Response of Tomato-Pseudomonas Pathosystem to Mild Heat Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Temperature Assay
2.2. Biomass and Chlorophyll Content
2.3. P. syringae Bioassay
2.4. Chromatographic Analysis
2.5. Gene Expression
2.6. Bacterial Acclimation Assays
2.7. Bacterial Mutants Growth Assay
2.8. Swimming Assays
2.9. Statistical Analysis
3. Results
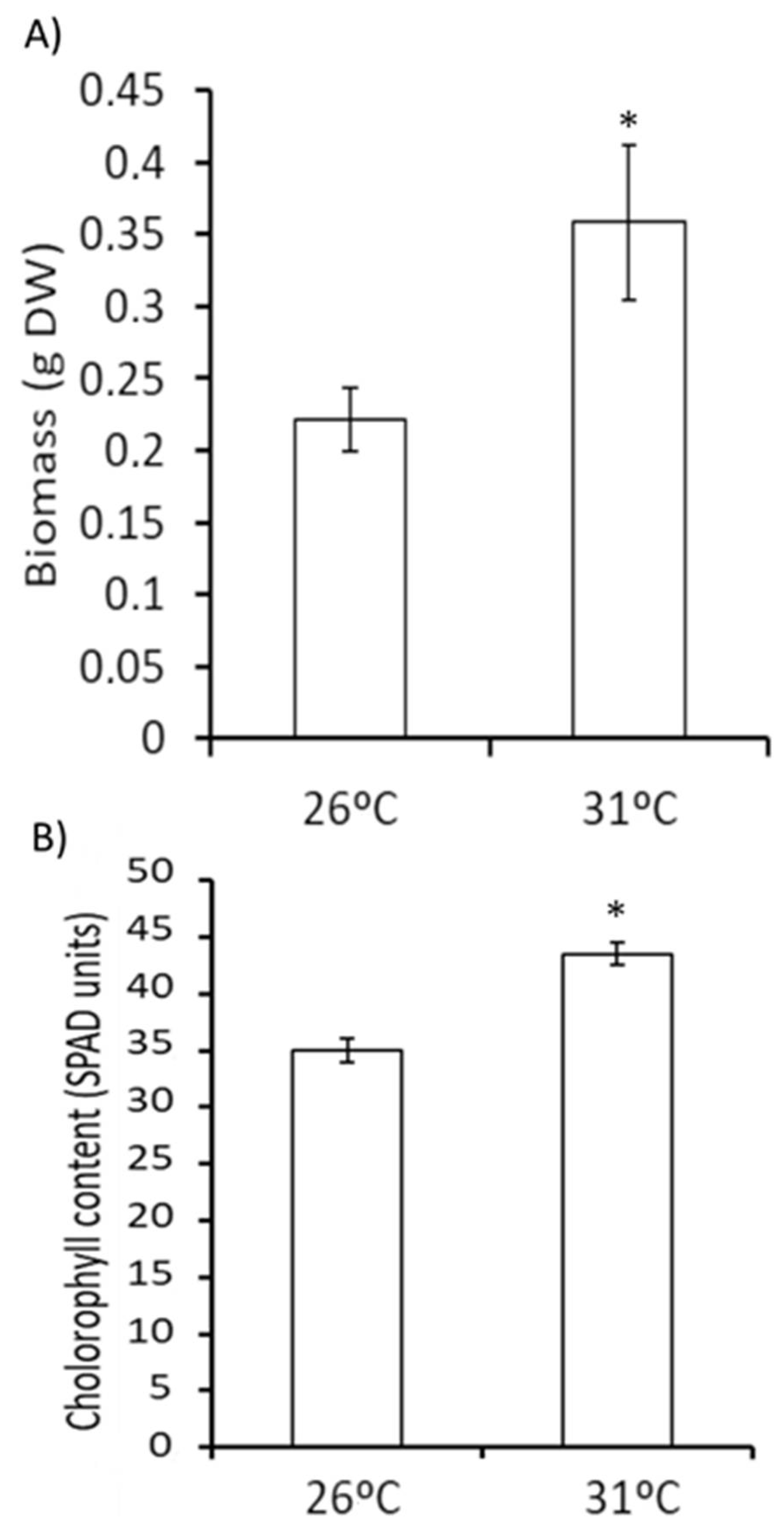
3.1. Effect of Moderate Temperature Stress on the Growth of Tomato Plants
3.2. Changes in the Amino Acid Profile as a Result of Mild Heat Stress
3.3. Effect of Mild Heat Stress on a Tomato–PstDC3000 Pathosystem
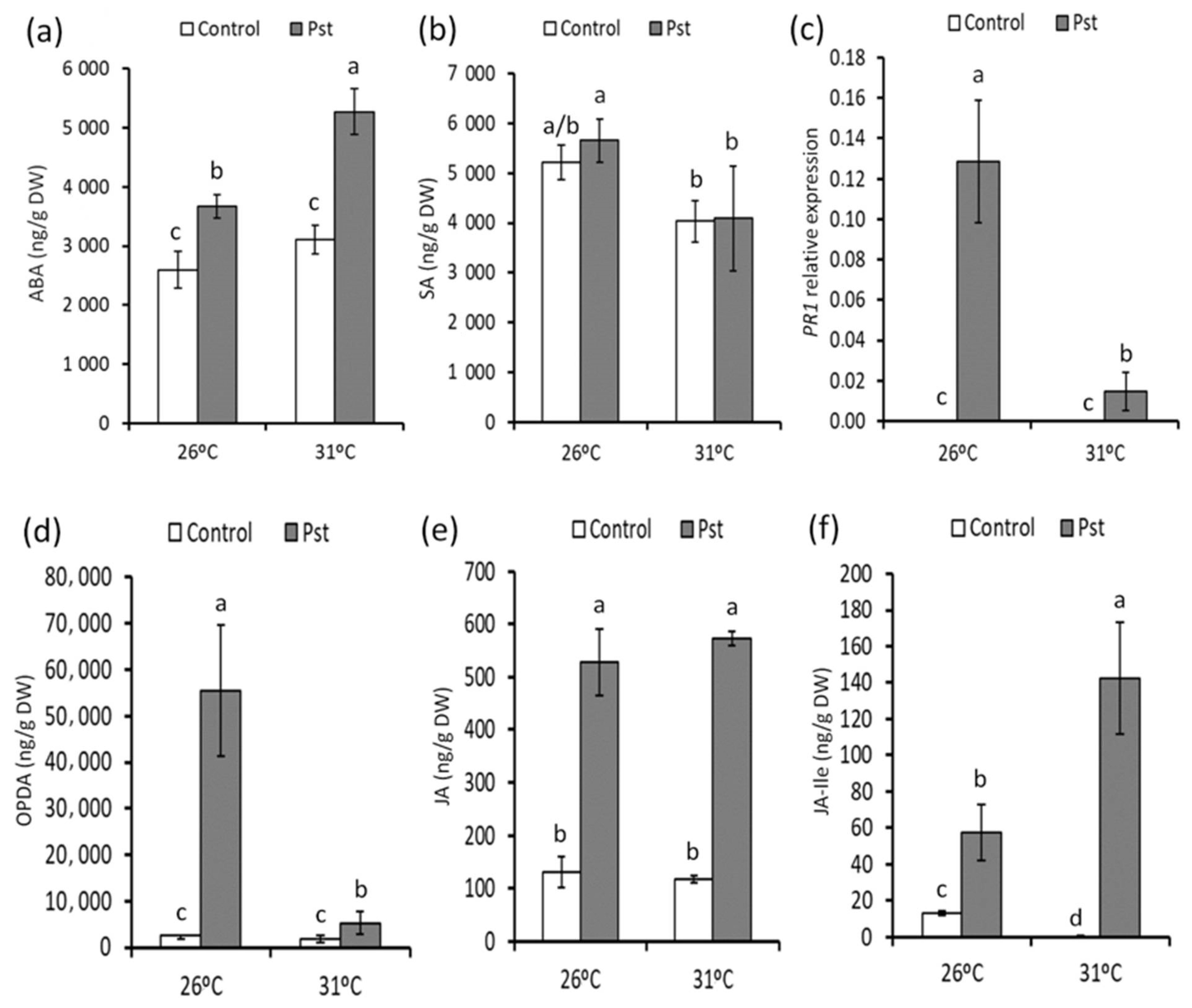
3.4. Changes in the Hormonal Response against PstDC3000 from Increased Temperatures
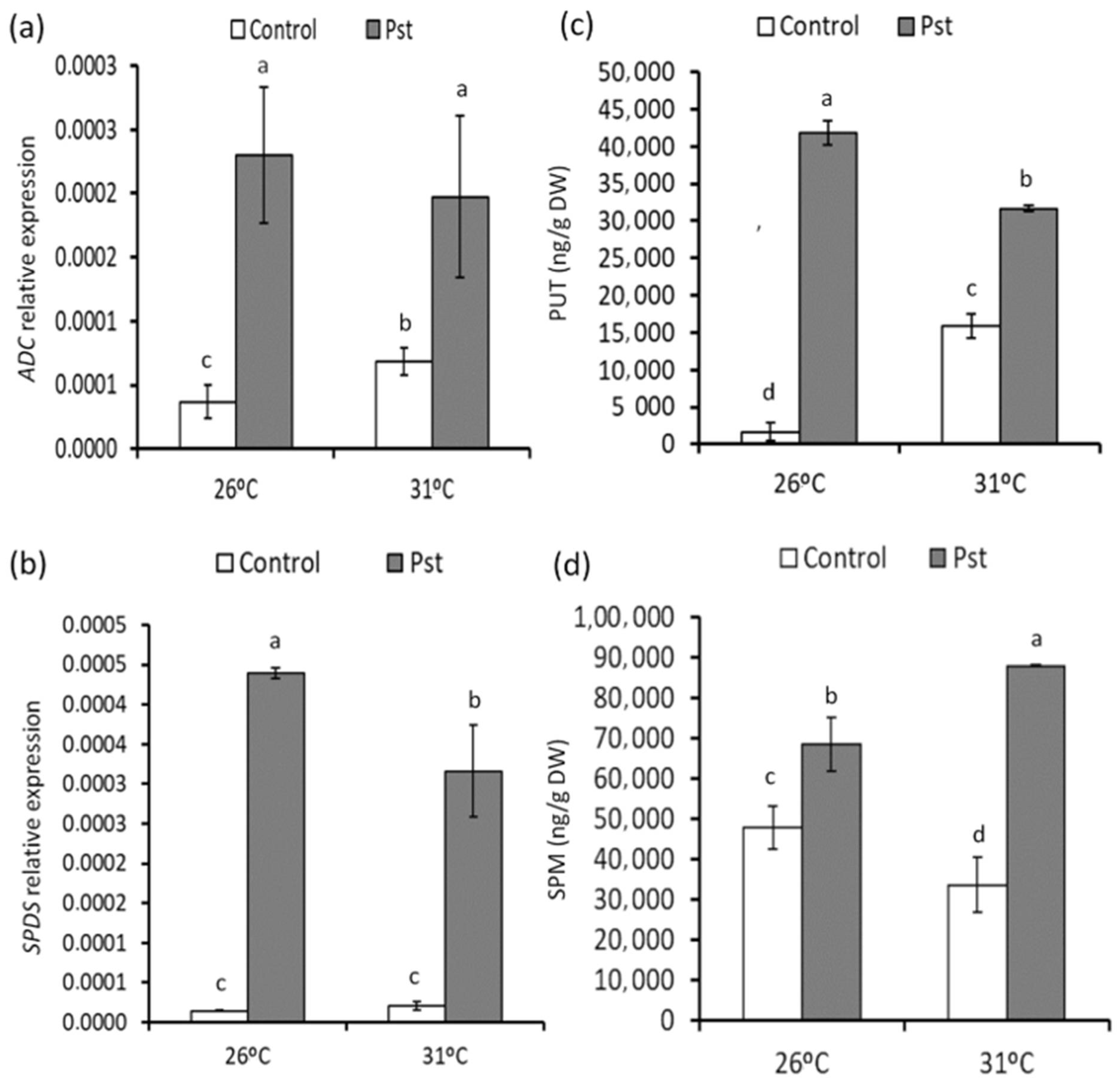
3.5. Changes in PA Accumulation in Healthy and Infected Plants from Mild Heat Stress
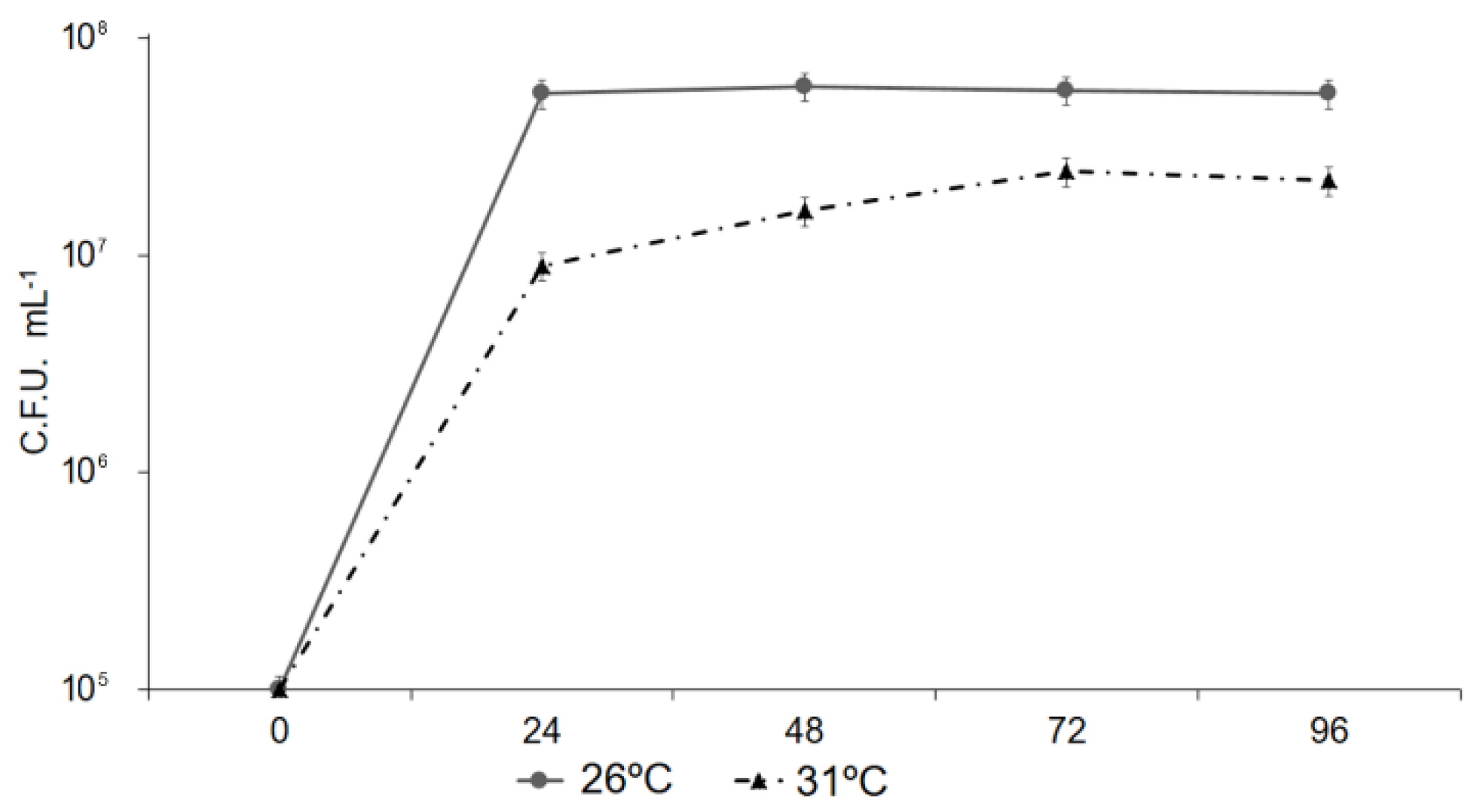
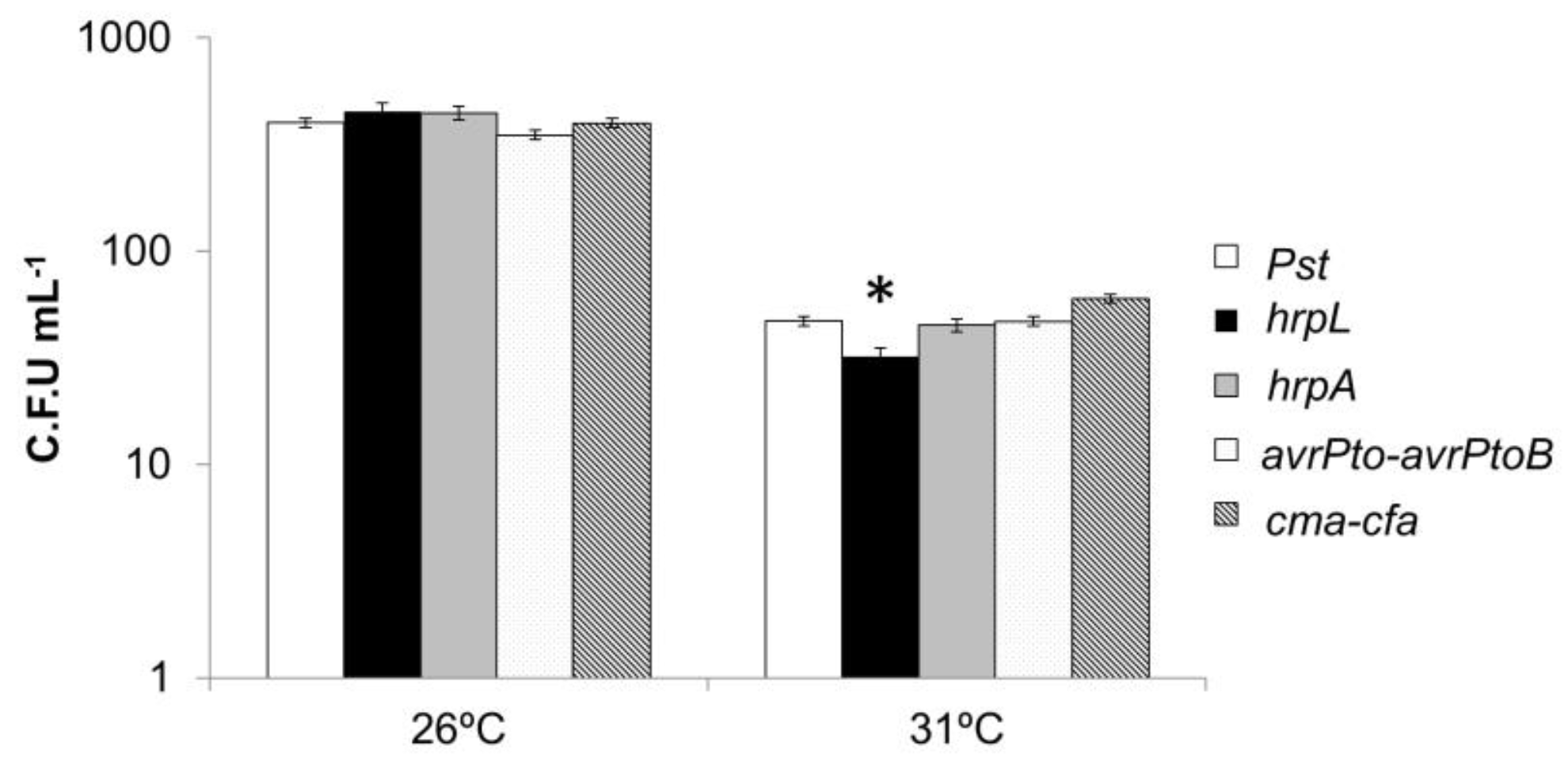
3.6. Effect of Mild Heat Stress on the Virulence of Pseudomonas in Plants
3.7. Response of Pseudomonas to Mild Temperatures In Vitro
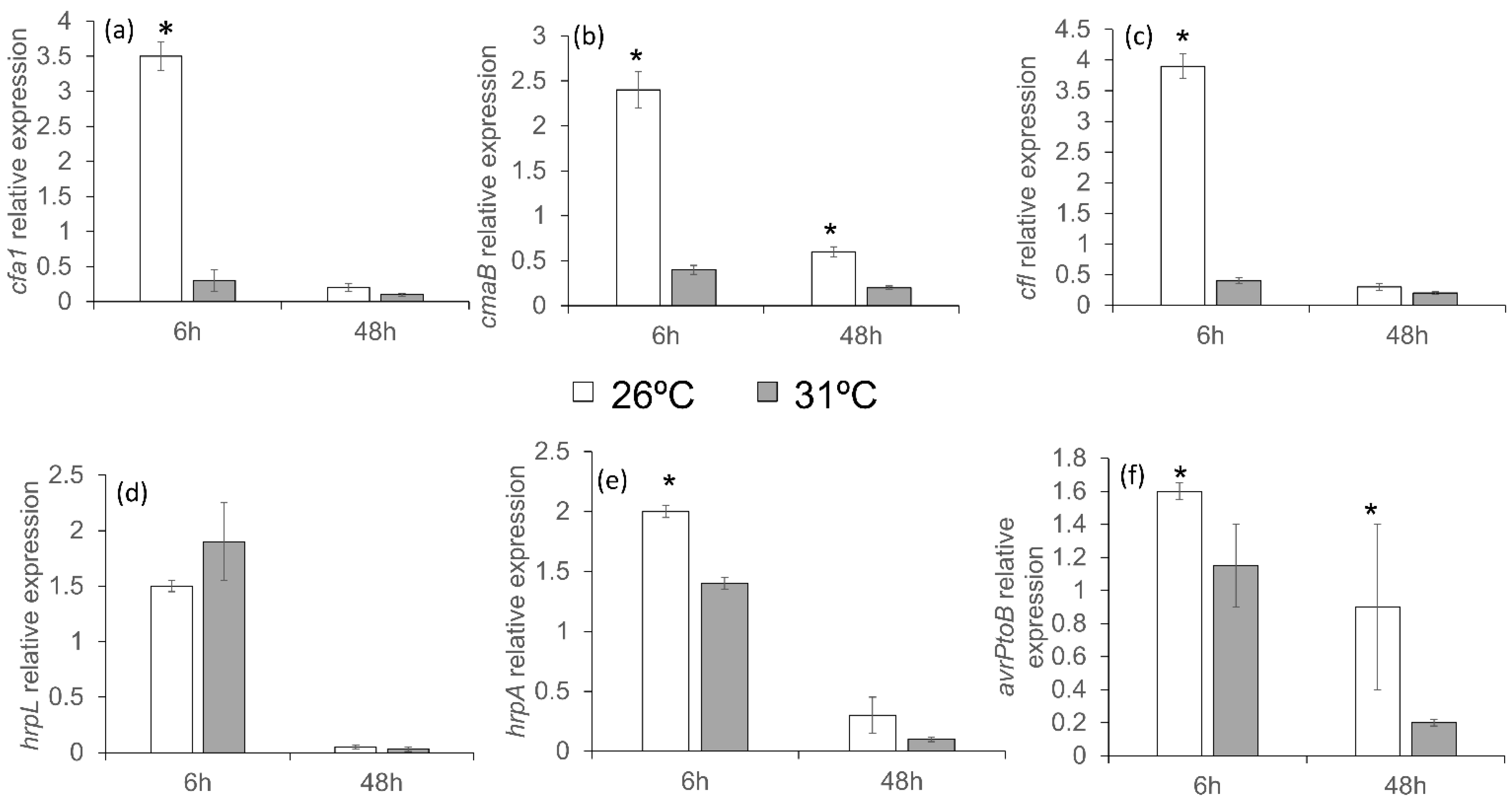
3.8. Response of Pseudomonas Mutants in Virulence Mechanisms to High Temperatures In Vitro
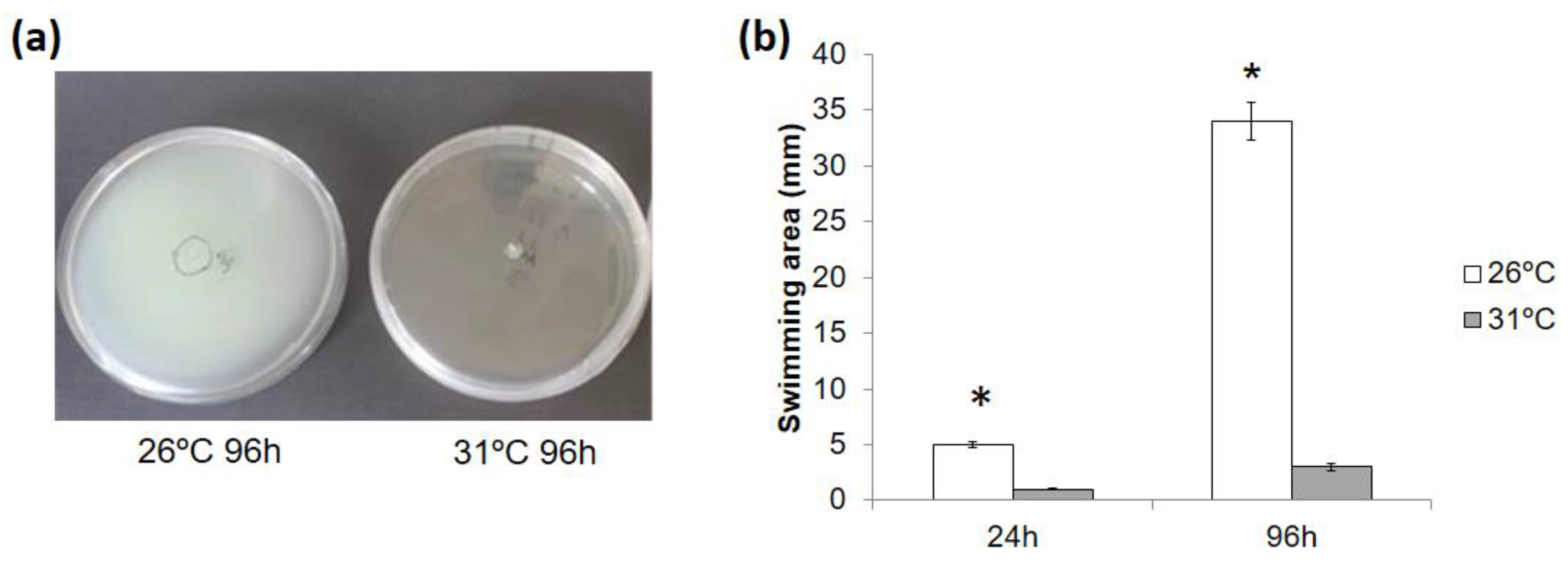
3.9. Mild Heat Stress Affected PstDC3000 Mobility In Vitro
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.T.; Yao, Y.T.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef] [PubMed]
- Field, C.B.; Barros, V.R.; Dokken, D.J.; Mach, K.J.; Mastrandrea, M.D.; Bilir, T.E. Cambio Climático 2014 Impactos, Adaptación y Vulnerabilidad; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Guy, C. Molecular responses of plants to cold shock and cold acclimation. J. Mol. Microbiol. Biotechnol. 1999, 1, 231–242. [Google Scholar] [PubMed]
- Mazzeo, M.F.; Cacace, G.; Iovieno, P.; Massarelli, I.; Grillo, S.; Siciliano, R.A. Response mechanisms induced by exposure to high temperature in anthers from thermo-tolerant and thermo-sensitive tomato plants: A proteomic perspective. PLoS ONE 2018, 13, e0201027. [Google Scholar] [CrossRef]
- Casal, J.J.; Balasubramanian, S. Thermomorphogenesis. Annu. Rev. Plant Biol. 2019, 70, 321–346. [Google Scholar] [CrossRef]
- Quint, M.; Delker, C.; Franklin, K.A.; Wigge, P.; Halliday, K.; Van Zanten, M. Molecular and genetic control of plant thermomorphogenesis. Nat. Plants 2016, 2, 15190. [Google Scholar] [CrossRef]
- Baniwal, S.K.; Chan, K.Y.; Scharf, K.-D.; Nover, L. Role of Heat Stress Transcription Factor HsfA5 as Specific Repressor of HsfA4. J. Biol. Chem. 2007, 282, 3605–3613. [Google Scholar] [CrossRef]
- Kotak, S.; Vierling, E.; Bäumlein, H.; von Koskull-Döring, P. A Novel Transcriptional Cascade Regulating Expression of Heat Stress Proteins during Seed Development of Arabidopsis. Plant Cell 2007, 19, 182–195. [Google Scholar] [CrossRef]
- Nievola, C.C.; Carvalho, C.P.; Carvalho, V.; Rodrigues, E. Rapid responses of plants to temperature changes. Temperature 2017, 4, 371–405. [Google Scholar] [CrossRef]
- Iba, K. Acclimative response to temperature stress in higher plants: Approaches of Gene Engineering for Temperature Tolerance. Annu. Rev. Plant Biol. 2002, 53, 225–245. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Sung, D.-Y.; Kaplan, F.; Lee, K.-J.; Guy, C.L. Acquired tolerance to temperature extremes. Trends Plant Sci. 2003, 8, 179–187. [Google Scholar] [CrossRef]
- Mirzaei, M.; Pascovici, D.; Atwell, B.J.; Haynes, P.A. Differential regulation of aquaporins, small GTPases and V-ATPases proteins in rice leaves subjected to drought stress and recovery. Proteomics 2012, 12, 864–877. [Google Scholar] [CrossRef] [PubMed]
- Anwar Hossain, M.; Hoque, M.A.; Burritt, D.J.; Fujita, M. Proline Protects Plants against Abiotic Oxidative Stress: Biochemical and Molecular Mechanisms. In Oxidative Damage to Plants: Antioxidant Networks and Signaling; Elsevier: Amsterdam, The Netherlands, 2014; pp. 477–522. [Google Scholar]
- Francl, L.J. The Disease Triangle: A Plant Pathological Paradigm Revisited. Plant Health Instr. 2001, 10. [Google Scholar] [CrossRef]
- Spoel, S.H.; Dong, X. How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 2012, 12, 89–100. [Google Scholar] [CrossRef]
- Zhou, J.M.; Zhang, Y. Plant Immunity: Danger Perception and Signaling. Cell 2020, 181, 978–989. [Google Scholar] [CrossRef]
- Beckers, G.J.M.; Spoel, S.H. Fine-Tuning Plant Defence Signalling: Salicylate versus Jasmonate. Plant Biol. 2006, 8, 1–10. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C.M. Hormonal Modulation of Plant Immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- Bürger, M.; Chory, J. Stressed Out About Hormones: How Plants Orchestrate Immunity. Cell Host Microbe 2019, 14, 163–172. [Google Scholar] [CrossRef]
- Takahashi, T.; Kakehi, J.-I. Polyamines: Ubiquitous polycations with unique roles in growth and stress responses. Ann. Bot. 2010, 105, 1–6. [Google Scholar] [CrossRef]
- Zeir, J. New insights into the regulation of plant immunity by amino acid metabolic pathways. Plant Cell Environ. 2013, 36, 2085–2103. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, A.C.; Castroverde, C.D.M.; He, S.Y. Plant-Pathogen Warfare under Changing Climate Conditions. Curr. Biol. 2018, 28, R619–R634. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, F.; Thilmony, R.; He, S.Y. The Arabidopsis thaliana-Pseudomonas syringae Interaction. Arab. Book 2002, 1, e0039. [Google Scholar] [CrossRef] [PubMed]
- Buell, C.R.; Joardar, V.; Lindeberg, M.; Selengut, J.; Paulsen, I.T.; Gwinn, M.L.; Dodson, R.J.; DeBoy, R.T.; Durkin, A.S.; Kolonay, J.F.; et al. The complete genome sequence of the Arabidopsis and tomato pathogen Pseudomonas syringae pv. tomato DC3000. Proc. Natl. Acad. Sci. USA 2003, 100, 10181–10186. [Google Scholar] [CrossRef]
- Melotto, M.; Underwood, W.; He, S.Y. Role of Stomata in Plant Innate Immunity and Foliar Bacterial Diseases. Annu. Rev. Phytopathol. 2008, 46, 101–122. [Google Scholar] [CrossRef]
- Chatterjee, A.; Cui, Y.; Hasegawa, H.; Chatterjee, A.K. PsrA, the Pseudomonas Sigma Regulator, Controls Regulators of Epiphytic Fitness, Quorum-Sensing Signals, and Plant Interactions in Pseudomonas syringae pv. tomato Strain DC3000. Appl. Environ. Microbiol. 2007, 73, 3684–3694. [Google Scholar] [CrossRef]
- He, S.Y.; Nomura, K.; Whittam, T.S. Type III protein secretion mechanism in mammalian and plant pathogens. Biochim. Et Biophys. Acta Mol. Cell Res. 2004, 1694, 181–206. [Google Scholar] [CrossRef]
- Coburn, B.; Sekirov, I.; Finlay, B.B. Type III Secretion Systems and Disease. Clin. Microbiol. Rev. 2007, 20, 535–549. [Google Scholar] [CrossRef]
- Cui, H.; Xiang, T.; Zhou, J.-M. Plant immunity: A lesson from pathogenic bacterial effector proteins. Cell. Microbiol. 2009, 11, 1453–1461. [Google Scholar] [CrossRef]
- Xin, X.-F.; Kvitko, B.; He, S.Y. Pseudomonas syringae: What it takes to be a pathogen. Nat. Rev. Genet. 2018, 16, 316–328. [Google Scholar] [CrossRef]
- King, E.O.; Ward, M.K.; Raney, D.E. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 1954, 44, 301–307. [Google Scholar] [PubMed]
- Scalschi, L.; Llorens, E.; González-Hernández, A.I.; Valcárcel, M.; Gamir, J.; Agustín, P.G.; Vicedo, B.; Camañes, G. 1-Methyltryptophan Modifies Apoplast Content in Tomato Plants Improving Resistance Against Pseudomonas syringae. Front. Microbiol. 2018, 9, 2056. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, J.; Camañes, G.; Flors, V.; Vicent, C.; Pastor, V.; Vicedo, B.; Cerezo, M.; García-Agustín, P. Underivatized polyamine analysis in plant samples by ion pair LC coupled with electrospray tandem mass spectrometry. Plant Physiol. Biochem. 2009, 47, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Lund, S.P.; Scott, R.A.; Greenwald, J.W.; Records, A.H.; Nettleton, D.; Lindow, S.E.; Gross, D.C.; Beattie, G.A. Transcriptional responses of Pseudomonas syringae to growth in epiphytic versus apoplastic leaf sites. Proc. Natl. Acad. Sci. USA 2013, 110, E425–E434. [Google Scholar] [CrossRef] [PubMed]
- Scalschi, L.; Camañes, G.; Llorens, E.; Fernández-Crespo, E.; López, M.M.; Agustín, P.G.; Vicedo, B. Resistance Inducers Modulate Pseudomonas syringae pv. Tomato Strain DC3000 Response in Tomato Plants. PLoS ONE 2014, 9, e106429. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.V.M.; Raghavendra, A.S.; Reddy, K.J. Physiology and Molecular Biology of Stress Tolerance in Plants; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Hildebrandt, T.M. Synthesis versus degradation: Directions of amino acid metabolism during Arabidopsis abiotic stress response. Plant Mol. Biol. 2018, 98, 121–135. [Google Scholar] [CrossRef]
- Hare, P.D.; Cress, W.A.; Van Staden, J. Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ. 1998, 21, 535–553. [Google Scholar] [CrossRef]
- McNeil, S.D.; Nuccio, M.L.; Hanson, A.D. Betaines and Related Osmoprotectants. Targets for Metabolic Engineering of Stress Resistance. Plant Physiol. 1999, 120, 945–949. [Google Scholar] [CrossRef]
- Diamant, S.; Eliahu, N.; Rosenthal, D.; Goloubinoff, P. Chemical Chaperones Regulate Molecular Chaperones in Vitro and in Cells under Combined Salt and Heat Stresses. J. Biol. Chem. 2001, 276, 39586–39591. [Google Scholar] [CrossRef]
- Bokszczanin, K.L.; Solanaceae Pollen Thermotolerance Initial Training Network (SPOT-ITN) Consortium; Fragkostefanakis, S. Perspectives on deciphering mechanisms underlying plant heat stress response and thermotolerance. Front. Plant Sci. 2013, 4, 315. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Alamri, S.; Siddiqui, M.H.; Al-Khaishany, M.Y.; Khan, M.N.; Ali, H.M.; Alakeel, K.A. Nitric oxide-mediated cross-talk of proline and heat shock proteins induce thermotolerance in Vicia faba L. Environ. Exp. Bot. 2019, 161, 290–302. [Google Scholar] [CrossRef]
- Tiburcio, A.F.; Altabella, T.; Bitrian, M.; Alcázar, R. The roles of polyamines during the lifespan of plants: From development to stress. Planta 2014, 240, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Crespo, E.; Camañes, G.; García-Agustín, P. Ammonium enhances resistance to salinity stress in citrus plants. J. Plant Physiol. 2012, 169, 1183–1191. [Google Scholar] [CrossRef]
- Alet, A.I.; Sanchez, D.H.; Cuevas, J.C.; Del Valle, S.; Altabella, T.; Tiburcio, A.F.; Marco, F.; Ferrando, A.; Espasandín, F.D.; González, M.E.; et al. Putrescine accumulation in Arabidopsis thaliana transgenic lines enhances tolerance to dehydration and freezing stress. Plant Signal. Behav. 2011, 6, 278–286. [Google Scholar] [CrossRef]
- Serrano, N.; Ling, Y.; Bahieldin, A.; Mahfouz, M.M. Thermopriming reprograms metabolic homeostasis to confer heat tolerance. Sci. Rep. 2019, 9, 181. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Yan, S.; Saleh, A.; Wang, W.; Ruble, J.; Oka, N.; Mohan, R.; Spoel, S.; Tada, Y.; Zheng, N.; et al. NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 2012, 486, 228–232. [Google Scholar] [CrossRef]
- Fonseca, S.; Chini, A.; Hamberg, M.; Adie, B.; Porzel, A.; Kramell, R.; Miersch, O.; Wasternack, C.; Solano, R. (+)-7-iso-Jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nat. Chem. Biol. 2009, 5, 344–350. [Google Scholar] [CrossRef]
- Kloek, A.P.; Verbsky, M.L.; Sharma, S.B.; Schoelz, J.E.; Vogel, J.; Klessig, D.F.; Kunkel, B.N. Resistance to Pseudomonas syringae conferred by an Arabidopsis thaliana coronatine-insensitive (coi1) mutation occurs through two distinct mechanisms. Plant J. 2001, 26, 509–522. [Google Scholar] [CrossRef]
- Jin, H.; Zhu, Z. Dark, Light, and Temperature: Key Players in Plant Morphogenesis. Plant Physiol. 2019, 180, 1793–1802. [Google Scholar] [CrossRef]
- Scalschi, L.; Vicedo, B.; Camanes, G.; Fernandez-Crespo, E.; Lapeña, L.; González-Bosch, C.; García-Agustín, P. Hexanoic acid is a resistance inducer that protects tomato plants against Pseudomonas syringae by priming the jasmonic acid and salicylic acid pathways. Mol Plant Pathol. 2013, 14, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Scalschi, L.; Llorens, E.; García-Agustín, P.; Vicedo, B. Role of Jasmonic Acid Pathway in Tomato Plant-Pseudomonas syringae Interaction. Plants 2020, 9, 136. [Google Scholar] [CrossRef] [PubMed]
- Mauch-Mani, B.; Mauch, F. The role of abscisic acid in plant-pathogen interactions. Curr. Opin. Plant Biol. 2005, 8, 409–414. [Google Scholar] [CrossRef] [PubMed]
- De Torres-Zabala, M.; Truman, W.; Bennett, M.H.; Lafforgue, G.; Mansfield, J.W.; Rodriguez Egea, P.; Bögre, L.; Grant, M. Pseudomonas syringae pv. tomato hijacks the Arabidopsis abscisic acid signalling pathway to cause disease. EMBO J. 2007, 26, 1434–1443. [Google Scholar] [CrossRef]
- Goritschnig, S.; Weihmann, T.; Zhang, Y.; Fobert, P.; McCourt, P.; Li, X. A Novel Role for Protein Farnesylation in Plant Innate Immunity. Plant Physiol. 2008, 148, 348–357. [Google Scholar] [CrossRef]
- Fernández-Crespo, E.; Scalschi, L.; Llorens, E.; García-Agustín, P.; Camañes, G. NH4+ protects tomato plants against Pseudomonas syringae by activation of systemic acquired acclimation. J. Exp. Bot. 2015, 66, 6777–6790. [Google Scholar] [CrossRef]
- Marco, F.; Busó, E.; Carrasco, P. Overexpression of SAMDC1 gene in Arabidopsis thaliana increases expression of defense-related genes as well as resistance to Pseudomonas syringae and Hyaloperonospora arabidopsidis. Front. Plant Sci. 2014, 5, 115. [Google Scholar] [CrossRef]
- Huot, B.; Castroverde, C.D.M.; Velásquez, A.C.; Hubbard, E.; Pulman, J.A.; Yao, J.; Childs, K.L.; Tsuda, K.; Montgomery, B.L.; He, S.Y. Dual impact of elevated temperature on plant defence and bacterial virulence in Arabidopsis. Nat. Commun. 2017, 8, 1808–1812. [Google Scholar] [CrossRef]
- Hirano, S.S.; Upper, C.D. Bacteria in the Leaf Ecosystem with Emphasis on Pseudomonas syringae—A Pathogen, Ice Nucleus, and Epiphyte. Microbiol. Mol. Biol. Rev. 2000, 64, 624–653. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, M.; Burgess, R.R. Adaptation in bacterial flagellar and motility systems: From regulon members to ‘foraging’-like behavior in E. coli. Nucleic Acids Res. 2007, 35, 4441–4452. [Google Scholar] [CrossRef]
- Hockett, K.L.; Burch, A.Y.; Lindow, S.E. Thermo-Regulation of Genes Mediating Motility and Plant Interactions in Pseudomonas syringae. PLoS ONE 2013, 8, e59850. [Google Scholar] [CrossRef] [PubMed]
- Weingart, H.; Stubner, S.; Schenk, A.; Ullrich, M.S. Impact of Temperature on In Planta Expression of Genes Involved in Synthesis of the Pseudomonas syringae Phytotoxin Coronatine. Mol. Plant-Microbe Interact. 2004, 17, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Dean, P.; Kenny, B. The effector repertoire of enteropathogenic E. coli: Ganging up on the host cell. Curr. Opin. Microbiol. 2009, 12, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Jin, L.; Shimada, M.; Kim, M.G.; Mackey, D. The phytotoxin coronatine is a multifunctional component of the virulence armament of Pseudomonas syringae. Planta 2014, 240, 1149–1165. [Google Scholar] [CrossRef]
- Diepold, A.; Armitage, J.P. Type III secretion systems: The bacterial flagellum and the injectisome. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20150020. [Google Scholar] [CrossRef]
- Marie, C.; Broughton, W.J.; Deakin, W.J. Rhizobium type III secretion systems: Legume charmers or alarmers? Curr. Opin. Plant Biol. 2001, 4, 336–342. [Google Scholar] [CrossRef]
- Mazurier, S.; Lemunier, M.; Siblot, S.; Mougel, C.; Lemanceau, P. Distribution and diversity of type III secretion system-like genes in saprophytic and phytopathogenic fluorescent pseudomonads. FEMS Microbiol. Ecol. 2004, 49, 455–467. [Google Scholar] [CrossRef][Green Version]




| Function | Gene | Primer |
|---|---|---|
| Pathogenesis-related protein 1 | PR1 | F 5′-CCGTGCAATTGTGGGTGTC-3′ R 5′-GAGTTGCGCCAGACTACTTGAGT-3′ |
| Arginine decarboxylase | ADC | F 5′-GGGCTTGGAATCGACTATGA-3′ R 5′-CCCGGTTTCAAAAATCAGAA-3′ |
| Spermidine synthase | SPDS | F 5′-GGTGACGGAGTTGCATTTTT-3′ R 5′-GCGGCAATTAGCAACAATTT-3′ |
| Elongation factor 1-alpha | EF1α | F 5′-GACAGGCGTTCAGGTAAGGA-3′ F 5′-GGGTATTCAGCAAAGGTCTC-3 |
| Function | Gene | Primer |
|---|---|---|
| Coronatine synthesis | cfa1 | F 5′-AAAACCATCGTCGACATTCTG-3′ R 5′-GTTGGCGTTGAGGTCGATA-3′ |
| cmaB | F 5′-AATTCGACACCCGACAAGAC-3′ R 5′-ACTAGGGGCTTCAGGTCCAT-3′ | |
| cfl | F 5′-ACAGCTGAAGCAGCACTTGA-3′ R 5′-CGAGGATCTCTCGGTAGTCG-3′ | |
| Type III secretion system, type III secretion system-associated pilus, and effector synthesis | hrpL | F 5′-TCTCCAGTGCGTGTTTCTTG-3′ R 5′-AGCTTTCCTGATACGGCTGA-3′ |
| hrpA | F 5′-CCTCCAAACTCACCAACCTT-3′ R 5′-CGGACTCTTTACTGGCCTTG-3′ | |
| avrPtoB | F 5′-ACCCTATCGCGTCACAATTC-3′ R 5′-CATGAACGCCAGGTCCTTAT-3′ | |
| Quorum sensing establishment | psyI | F 5′-GGCTTGAATGGAATGTTCGT-3′ R 5′-CAGGTGTTGATCAGCCGTAA-3′ |
| Flagellin synthesis | fliC | F 5′-ATCTGAACGGCAAGAACCTG-3′ R 5′-TGCGCTCAAAGTCAGAGAGA-3′ |
| Internal reference | recA | F 5′-CGGCAAGGGTATCTACCTCA-3′ R 5′-CTTTGCAGATTTCCGGGTTA-3′ |
| 26 °C (ng/g FW) | 31 °C (ng/g FW) | ||||
|---|---|---|---|---|---|
| Major amino acids | ASN | 7278.02 ± 1711.20 | 7251.85 ± 3026.76 | ||
| GLU | 26,255.48 ± 5657.45 | 30,944.55 ± 4721.04 | |||
| Minor amino acids | From shikimate | TRP | 659.30 ± 2.07 | 691.99 ± 24.77 | |
| Metil-TRP | 750.31 ± 18.34 | 738.04 ± 23.40 | |||
| PHE | 1429.29 ± 12.45 | 1487.42 ± 56.38 | |||
| TYR | 555.24 ± 112.50 | 484.78 ± 59.78 | |||
| Branched chain | VAL | 498.15 ± 69.06 | 777.08 ± 67.92 | * | |
| From aspartate | THR | 2265.65 ± 84.96 | 2894.86 ± 772.17 | ||
| MET | 240.70 ± 48.96 | 198.70 ± 18.22 | |||
| LYS | 37,144.92 ± 2827.00 | 41,975.57 ± 1613.30 | |||
| Pip | 173,912.30 ± 20,894.94 | 231,928.91 ± 19,346.29 | |||
| From glutamate | HIS | 929.24 ± 28.03 | 937.74 ± 59.81 | ||
| PRO | 15,879.56 ± 3711.11 | 52,493.88 ± 6191.67 | * | ||
| SER | 13,013.59 ± 1553.86 | 35,274.43 ± 6943.60 | * | ||
| ALA | 20,360.30 ± 4401.66 | 23,096 ± 5773.60 | |||
| GLY | 54,403.19 ± 3750.53 | 56,181.24 ± 3048.50 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scalschi, L.; Fernández-Crespo, E.; Pitarch-Marin, M.; Llorens, E.; González-Hernández, A.I.; Camañes, G.; Vicedo, B.; García-Agustín, P. Response of Tomato-Pseudomonas Pathosystem to Mild Heat Stress. Horticulturae 2022, 8, 174. https://doi.org/10.3390/horticulturae8020174
Scalschi L, Fernández-Crespo E, Pitarch-Marin M, Llorens E, González-Hernández AI, Camañes G, Vicedo B, García-Agustín P. Response of Tomato-Pseudomonas Pathosystem to Mild Heat Stress. Horticulturae. 2022; 8(2):174. https://doi.org/10.3390/horticulturae8020174
Chicago/Turabian StyleScalschi, Loredana, Emma Fernández-Crespo, Marcel Pitarch-Marin, Eugenio Llorens, Ana Isabel González-Hernández, Gemma Camañes, Begonya Vicedo, and Pilar García-Agustín. 2022. "Response of Tomato-Pseudomonas Pathosystem to Mild Heat Stress" Horticulturae 8, no. 2: 174. https://doi.org/10.3390/horticulturae8020174
APA StyleScalschi, L., Fernández-Crespo, E., Pitarch-Marin, M., Llorens, E., González-Hernández, A. I., Camañes, G., Vicedo, B., & García-Agustín, P. (2022). Response of Tomato-Pseudomonas Pathosystem to Mild Heat Stress. Horticulturae, 8(2), 174. https://doi.org/10.3390/horticulturae8020174






