Shortening the Vegetative Growth Stage of Phalaenopsis Queen Beer ‘Mantefon’ by Controlling Light with Calcium Ammonium Nitrate Levels under Enriched CO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. The Combination of Light and Calcium Ammonium Nitrogen Levels
2.3. Measurements of Leaf Growth
2.4. Measurements of Gas Exchange and Chlorophyll a Fluorescence
2.5. Stomatal Aperture Width, Length, Maximum Stomatal Aperture, and Stomatal Index Measurements
2.6. Statistical Analysis
3. Results
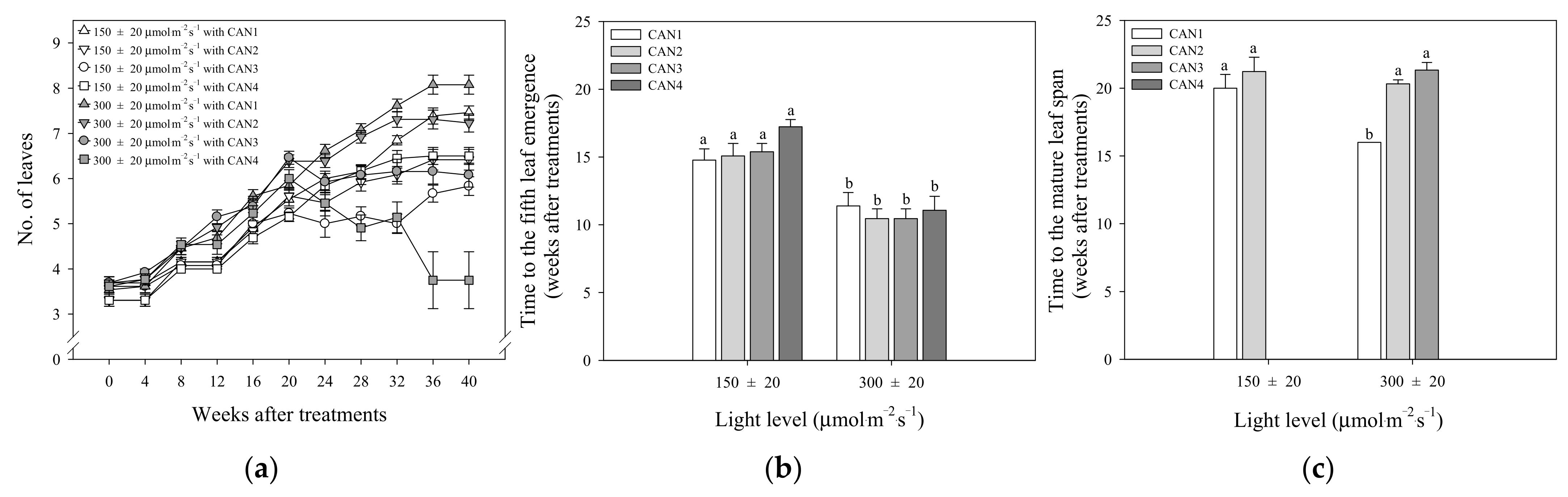
3.1. Leaf Growth Characteristics
3.2. Photosynthetic Characteristics
3.3. Chlorophyll a Fluorescence
3.4. Stomatal Aperture Width, Length, Maximum Stomatal Aperture, and Stomatal Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tsai, C.C. Molecular Phylogeny and Biogeography of Phalaenopsis Species; World Scientific: Singapore, 2011; pp. 1–24. [Google Scholar]
- Guo, W.J.; Lin, Y.Z.; Lee, N. Photosynthetic light requirements and effects of low irradiance and daylength on Phalaenopsis amabilis. J. Am. Soc. Hortic. Sci. 2012, 137, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.C.; Liu, C.H.; Lin, Y.C.; Lu, C.H.; Chen, W.H.; Wang, H.L. Effect of low irradiance on the photosynthetic performance and spiking of Phalaenopsis. Photosynthetica 2016, 54, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Bernier, G.; Kinet, J.M.; Sachs, R.M. Control by Nutrition and Water Stress; CRC Press: Boca Raton, FL, USA, 1981; pp. 13–20. [Google Scholar]
- Huang, C.H.; Chu, C.Y. The flower development and photoperiodism of native Kalanchoe spp. in Taiwan. Sci. Hortic. 2012, 146, 59–64. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, H.J.; Kim, K.S. Night interruption promotes vegetative growth and flowering of Cymbidium. Sci. Hortic. 2011, 130, 887–893. [Google Scholar] [CrossRef]
- Cameron, A.; Yuan, M.; Heins, R.; Carlson, W. Juvenility: Your perennial crop’s age affects flowering. GrowerTalks 1996, 60, 30–32. [Google Scholar]
- Blanchard, M.; Lopez, R.; Runkle, E.; Wang, Y.T.; Lopez, R. Growing the best Phalaenopsis. Orchids 2007, 4, 266–271. [Google Scholar]
- Purvis, O.N. An analysis of the influence of temperature during germination on the subsequent development of certain winter cereals and its relation to the effect of length of day. Ann. Bot. 1934, 48, 919–955. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, Y.D.; Kim, S.Y.; Kim, C.U.; Park, K.S. Study on GHP-based integrated energy generation system for horticulture. Korean Soc. Agric. Mach. 2015, 20, 393–394. [Google Scholar]
- Cho, A.R.; Choi, S.H.; Kim, Y.J. Flowering and photosynthetic responses of Phalaenopsis under elevated CO2 and nutrient supply. Hortic. Sci. Technol. 2020, 38, 595–607. [Google Scholar]
- Kim, H.J.; Cho, A.R.; Park, K.S.; Kim, Y.J. Effect of CO2 enrichment on growth and flowering of Phalaenopsis. Hortic. J. 2017, 86, 389–394. [Google Scholar]
- Yun, D.L.; Kim, H.J.; Kim, Y.J. CO2 enrichment increased leaf initiation and photosynthesis in Doritaenopsis Queen Beer ‘Mantefon’ orchids. Hortic. Environ. Biotechnol. 2018, 59, 159–165. [Google Scholar] [CrossRef]
- Song, S.J.; Yun, D.L.; Cho, A.R.; Kim, Y.J. Photosynthetic and growth response of Phalaenopsis Queen Beer ‘Mantefon’ to variable CO2 concentrations at different vegetative growth stages. Flower Res. J. 2019, 27, 9–16. [Google Scholar] [CrossRef]
- Ota, K.; Morioka, K.; Yamamoto, Y. Effects of leaf age, inflorescence, temperature, light intensity and moisture conditions on CAM photosynthesis in Phalaenopsis. J. Jpn. Soc. Hortic. Sci. 1991, 60, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Lootens, P.; Heursel, J. Irradiance, temperature, and carbon dioxide enrichment affect photosynthesis in Phalaenopsis hybrids. HortScience 1998, 33, 1183–1185. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.J.; Hsu, B.D. Photosynthetic plasticity of Phalaenopsis in response to different light environments. J. Plant Physiol. 2004, 161, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.H.; Jeon, S.M.; Park, J.S.; Kim, C.K. Combined effects of supplementary light and CO2 on rose growth and the production of good quality cut flowers. Can. J. Plant Sci. 2016, 96, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Mortensen, L.M.; Moe, R. Growth responses of some greenhouse plants to environment. V. Effect of CO2, O2 and light on net photosynthetic rate in Chrysanthemum morifolium Ramat. Sci. Hortic. 1983, 19, 133–140. [Google Scholar] [CrossRef]
- Cho, A.R.; Song, S.J.; Chung, S.W.; Kim, Y.J. CO2 enrichment with higher light level improves flowering quality of Phalaenopsis Queen Beer ‘Mantefon’. Sci. Hortic. 2019, 247, 356–361. [Google Scholar] [CrossRef]
- Wang, Y.T. Effects of six fertilizers on vegetative growth and flowering of Phalaenopsis orchids. Sci. Hortic. 1996, 65, 191–197. [Google Scholar] [CrossRef]
- Wang, Y.T.; Gregg, L.L. Medium and fertilizer affect the performance of Phalaenopsis orchids during two flowering cycles. HortScience 1994, 29, 269–271. [Google Scholar] [CrossRef]
- Prior, S.A.; Runion, G.B.; Mitchell, R.J.; Rogers, H.H.; Amthor, J.S. Effects of atmospheric CO2 on longleaf pine: Pproductivity and allocation as influenced by nitrogen and water. Tree Physiol. 1997, 17, 397–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, K.R.; Zhao, D. Interactive effects of elevated CO2 and potassium deficiency on photosynthesis, growth, and biomass partitioning of cotton. Field Crops Res. 2005, 94, 201–213. [Google Scholar] [CrossRef]
- Seneweera, S.P.; Conroy, J.P. Growth, grain yield and quality of rice (Oryza sativa L.) in response to elevated CO2 and phosphorus nutrition. Soil Sci. Plant Nutr. 1997, 43, 1131–1136. [Google Scholar] [CrossRef]
- Sánchez-Guerrero, M.C.; Lorenzo, P.; Medrano, E.; Baille, A.; Castilla, N. Effects of EC-based irrigation scheduling and CO2 enrichment on water use efficiency of a greenhouse cucumber crop. Agric. Water Manag. 2009, 96, 429–436. [Google Scholar] [CrossRef]
- Thyssenkrupp. 2019. Available online: https://insights.thyssenkrupp-industrial-solutions.com/story/ammonium-nitrate-fertilizer-and-why-it-is-the-preferred-choice-of-european-farmers/ (accessed on 9 December 2021).
- Gao, H.; Jia, Y.; Guo, S.; Lv, G.; Wang, T.; Juan, L. Exogenous calcium affects nitrogen metabolism in root-zone hypoxia-stressed muskmelon roots and enhances short-term hypoxia tolerance. J. Plant Physiol. 2011, 168, 1217–1225. [Google Scholar] [CrossRef]
- Chen, C.T.; Lee, C.L.; Yeh, D.M. Effects of nitrogen, phosphorus, potassium, calcium, or magnesium deficiency on growth and photosynthesis of Eustoma. HortScience 2018, 53, 795–798. [Google Scholar] [CrossRef]
- Xu, S.; Yuan, X.; Zhang, Y.; Ye, Q. Effect of elevated CO2 combined with two nitrogen levels on photosynthesis, growth and leaf structure in cam orchid Phalaenopsis sp. Int. J. Agric. Biol. 2019, 22, 882–890. [Google Scholar]
- Farage, P.K.; McKee, I.F.; Long, S.P. Does a low nitrogen supply necessarily lead to acclimation of photosynthesis to elevated CO2? Plant Physiol. 1998, 118, 573–580. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Zhu, X.; Li, C.; Ye, Q. Effects of CO2 enrichment on photosynthesis and growth in Gerbera jamesonii. Sci. Hortic. 2014, 177, 77–84. [Google Scholar] [CrossRef]
- Zha, T.S.; Wu, Y.J.; Jia, X.; Zhang, M.Y.; Bai, Y.J.; Liu, P.; Ma, J.Y.; Bourque, C.P.-A.; Peltola, H. Diurnal response of effective quantum yield of PSII photochemistry to irradiance as an indicator of photosynthetic acclimation to stressed environments revealed in a xerophytic species. Ecol. Indic. 2017, 74, 191–197. [Google Scholar] [CrossRef]
- Wang, Y.T.; Chang, Y.C.A. Effects of nitrogen and the various forms of nitrogen on Phalaenopsis orchid-A review. HortTechnology 2017, 27, 144–149. [Google Scholar] [CrossRef] [Green Version]
- Pollet, B.; Steppe, K.; Van Labeke, M.-C.; Lemeur, R. Diurnal cycle of chlorophyll fluorescence in Phalaenopsis. Photosynthetica 2009, 47, 309–312. [Google Scholar] [CrossRef]
- Oh, W.; Rhie, Y.H.; Park, J.H.; Runkle, E.S.; Kim, K.S. Flowering of cyclamen is accelerated by an increase in temperature, photoperiod, and daily light integral. J. Hortic. Sci. Biotechnol. 2008, 83, 559–562. [Google Scholar] [CrossRef]
- Pritchard, S.G.; Rogers, H.H.; Prior, S.A.; Peterson, C.M. Elevated CO2 and plant structure: a review. Glob. Chang. Biol. 1999, 5, 807–837. [Google Scholar] [CrossRef] [Green Version]
- Kinsman, E.A.; Lewis, C.; Davies, M.S.; Young, J.E.; Francis, D.; Vilhar, B.; Ougham, H.J. Elevated CO2 stimulates cells to divide in grass meristems: A differential effect in two natural populations of Dactylis glomerata. Plant Cell Environ. 1997, 20, 1309–1316. [Google Scholar] [CrossRef]
- Becker, D.W.; Bain, G.; Norman, J.; Moholt-Siebert, M. Two Sites of Heat-Induced Damage to Photosystem II; Springer: Berlin/Heidelberg, Germany, 1990; pp. 3499–3502. [Google Scholar]
- Björkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef]
- Krause, G.H.; Winter, K. Photoinhibition of photosynthesis in plants growing in natural tropical forest gaps. A chlorophyll fluorescence study. Bot. Acta 1996, 109, 456–462. [Google Scholar]
- Ač, A.; Malenovský, Z.; Olejníčková, J.; Gallé, A.; Rascher, U.; Mohammed, G. Meta-analysis assessing potential of steady-state chlorophyll fluorescence for remote sensing detection of plant water, temperature and nitrogen stress. Remote Sens. Environ. 2015, 168, 420–436. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.J.; Zha, T.S.; Jia, X.; Qin, S.G.; Li, Y.; Wang, B. Temporal variation and controlling factors of photochemical efficiency and non-photochemical quenching in Artemisia ordosica. Chin. J. Ecol. 2015, 34, 319–325. [Google Scholar]
- Lake, J.A.; Quick, W.; Beerling, D.J.; Woodward, F.I. Signals from mature to new leaves. Nature 2001, 411, 154. [Google Scholar] [CrossRef]
- O’Carrigan, A.; Hinde, E.; Lu, N.; Xu, X.-Q.; Duan, H.; Huang, G.; Mak, M.; Bellotti, B.; Chen, Z.H. Effects of light irradiance on stomatal regulation and growth of tomato. Environ. Exp. Bot. 2014, 98, 65–73. [Google Scholar] [CrossRef]
- Uprety, D.C.; Dwivedi, N.; Jain, V.; Mohan, R. Effect of elevated carbon dioxide concentration on the stomatal parameters of rice cultivars. Photosynthetica 2002, 40, 315–319. [Google Scholar] [CrossRef]
- Dilcher, D.L.; Kuerschner, W.M.; Visscher, H.; Wagner, F. Fossil leaves as biosensors of eocene paleoatmospheric CO2. Environ. Geosci. 1999, 6, 151–152. [Google Scholar] [CrossRef]
- James, S.A.; Bell, D.T. Influence of light availability on leaf structure and growth of two Eucalyptus globulus ssp globulus provenances. Tree Physiol. 2000, 20, 1007–1018. [Google Scholar] [CrossRef]
- Fanourakis, D.; Hyldgaard, B.; Giday, H.; Aulik, I.; Bouranis, D.; Körner, O.; Ottosen, C. Stomatal anatomy and closing ability is affected by supplementary light intensity in rose (Rosa hybrida L.). Hortic. Sci. 2019, 46, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Saure, M.C. Why calcium deficiency is not the cause of blossom-end rot in tomato and pepper fruit–a reappraisal. Sci. Hortic. 2014, 174, 151–154. [Google Scholar] [CrossRef]
- Sonneveld, C.; De Kreij, C. Response of cucumber (Cucumis sativus L.) to an unequal distribution of salts in the root environment. Plant Soil 1999, 209, 47–56. [Google Scholar] [CrossRef]
- Xing, Y.; Zhu, Z.L.; Wang, F.; Zhang, X.; Li, B.Y.; Liu, Z.X.; Wu, X.X.; Ge, S.F.; Jiang, Y.M. Role of calcium as a possible regulator of growth and nitrate nitrogen metabolism in apple dwarf rootstock seedlings. Sci. Hortic. 2021, 276, 109740. [Google Scholar] [CrossRef]
- Kaya, C.; Higgs, D.; Kirnak, H.; Tas, I. Ameliorative effect of calcium nitrate on cucumber and melon plants drip irrigated with saline water. J. Plant Nutr. 2003, 26, 1665–1681. [Google Scholar] [CrossRef]

| Level | Total N (mg·L−1) | Elements (mmol·L−1) | EC (dS·m−1) | pH | |||||
|---|---|---|---|---|---|---|---|---|---|
| Ca | NH4 | NO3 | P | K | S | ||||
| CAN1 | 200 | 0.90 | 0.55 | 2.97 | 2.74 | 4.37 | 0.81 | 1.6 ± 0.1 | 5.9 |
| CAN2 | 400 | 8.63 | 1.11 | 6.05 | 2.74 | 4.37 | 0.81 | 2.1 ± 0.1 | 6.0 |
| CAN3 | 600 | 12.80 | 1.72 | 9.13 | 2.74 | 4.37 | 0.81 | 3.0 ± 0.1 | 6.1 |
| CAN4 | 800 | 18.80 | 2.27 | 12.20 | 2.74 | 4.37 | 0.81 | 4.9 ± 0.1 | 6.1 |
| Light Levels (μmol·m−2·s−1) | CAN Levels | Fv/Fm | ΦPSΙΙ | NPQ |
|---|---|---|---|---|
| 150 ± 20 | CAN1 | 0.709 ± 0.011 ab z | 0.299 ± 0.009 ab | 1.101 ± 0.084 a |
| CAN2 | 0.715 ± 0.001 a | 0.330 ± 0.025 a | 0.896 ± 0.189 a | |
| CAN3 | 0.697 ± 0.019 ab | 0.256 ± 0.016 bc | 1.010 ± 0.051 a | |
| CAN4 | 0.688 ± 0.053 ab | 0.251 ± 0.026 bc | 0.862 ± 0.089 a | |
| 300 ± 20 | CAN1 | 0.647 ± 0.015 ab | 0.211 ± 0.018 cd | 0.835 ± 0.139 a |
| CAN2 | 0.673 ± 0.034 ab | 0.230 ± 0.017 cd | 0.890 ± 0.147 a | |
| CAN3 | 0.671 ± 0.021 ab | 0.233 ± 0.022 cd | 1.081 ± 0.032 a | |
| CAN4 | 0.617 ± 0.030 b | 0.183 ± 0.024 d | 0.839 ± 0.274 a | |
| Significance y | ||||
| Light | * | * | ns | |
| CAN | ns | ns | ns | |
| Light × CAN | ns | ns | ns | |
| Light Levels (μmol·m−2·s−1) | CAN Levels | Stomatal Aperture Width (μm) | Stomatal Aperture Length (μm) | Maximum Stomatal Aperture (μm2) | Stomatal Index (%) |
|---|---|---|---|---|---|
| 150 ± 20 | CAN1 | 3.0 ± 0.4 b z | 12.6 ± 0.6 bc | 27 ± 5 b | 6.23 ± 0.15 a |
| CAN2 | 3.3 ± 0.3 b | 12.9 ± 0.4 bc | 29 ± 3 b | 5.07 ± 0.22 c | |
| CAN3 | 2.7 ± 0.4 bc | 14.1 ± 0.3 ab | 28 ± 3 b | 5.93 ± 0.28 ab | |
| CAN4 | 1.9 ± 0.1 c | 13.6 ± 0.2 bc | 20 ± 1 b | 5.91 ± 0.10 ab | |
| 300 ± 20 | CAN1 | 5.7 ± 0.3 a | 13.8 ± 0.5 ab | 58 ± 5 a | 6.18 ± 0.26 a |
| CAN2 | 6.1 ± 0.5 a | 15.1 ± 0.5 a | 70 ± 10 a | 5.25 ± 0.09 bc | |
| CAN3 | 3.0 ± 0.1 b | 11.0 ± 0.8 d | 23 ± 3 b | 4.03 ± 0.55 d | |
| CAN4 | 3.1 ± 0.5 b | 12.1 ± 0.5 cd | 26 ± 5 b | 6.08 ± 0.16 ab | |
| Significance y | |||||
| Light | *** | ns | *** | * | |
| CAN | *** | * | *** | *** | |
| Light × CAN | ** | *** | *** | ** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, A.R.; Chung, S.W.; Kim, Y.J. Shortening the Vegetative Growth Stage of Phalaenopsis Queen Beer ‘Mantefon’ by Controlling Light with Calcium Ammonium Nitrate Levels under Enriched CO2. Horticulturae 2022, 8, 157. https://doi.org/10.3390/horticulturae8020157
Cho AR, Chung SW, Kim YJ. Shortening the Vegetative Growth Stage of Phalaenopsis Queen Beer ‘Mantefon’ by Controlling Light with Calcium Ammonium Nitrate Levels under Enriched CO2. Horticulturae. 2022; 8(2):157. https://doi.org/10.3390/horticulturae8020157
Chicago/Turabian StyleCho, Ah Ram, Sun Woo Chung, and Yoon Jin Kim. 2022. "Shortening the Vegetative Growth Stage of Phalaenopsis Queen Beer ‘Mantefon’ by Controlling Light with Calcium Ammonium Nitrate Levels under Enriched CO2" Horticulturae 8, no. 2: 157. https://doi.org/10.3390/horticulturae8020157
APA StyleCho, A. R., Chung, S. W., & Kim, Y. J. (2022). Shortening the Vegetative Growth Stage of Phalaenopsis Queen Beer ‘Mantefon’ by Controlling Light with Calcium Ammonium Nitrate Levels under Enriched CO2. Horticulturae, 8(2), 157. https://doi.org/10.3390/horticulturae8020157






