2 | 5.52 ± 0.07 | 3.79 ± 0.01 | |
| Rosa canina | Fr | 66.62 ± 5.24 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 9.14 ± 0.10 |
| Lv | n.d. | 88.65 ± 4.52 | 198.00 ± 10.67 | 39.23 ± 1.48 | 0.32 ± 0.01 | n.d. | 21.14 ±0.05 | 96.22 ± 5.2 | 279.92 ± 7.6 | 6.62 ± 0.04 | 293.39 ± 24.50 | |
| Fl | 23.77 ± 1.14 | 261.61 ± 20.4 | 84.25 ± 6.45 | 17.73 ± 1.57 | 29.40 ± 1.64 | n.d. | 66.01 ± 4.34 | 103.07 ± 0.47 | 25.47 ± 2.84 | 639.97 ± 10.25 | 278.17 ± 11.23 | |
| Crataegus pentagyna | Fr | 0.43 ± 0.01 | 95.57 ± 9.12 | 41.66 ± 2.65 | n.d. | 35.5 ± 2.48 | n.d. | 0.40 ± 0.01 | 0.65 ± 0.01 | n.d. | 9.79 ± 0.45 | 33.56 ± 2.30 |
| Lv | n.d. | 9.14 ± 1.23 | 214.73 ± 11.11 | 16.38 ± 1.12 | 51.60 ± 4.26 | n.d. | 3.74 ± 0.01 | 28.87 ± 1.14 | n.d. | 864.30 ± 26.12 | 85.93 ± 4.62 | |
| Fl | 1.86 ± 0.01 | 633.19 ± 35.1 | 246.92 ± 15.23 | n.d. | 50.86 ± 2.27 | n.d. | 0.33 ± 0.01 | n.d. | n.d. | 90.79 ± 5.27 | n.d. | |
| Crataegus monogyna | Fr | 7.92 ± 0.56 | 10.54 ± 1.21 | 41.75 ± 3.78 | 0.21 ± 0.01 | n.d. | n.d. | n.d. | n.d. | n.d. | 22.70 ± 1.26 | 107.76 ± 9.45 |
| Lv | n.d. | 37.55 ± 2.30 | 508.32 ± 24.74 | 11.86 ± 0.70 | 24.80 ± 1.31 | n.d. | 28.18 ± 1.45 | 55.07 ± 6.21 | n.d. | 250.90 ± 14.52 | 8.58 ± 0.16 | |
| Fl | 4.15 ± 0.23 | 203.90 ± 15.2 | 226.33 ± 18.31 | n.d. | 30.06 ± 1.94 | n.d. | n.d. | 0.09 ± 0.01 | n.d. | 0.36 ± 0.01 | n.d. | |
| Sambucus nigra | Fr | 122.14 ± 14.8 | n.d. | 4.38 ± 0.35 | 1.80 ± 0.01 | 5.07 ± 0.25 | n.d. | n.d. | 7.45 ± 0.60 | n.d. | n.d. | n.d. |
| Lv | n.d. | n.d. | 151.50 ± 10.50 | 4.84 ± 0.10 | 11.67 ± 0.97 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Fl | 6.95 ± 0.56 | 99.64 ± 5.28 | 807.42 ± 20.87 | n.d. | 11.44 ± 0.84 | n.d. | n.d. | 3.56 ± 0.21 | n.d. | 303.13 ± 8.64 | 26.90 ± 1.17 | |
| Rubus caesius | Fr | 25.15 ± 2.73 | n.d. | n.d. | n.d. | 1.24 ± 0.02 | n.d. | 9.55 ± 0.21 | 8.17 ± 0.51 | n.d. | n.d. | n.d. |
| Lv | 42.35 ± 3.25 | 20.74 ± 1.27 | n.d. | n.d. | 6.20 ± 0.52 | n.d. | n.d. | 7.24 ± 1.01 | n.d. | n.d. | 91.21 ± 4.02 | |
| Fl | n.d. | 12.18 ± 1.26 | n.d. | 1.33 ± 0.02 | 7.11 ± 0.64 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Rubus fruticosus | Fr | 58.28 ± 6.12 | 16.48 ± 2.15 | n.d. | 17.32 ± 0.23 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Lv | 21.06 ± 1.61 | 78.63 ± 6.45 | 21.06 ± 1.61 | 78.63 ± 6.45 | 21.06 ± 1.61 | 78.63 ± 6.4 | 29.04 ± 1.10 | 5.89 ± 0.01 | n.d. | n.d. | 43.68 ± 1.00 | |
| Fl | 2.68 ± 0.02 | 141.02 ± 13.8 | n.d. | n.d. | 25.77 ± 0.36 | n.d. | 17.76 ± 0.56 | 19.54 ± 1.43 | n.d. | n.d. | 9.61 ± 0.56 | |
| Fragaria viridis | Fr | 122.02 ± 18.4 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 351.96 ± 20.05 |
| Lv | 57.65 ± 4.23 | 9.30 ± 1.31 | 23.46 ± 1.15 | 15.18 ± 0.64 | n.d. | 131.01 ± 9.4 | 67.99 ± 4.2 | 86.46 ± 4.6 | 121.69 ± 9.1 | 140.19 ± 10.2 | 549.58 ± 41.2 | |
| Fl | 5.16 ± 0.68 | 5.08 ± 0.25 | n.d. | n.d. | n.d. | 29.05 ± 1.2 | n.d. | 229.79 ± 2.42 | n.d. | 10.60 ± 1.25 | 8.61 ± 0.98 | |
| Cornus mas | Fr | 76.47 ± 11.10 | 16.93 ± 2.63 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 175.15 ± 15.30 |
| Lv | 145.84 ± 10.4 | 40.45 ± 2.43 | 31.54 ± 1.46 | 95.22 ± 2.48 | 77.16 ± 1.64 | n.d. | 6.23 ± 0.01 | n.d. | n.d. | n.d. | 47.38 ± 12.21 | |
| Prunus spinosa | Fr | 109.06 ± 20.2 | 34.20 ± 4.52 | n.d. | n.d. | 12.20 ± 0.94 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Lv | n.d. | 62.98 ± 5.87 | 310.98 ± 18.24 | 30.99 ± 0.50 | 5.35 ± 0.09 | n.d. | 8.86 ± 0.09 | 12.15 ± 1.12 | n.d. | n.d. | 16.25 ± 1.24 | |
| Fl | 3.99 ± 0.74 | 151.68 ± 10.8 | 583.39 ± 14.69 | n.d. | n.d. | n.d. | n.d. | 26.48 ± 3.14 | 0.09 ± 0.01 | 6.16 ± 0.84 | n.d. | |
| Bd | 4.11 ± 0.21 | n.d. | 19.10 ± 2.13 | n.d. | n.d. | n.d. | 0.75 ± 0.01 | 16.24 ± 2.49 | n.d. | 10.85 ± 0.67 | n.d. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoenescu, A.-M.; Trandafir, I.; Cosmulescu, S. Determination of Phenolic Compounds Using HPLC-UV Method in Wild Fruit Species. Horticulturae 2022, 8, 84. https://doi.org/10.3390/horticulturae8020084
Stoenescu A-M, Trandafir I, Cosmulescu S. Determination of Phenolic Compounds Using HPLC-UV Method in Wild Fruit Species. Horticulturae. 2022; 8(2):84. https://doi.org/10.3390/horticulturae8020084
Chicago/Turabian StyleStoenescu, Ana-Maria, Ion Trandafir, and Sina Cosmulescu. 2022. "Determination of Phenolic Compounds Using HPLC-UV Method in Wild Fruit Species" Horticulturae 8, no. 2: 84. https://doi.org/10.3390/horticulturae8020084
APA StyleStoenescu, A.-M., Trandafir, I., & Cosmulescu, S. (2022). Determination of Phenolic Compounds Using HPLC-UV Method in Wild Fruit Species. Horticulturae, 8(2), 84. https://doi.org/10.3390/horticulturae8020084







