Abstract
Medicinal herbs are potential sources of biomolecules and their analogues that have great relevance in the preparation of modern medicines. Calligonum crinitum, a perennial shrub growing in the United Arab Emirates, has been utilized in the study to validate the therapeutic properties exploited in the traditional medicinal system of UAE. The phytochemical screening of the plant employing different solvents of methanolics and ethyl-acetates, expressed varied proportions of monosaccharides and carbohydrates along with tannins and saponins, which are high potency molecules for therapeutic applications. The presence of total phenol and flavonoid contents derived from methanolic extracts indicates antioxidant potentials and the defense mechanisms of the plant. Proximate and mineral nutrient analysis validates the significance of the extracts with a high amount of carbohydrates and proteins along with significantly high amounts of Zn, Fe, Mn, Ca, Mg and K involved in various metabolic reactions. Similarly, the ABTS radical scavenging activity varied significantly (p < 0.05) and ranged from 10 to 160 µg GAE/g in the methanolic extract. DPPH free radical scavenging activity exhibited a significantly high DPPH activity in methanolic extracts with free radical scavenging activity of 72%. Hydroxyl radicals scavenging activity was also found to be high in the Calligonum extracts along with SOD (49–83%, compared to the standard GAE 37–58%). Nitric oxide scavenging was also found to be high in the extracts, thereby decreasing the content of NO. Thus, our results confirm that the derived extracts have potential antioxidants, and this legitimizes their use in folkloric medicine. These results are highly significant as they can pave the way for future scientific validation of the traditional knowledge of this important medicinal plant.
1. Introduction
The therapeutic properties of plants have been examined in the current scientific world, as a result of their strong antioxidant values, absence of side effects and economic viability [1]. The alternative medicinal systems are always trustworthy in terms of their efficiency and non-side effects in therapeutics. As per World Health Organization (WHO) reports, throughout the globe, approximately 21,000 plants are used in alternative medicine [2], and more than 50,000 plant species are utilized as a part of traditional medicines [3]. The medications are derived from the entire plant or from various parts such as the leaves, stem, bark, root, flower, tuber and seed and so on. Over 30% of the total plant species, at one time or another, have been utilized for medicinal purposes. Conventional drug practice in the treatment of diseases and infections has accepted a more scientific and more extensive measurement compared with ethnomedicine’s use in practice, particularly in the developing nations [4].
Most of the plants used in traditional medicines have shown potent antioxidant actions [5]. Phytochemicals are among many other secondary metabolites that provide the therapeutic power for medicinal plants [6]. Recently, the importance of studies related to phenolic compounds revealed many of their conceivable medical advantages. The anticarcinogenic, antimutagenic and cardioprotective impacts of phenolic mixes are connected with their antioxidant properties of scavenging free radicals and easing lipid peroxidation [7]. The extraction process does affect the yield and, to some extent, affects the stability of polyphenols [8]. An estimation of the nutrient composition and proximate values of plants gives the idea of their nutritional significance. If the plant standardizes all of the parameters of proximate composition, then it is quite safe to be used as a dietary supplement or as herbal drug [9]. The WHO underline the need for and significance of deciding the proximate and micronutrient content of natural plants in order to standardize the herbs [10].
The stress from free radicals starts when they try to interact with the biological macromolecules such as proteins and lipids through electron pairing [11]. The major free radicals formed in the human body are superoxide radical (O2−), hydroxyl radical (OH), singlet oxygen (1O2), hydrogen peroxide (H2O2), hypochlorous acid (HOCl), peroxyl (ROO), alkoxyl radical (RO), radicals of nitric oxide (NO), nitrogen dioxide (NO2), peroxynitrite (O NOO−), ozone (O3) and toxic nonradical derivatives of oxygen [12]. Synthetic antioxidants have been used as supplements for quite a while; however the details provided regarding their inclusion in chronic infections have restricted their utilization in nourishments. Therefore, universal consideration has been centered around characteristic antioxidants, essentially from plant sources [13].
The medicinal value of plants is mainly due to the fact that they produce substances that have defense properties created in them as a result of metabolic processes. Conventional native plant use in UAE traditional medicine is very common. Around 678 plant species are reported to be from the UAE, huge numbers of which show tolerance to live in extreme environmental conditions, and only some of them have been screened for medicinal properties [14]. In a review by Sakkir et al. [15], a total of 132 plant species were found to have therapeutic properties. Among these medicinal plants, the plants in the family Polygonaceae are utilized by traditional people. The desert of the Abu Dhabi area of the UAE is a rich source of a wide variety of plants; however, the vast majority of these has scarcely been contemplated from the view of their proximate and nutritional profile [16].
Calligonum is a plant genus in the Polygonaceae family with about 80 species across the Mediterranean region, Asia and North America. Calligonum crinitum Boiss (locally known as Abal) is a perennial plant, similar to C. comosum but having white and pink flowers, mainly distributed in the deserts of the UAE and some other Gulf countries and India [17]. The woody shrub is 2.5 m tall and prefers higher and well drained sandy areas [18]. It is one of the few plants able to grow on large mobile dunes, even on the leeward slopes.
Recently, a few reviews affirmed the phytochemical studies in C. polygonoides [19,20,21,22]. Earlier, we reported the phytochemical constituents of C. comosum [23] collected from the deserts of the UAE. Apart from studies focused on the sorbent properties of C. crinitum for the removal of metal ions in water remediation processes [24], studies about C. crinitum are scant, mainly in terms of the medicinal property evaluation. Thus, the objectives of this study were to estimate the phytochemicals, total phenol, flavonoid contents, proximate and mineral analysis and free radical scavenging activities by different in vitro antioxidant assays from C. crinitum growing in the UAE in order to have scientific validation of the traditional medicinal uses.
2. Materials and Methods
2.1. Plant Material
The completely developed aerial parts of Calligonum crinitum were gathered during summer season from Al Foah Experimental Station of College of Agriculture and Veterinary Medicine, United Arab Emirates University. The plants were identified and validated at United Arab Emirates University, UAE.
2.2. Preparation of Extracts
Plants were uprooted and collected in separated labelled plant collection bags. The shoots were washed altogether with water and dried under the shade at room temperature. The dried shoots were powdered by utilizing blender to get the coarse powder so that all the material passed through a mesh not larger than 0.5 mm. The powdered materials of each plant (500 g) were soaked in 1.5 L of petroleum ether (Merck Co., Darmstadt, Germany) for 1 day, and the steps were sequentially extracted by petroleum ether, chloroform, ethyl acetate, acetone and methanol for 72 h. At the end of extraction, it was passed through Whatman filter paper No.1 (Whatman Ltd., Maidstone, UK). The plant extracts were concentrated to dryness under vacuum on rotary evaporator at 40 °C, then reconstituted in minimum amount of DMSO and stored at 4 °C for further use [25].
2.3. Preliminary Phytochemical Screening
Phytochemical tests were performed on the different extracts using standard qualitative methods [26,27] and as explained by Prakash et al. [28].
2.4. Total Phenolic and Flavonoid Contents
Total phenolic contents were determined by the Folin–Ciocalteu reagent method [29] and expressed in terms of gallic acid equivalent (mg/g of dried sample). Total flavonoids were determined in the method of Zhishen et al. [30] and expressed as quercetin equivalents.
2.5. Proximate Analysis
Proximate analysis (total ash, dry matter, crude fiber, protein and fat contents) was conducted by standard methods of the Association of Official Analytical Chemists [31,32,33].
2.6. Microelements and Macro Elements
Micro and macro elements were analyzed from samples based on standard methodology [34].
2.7. In Vitro Antioxidant Analysis
Six different in vitro assays were performed to estimate the antioxidant activity of the extracts: 2,2′-Azinobis-(3-eythylbenzothiazoline-6-sulfonic acid) (ABTS●+) radical cation decolorization assay [35], DPPH● radical scavenging assay [36], the hydroxyl radical scavenging activity [37], superoxide anion scavenging activity [38], nitric oxide scavenging activity [39] and the reducing power of plant extracts [40] as explained previously [41].
2.8. Statistical Analysis
All the experiments were carried out in triplicate, and the results were expressed as mean ± SD. Statistical analysis was performed using SPSS 13.0 and Excel 2003. The p value less than 0.05 was considered to be statistically significant.
3. Results and Discussion
3.1. Percentage Yield and Preliminary Phytochemical Screening
The partitioning of crude extract with different solvents such as petroleum ether, chloroform, ethyl acetate, acetone and methanol are shown in Table 1 with their extract yield percentage.

Table 1.
Extraction yield from Calligonum crinitum aerial parts with various solvents.
The results of the phytochemical composition of the aerial parts of C. crinitum in solvent extracts were given in Table 2. From phytochemical screening, we observed that the methanolic and ethyl acetate extracts gave a positive result with the Molisch test, which indicated the presence of monosaccharides and carbohydrates in the different extracts.

Table 2.
Phytochemical screening of crude extracts of Calligonum crinitum.
The methanol extract of C. crinitum gave a higher percentage of yield when compared to other extracts. This might result from the difference in polarity of the methanolic solvent when compared to other extracts used in this study, and might have elaborated more individual contents from plants [42]. Tannins are famous for their usage in the treatment of inflamed or ulcerated tissues and they have anticancer properties as well [43]. Thus, the Calligonum species, which are high in tannin, can be a good candidate for cancer treatment. Saponins have been reported to exhibit many properties such as anti-fungal and molluscicidal activities [44].
3.2. Total Phenolic and Flavonoid Contents
The result of the total phenol and flavonoid contents is given in Table 3. Methanolic extract has the highest content with a value of 53.03 mg of GAE/g of dry extract phenol compared with the other four extracts. Similarly, the total flavonoid content in methanolic extract (45.17 mg of GAE/g of dry extract) was significantly higher (p < 0.05) than that of other extracts.

Table 3.
Total phenolics content of various extracts of the aerial parts of Calligonum crinitum.
There were significant phenol contents in C. crinitum extracts. Generally, phenolics are directly proportional to the antioxidative potential of the extracts, the number of hydroxyl groups in the phenol molecule structure playing an important role in this activity [45]. There are many studies on the activity of phenolics in antioxidant potentials and the plant defense reactions of medicinal plants [46]. All of the extracts of both of the plants showed the presence of significant amounts of flavonoids. The role of flavonoids in human nutrition and health is inevitable due to their antioxidant activity. Their assistance in the chelating process is the basic mechanism of action of flavonoids [47], and they can suppress ROS formation and protect cells through antioxidant defenses [48]. There are reports showing that a flavonoid rich diet could reduce some human diseases [49]. The significant phenol and flavonoid contents may be the reason for antioxidant activity in C. crinitum plants.
3.3. Proximate Analysis
Table 4 shows the proximate compositions of the aerial parts of C. crinitum. The determination of the proximate compositions was performed in duplicates. All of the data obtained were from a dry basis and expressed in percentage (%). The fibre and ash contents were also high. The proximate compositions and calorific values were calculated for the dry weight of the samples.

Table 4.
Proximate analysis of Calligonum crinitum.
The measurement of moisture content is very important in the processing, preservation and storage of food [50]. Protein content analysis is basic for the characterization of food type [51]. C. crinitum that are high in carbohydrate content are more advantageous. All of the aerial parts of C. crinitum showed rich contents of crude fibre and fell in the range of the standard recommendation for fiber in diet. There is a positive correlation between fats and ash, and C. crinitum showed moderate values, which are in accordance with the standard recommended by World Health Organization for herbal preparations [52].
3.4. Mineral Analysis
Nutritional composition was analysed by measuring the elemental contents in the aerial parts of plant species. Fe, Mn, Ca, K, Mg, S and Zn were presented in appreciable quantities, and low concentrations of Pb, Co, Cd and Cu were observed in C. crinitum. The results of elemental analysis are given in Table 5. It is to be noted that each outcome is an average of no less than three autonomous estimations with an accuracy of about ±1%.

Table 5.
Mineral composition of Calligonum crinitum.
3.5. Microelements and Macroelements
Almost all herbal plants provide health benefits that can be well correlated to their nutritional content. Calcium is an important mineral in plants and has many vital roles in human and animal life, and the deficiency causes poor development, growth and different abnormalities [53]. Manganese is considered as an antioxidant nutrient and is essential for the breakdown of fats and cholesterol [54]. Magnesium has a great role in the activities of various enzymes that are involved in carbohydrate metabolism and glucose oxidation [55]. In the present study, the concentrations of K and Ca were found to be high followed by the remaining elements in trace levels. Zinc is a component of many metalloenzymes and aids wound healing [56]. In diet and herbal therapy, the high content of minerals is considered as beneficial as they have profound effects on nutritional and therapeutic aspects [57].
3.6. In Vitro Antioxidant Activity
3.6.1. ABTS Radical Scavenging Activity
The plant extracts were faster and more effective in scavenging ABTS radical (Figure 1) with comparable extent than GAE. The percentage of inhibition was 61% and 71% for the plant extract and GAE, respectively, at 160 µg/mL concentration. In ABTS+ scavenging activity, the values varied significantly (p < 0.05) and ranged from 10 to 160 µg GAE/g extract. The ABTS radical scavenging activities of C. crinitum were higher for the methanolic extract, while the least activity was observed for the acetone extract.
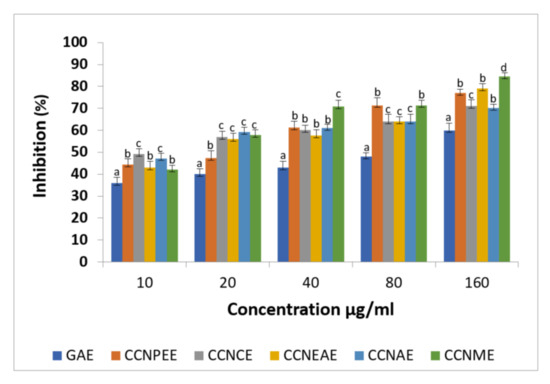
Figure 1.
ABTS free radical scavenging activity of the solvent extracts of Calligonum crinitum in comparison with gallic acid. CCN—Calligonum crinitum, GAE—Gallic acid extract, CCNPEE—Petroleum ether extract, CCNCE—Chloroform extract, CCNEAE—Ethyl acetate extract, CCNAE—Acetone extract, CCNME—Methanol extract. Values are given as mean ± SD of six experiments in each group. Values that are not sharing a common superscript (a,b,c,d) differ significantly at p ≤ 0.05 (DMRT).
The results suggest that C. crinitum has moderate to potent free radical scavenging activity. In biological systems, the production of free radicals is spontaneous with the various metabolic processes. The production of free radicals can create many damages and diseases including cancer [58]. The ABTS assay gives an ABTS radical cation, which has blue-green chromophore absorption prior to the addition of antioxidants [59]. Plant extracts can cause radical cation reduced to ABTS in a concentration-dependent manner. The ABTS activities of the two plant extracts were similar and comparable to that of GAE and the other extracts used; the quenching also increased. ABTS is widely used to assess the free radical ameliorating efficiency of plant extracts [60].
3.6.2. DPPH Free Radical Scavenging Activity
The different solvent extracts of C. crinitum exhibited significant DPPH activity, as shown in Figure 2. The methanolic extracts of C. crinitum showed free radical scavenging activity of 72%. This result demonstrated that the C. crinitum extract has inhibitory activity against the DPPH radical.
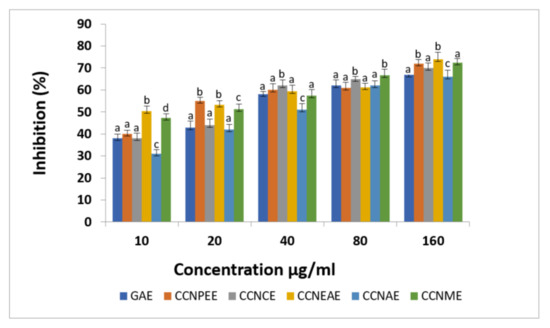
Figure 2.
DPPH radical scavenging activity of the different extracts of Calligonum crinitum in comparison with gallic acid. CCN—Calligonum crinitum, GAE—Gallic acid extract, CCNPEE—Petroleum ether extract, CCNCE—Chloroform extract, CCNEAE—Ethyl acetate extract, CCNAE—Acetone extract, CCNME—Methanol extract. Values are given as mean ± SD of six experiments in each group. Values that are not sharing a common superscript (a,b,c,d) differ significantly at p ≤ 0.05 (DMRT).
The extracts of C. crinitum showed significant antioxidant activities when analyzed with DPPH, a stable free radical. As DPPH attracts one electron in combination with a free radical quenching substance, the absorption decreases and the discoloration can be measured [61]. In the presence of an antioxidant, possibly in the extract DPPH’s radical form, there is a major force for antioxidant activity [62]. The different extracts of C. crinitum showed potent activities in DPPH assay. Among the various solvent extracts tested, the methanol extracts of the aerial parts of C. crinitum exhibited higher DPPH• radical scavenging activity.
3.6.3. Hydroxyl Radical Scavenging Activity
The antioxidant activity is shown in Figure 3 for hydroxyl radical scavenging of C. crinitum at various concentrations.
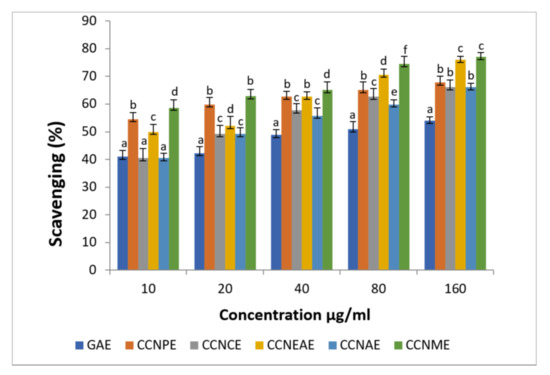
Figure 3.
Hydroxyl radical scavenging action of different extracts of Calligonum crinitum in comparison with gallic acid. CCN—Calligonum crinitum, GAE—Gallic acid extract, CCNPEE—Petroleum ether extract, CCNCE—Chloroform extract, CCNEAE—Ethyl acetate extract, CCNAE—Acetone extract, CCNME—Methanol extract. Values are given as mean ± SD of six experiments in each group. Values that are not sharing a common superscript (a,b,c,d,e,f) differ significantly at p ≤ 0.05 (DMRT).
Another important and most deleterious free radical is hydroxyl radical. It can be found in almost every molecule in living cells [63]. Hydroxyl radicals can cause breaks in DNA and can create carcinogenesis, mutagenesis, etc., [64]. Recently many studies reported the action of many of the plant extracts in scavenging this radical [65]. The extracts from plants contain a lot of free radical scavenging organic molecules, such as flavonoids, phenols, etc., which can help in the quenching of this type of radical [66].
3.6.4. Superoxide Radicals Scavenging Activity
At 10–160 μg, the superoxide scavenging activity of the different extracts of the plant were 49–83%, and that of the standard GAE was 37–58%. The superoxide scavenging activity and standard gallic acid is shown in Figure 4. The percentage inhibition increased with the sample concentration of the extracts.
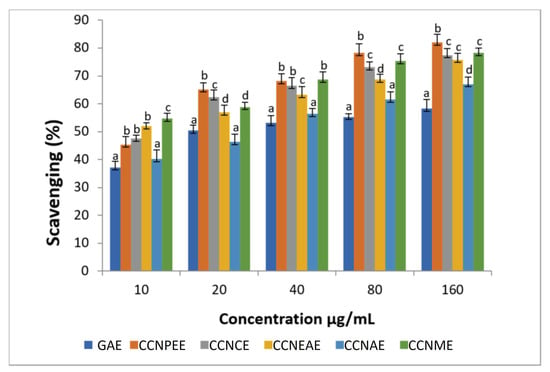
Figure 4.
Superoxide radical scavenging activity of the different extracts of Calligonum crinitum from the aerial parts of plants. CCN—Calligonum crinitum, GAE—Gallic acid extract, CCNPEE—Petroleum ether extract, CCNCE—Chloroform extract, CCNEAE—Ethyl acetate extract, CCNAE—Acetone extract, CCNME—Methanol extract. Values are given as mean ± SD of six experiments in each group. Values that are not sharing a common superscript (a,b,c,d) differ significantly at p ≤0.05 (DMRT).
Superoxide anions are among the most important agents for the formation of reactive oxygen species and create oxidative damage in cells [67]. Inhibition of superoxide generation by Calligonum may be due to the presence of flavonoids and polyphenols [63]. The production of reactive oxygen and nitrogen species can act as initiators of apoptosis by increasing mitochondrial membrane permeability, which results in cytochrome c release and the induction of apoptosis [68].
3.6.5. Nitric Oxide Scavenging
The scavenging of nitric oxide increased in a dose dependent manner by C. crinitum extracts. The ethyl acetate extract showed moderate nitric oxide scavenging activity (Figure 5).
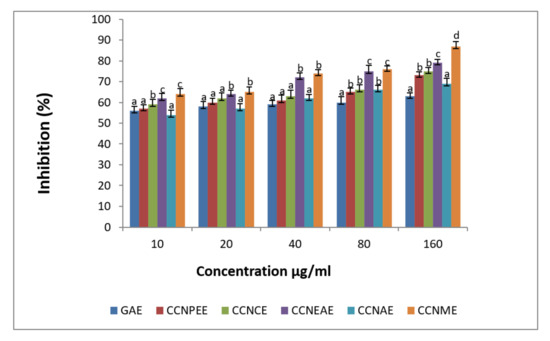
Figure 5.
Scavenging effect of the different extracts of Calligonum crinitum and standard gallic acid on nitric oxide radical. CCN—Calligonum crinitum, GAE—Gallic acid extract, CCNPEE—Petroleum ether extract, CCNCE—Chloroform extract, CCNEAE—Ethyl acetate extract, CCNAE—Acetone extract, CCNME—Methanol extract. Values are given as mean ± SD of six experiments in each group. Values that are not sharing a common superscript (a,b,c,d,e,f) differ significantly at p ≤ 0.05 (DMRT).
The important reactive radical nitric oxide (NO) is produced in the living system by means of many of the stresses and is deleterious in its effect in creating many undesirable effects [69]. In the present study, the extracts of C. crinitum significantly reduced this radical. Jagetia and Baliga [70] reported the nitric oxide scavenging activity in some of the Indian medicinal plants. Saha et al. [71] showed antioxidant and nitric oxide inhibitory activities from a variety of medicinal plants grown in Malaysia. In this study, there was significant scavenging of nitric oxide by the extracts of C. crinitum.
3.6.6. Reducing Power
Figure 6 shows the reducing power of the different extracts of C. crinitum. The concentrations tested were in a range from 10 to 160 µg/mL compared to GAE. When compared to the standard, the reducing power was highly significant in all of the extracts. Among the extracts, the methanolic concentrate of C. crinitum displayed a significant dose-dependent inhibition of reducing power movement.
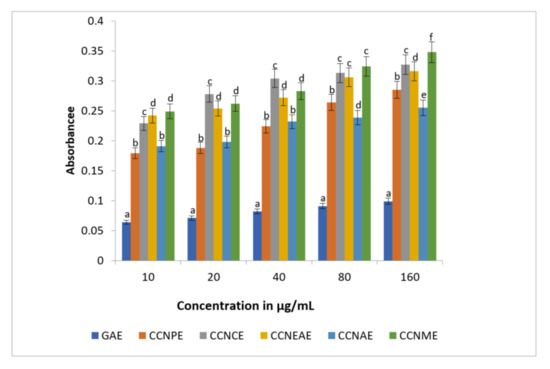
Figure 6.
Reducing power of methanolic extracts of the different extracts of Calligonum crinitum as compared to gallic acid. CCN—Calligonum crinitum, GAE—Gallic acid extract, CCNPEE—Petroleum ether extract, CCNCE—Chloroform extract, CCNEAE—Ethyl acetate extract, CCNAE—Acetone extract, CCNME—Methanol extract. Values are given as mean ± SD of six experiments in each group. Values that are not sharing a common superscript (a,b,c,d,e,f) differ significantly at p ≤ 0.05 (DMRT).
The antioxidant activity of a given extract or molecule can be evaluated based on reducing capacity [72]. The breaking of the free radical chain with the provision of a donation of a hydrogen atom is the general property of the compound in inducing the reducing power [73]. The absorbance of C. crinitum clearly increased due to the formation of the Fe2+-TPTZ complex with higher contents. This means they are donating electrons to free radicals. As shown in the result, the reducing power of the plant extract was higher when compared with the standard gallic acid. In this study, the reducing power of C. crinitum was increased with increasing concentration.
4. Conclusions
The screening of medicinal plants for potential biological activities and compounds is really important for the production of new drugs. In this study, C. crinitum’s biological extracts were analyzed to elucidate their medicinal potentials with an aim to scientifically validate traditional medicinal uses. The results throw light on some potential phytochemicals and antioxidant activities of the solvent extracts. Apart from the above, the proximate analysis and mineral nutritional content also showed significance in terms of providing health benefits that can well be correlated to their nutritional content. Therefore, in the future, more studies have to be taken up to precisely characterize how these compounds interact in the metabolic process.
Author Contributions
Conceptualization: A.J.; Formal analysis: K.M.A.A.N., K.K.; Funding acquisition: A.J.; Investigation: K.M.A.A.N., K.K.; Methodology: K.K.; Project administration: A.J.; Supervision: A.J., M.A.M.A., S.S.K.; Validation: A.J., M.A.M.A., S.S.K.; Writing—original draft: K.M.A.A.N., K.K.; Writing—review and editing: A.J., S.S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding except the support from MS Horticulture Fund of K.M.A.A.N. as part of his Master’s of Science in Horticulture Degree in the Department of Integrative Agriculture at UAEU, under the major supervision of A.J. and co-supervision of M.A.M.A. and S.S.K. The APC was funded by Ph.D. student fund #31F152 of K.M.A.A.N.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
The assistance from Arshed M. Eldaly (Agriculture Engineer, Al Foah Experimental Station, CAVM, UAEU) for the collection of samples is greatly acknowledged. We greatly appreciate the sincere help of Abou Messallam Azab (Lab Specialist, E3: Horticulture Lab), Felix T. Labata (Lab Specialist, E3: Animal Nutrition Lab) for lab analysis part.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Auddy, B.; Ferreira, M.; Blasina, F.; Lafon, L.; Arredondo, F.; Dajas, F.; Mukherjee, B. Screening of antioxidant activity of three Indian medicinal plants, traditionally used for the management of neurodegenerative diseases. J. Ethnopharmacol. 2003, 84, 131–138. [Google Scholar] [CrossRef]
- Cathrine, L.; Nagarajan, N.P. Preliminary phytochemical analysis and antibacterial activity of leaf extracts of Vitex leucoxylon LF. Int. J. Curr. Pharm. Res. 2011, 3, 71–73. [Google Scholar]
- Schippmann, U.; Leaman, D.J.; Cunningham, A.B. Impact of cultivation and gathering of medicinal plants on biodiversity: Global trends and issues. In Biodiversity and the Ecosystem Approach in Agriculture, Forestry and Fisheries; Inter-Department Working Group on Biology Diversity for Food and Agriculture, FAO: Rome, Italy, 2002. [Google Scholar]
- Falodun, A.; Irabor, E.E. Phytochemical, proximate, antioxidant and free radical scavenging evaluations of Calliandria surinamensis. Acta Pol. Pharm. Drug Res. 2008, 65, 571–575. [Google Scholar]
- Sivakumar, K.; Mohandass, S.; Devika, V. In vitro antioxidant and free radical scavenging activity of root extracts of Uraria lagopoides. Int. J. Pharma Bio. Sci. 2012, 3, B1–B9. [Google Scholar]
- Raj, X.J.; Chaurasia, O.P.; Vajpayee, P.K.; Murugan, M.P.; Bala, S.S. Antioxidative activity and phytochemical investigation on a high altitude medicinal plant Dracocephalum heterophyllum Benth. Pharmacogn. J. 2010, 2, 112–117. [Google Scholar]
- Potter, J.D. Vegetables, fruit, and cancer. Lancet 2005, 366, 527–530. [Google Scholar] [CrossRef]
- Marete, E.N.; Jacquier, J.C.; O’Riordan, D. Effects of extraction temperature on the phenolic and parthenolide contents, and colour of aqueous feverfew (Tanacetum parthenium) extracts. Food Chem. 2009, 117, 226–231. [Google Scholar] [CrossRef]
- Pandey, M.; Abidi, A.B.; Singh, S.; Singh, R.P. Nutritional evaluation of leafy vegetable paratha. J. Hum. Ecol. 2006, 19, 155–156. [Google Scholar] [CrossRef]
- Rajani, M.; Kanaki, N.S. Phytochemical standardization of herbal drugs and polyherbal formulations. In Bioactive Molecules and Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2008; pp. 349–369. [Google Scholar]
- Sun, J.; Liu, S.F.; Zhang, C.S.; Yu, L.N.; Bi, J.; Zhu, F.; Yang, Q.L. Chemical composition and antioxidant activities of Broussonetia papyrifera fruits. PLoS ONE 2012, 7, e32021. [Google Scholar] [CrossRef] [Green Version]
- Weijl, N.I.; Cleton, F.J.; Osanto, S. Free radicals and antioxidants in chemotherapy induced toxicity. Cancer Treat. Rev. 1997, 23, 209–240. [Google Scholar] [CrossRef]
- Manzano, P.; Hernández, J.; Quijano-Avilés, M.; Barragán, A.; Chóez-Guaranda, I.; Viteri, R.; Valle, O. Polyphenols extracted from Theobroma cacao waste and its utility as antioxidant. Emir. J. Food Agric. 2017, 29, 45. [Google Scholar] [CrossRef] [Green Version]
- Shahin, S.M.; Jaleel, A.; Alyafei, M.A.M. The Essential Oil-Bearing Plants in the United Arab Emirates (UAE): An Overview. Molecules 2021, 26, 6486. [Google Scholar] [CrossRef]
- Sakkir, S.; Kabshawi, M.; Mehairbi, M. Medicinal plants diversity and their conservation status in the United Arab Emirates (UAE). J. Med. Plants Res. 2012, 6, 1304–1322. [Google Scholar]
- Senthilkumar, A.; Karuvantevida, N.; Rastrelli, L.; Kurup, S.S.; Jaleel, A. Traditional uses, pharmacological efficacy, and phytochemistry of Moringa peregrina (Forssk.) Fiori.—A review. Front. Pharmacol. 2018, 9, 465. [Google Scholar] [CrossRef] [Green Version]
- Purohit, C.S.; Kumar, R. A review on genus Calligonum L. (Polygonaceae) from India and report Calligonum crinitum an addition for Flora of India. J. Asia-Pac. Biodivers. 2020, 13, 319–324. [Google Scholar] [CrossRef]
- Mandaville, J.P. Plant life in the Rub’al-Khali (the Empty Quarter), south-central Arabia. Proc. R. Soc. Edinburgh Sect. B Biol. Sci. 1986, 89, 147–157. [Google Scholar] [CrossRef]
- Ahmed, M.N.; Gowan, M.; Azam, M.N.K.; Mannan, M.A.; Rahman, M.M. Clinical appraisals and phytochemical potential of ethnomedicinal pteridophyte: Drynaria quercifolia (L.) J. Smith (Polypodiaceae). Pharmacol. Online 2015, 1, 4–17. [Google Scholar]
- Ahmed, H.; Moawad, A.; Owis, A.; AbouZid, S.; Ahmed, O. Flavonoids of Calligonum polygonoides and their cytotoxicity. Pharm. Biol. 2016, 54, 2119–2126. [Google Scholar] [CrossRef] [Green Version]
- Degheidy, N.S.; Sharaf, E.M.; Fathi, S.M. Field evaluation of anthelmentic efficacy of Calligonum comosum against Fasciolosis in sheep at Taif KSA. Glob. Vet. 2013, 11, 377–384. [Google Scholar]
- Abdo, W.; Hirata, A.; Shukry, M.; Kamal, T.; Abdel-Sattar, E.; Mahrous, E.; Yanai, T. Calligonum comosum extract inhibits diethylnitrosamine-induced hepatocarcinogenesis in rats. Oncol. Lett. 2015, 10, 716–722. [Google Scholar] [CrossRef]
- Jaleel, A.; Al Naqbi, K.M.; El-Kaabi, A.A.A.; Odeh, O.W.; Kandhan, K.; Maqsood, S.; Kurup, S.S.; Sakkir, S. In vitro antioxidant activities and screening of phytochemicals from methanolic and ethyl acetate extracts of Calligonum comosum L’Her. Orient. Pharm. Exp. Med. 2016, 16, 209–215. [Google Scholar]
- Safri, A.; Fletcher, A.J.; Abdel-Halim, E.; Ismail, M.A.; Hashem, A. Calligonum crinitum as a novel sorbent for sorption of Pb (II) from aqueous solutions: Thermodynamics, kinetics, and isotherms. J. Polym. Environ. 2021, 29, 1505–1515. [Google Scholar] [CrossRef]
- Sawangjaroen, N.; Sawangjaroen, K. The effects of extracts from anti-diarrheic Thai medicinal plants on the in vitro growth of the intestinal protozoa parasite: Blastocystis hominis. J. Ethnopharmacol. 2005, 98, 67–72. [Google Scholar] [CrossRef]
- Harborne, A.J. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis; Springer Science Business Media: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Edeoga, H.O.; Okwu, D.E.; Mbaebie, B.O. Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol. 2005, 4, 685–688. [Google Scholar] [CrossRef]
- Prakash, D.; Suri, S.; Upadhyay, G.; Singh, B.N. Total phenol, antioxidant and free radical scavenging activities of some medicinal plants. Int. J. Food Sci. Nutr. 2007, 58, 18–28. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Association of Analytical Chemists. Official Methods of Analysis, 15th ed.; AOAC: Washington, DC, USA, 1990; pp. 1121–1180. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis; Horwitz, W., Ed.; AOAC: Washington, DC, USA, 1995. [Google Scholar]
- Knevel, A.L.; Digangi, F.F. Jenkin’s Quantitative Pharmaceutical Chemistry; McGraw-Hill: New York, NY, USA, 1977. [Google Scholar]
- USEPA United States Environmental Protection Agency. Revision 5.4 Determination of Trace Elements in Waters and Wastes by Inductively Coupled Plasma–Mass Spectrometry; Method 200.8; USEPA United States Environmental Protection Agency: Washington, DC, USA, 1994. [Google Scholar]
- Wolfenden, B.S.; Willson, R.L. Radical-cations as reference chromogens in kinetic studies of ono-electron transfer reactions: Pulse radiolysis studies of 2, 2′-azinobis-(3-ethylbenzthiazoline-6-sulphonate). J. Chem. Soc. Perkin Trans. 2 1982, 7, 805–812. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.; Aruoma, O.I. The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal. Biochem. 1987, 165, 215–219. [Google Scholar] [CrossRef]
- Nishimiki, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulphate and molecular oxygen. Biochem. Biophys. Res. Comm. 1972, 46, 849–853. [Google Scholar] [CrossRef]
- Marcocci, L.; Maguire, J.J.; Droy-Lafaix, M.T.; Packer, L. The nitric oxide scavenging property of Ginkgo biloba extracts EGb 761. Biochem. Biophys. Res. Commun. 1994, 201, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, I.C.; Baptista, P.; Vilas-Boas, M.; Barros, L. Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: Individual cap and stipe activity. Food Chem. 2007, 100, 1511–1516. [Google Scholar] [CrossRef]
- Paulsamy, S.; Jeeshna, M.V. Preliminary phytochemistry and antimicrobial studies of an endangered medicinal herb Exacum bicolor Roxb. Res. J. Pharm. Biol. Chem. Sci. 2011, 2, 447–457. [Google Scholar]
- De Jesus, N.Z.T.; Falcão, H.d.S.; Gomes, I.F.; Leite, T.J.d.A.; Lima, G.R.d.M.; Barbosa-Filho, J.M.; Tavares, J.F.; Silva, M.S.d.; Athayde-Filho, P.F.d.; Batista, L.M. Tannins, Peptic Ulcers and Related Mechanisms. Int. J. Mol. Sci. 2012, 13, 3203–3228. [Google Scholar] [CrossRef] [Green Version]
- Sparg, S.; Light, M.E.; Van Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef]
- Pop, A.; Fizeșan, I.; Vlase, L.; Rusu, M.E.; Cherfan, J.; Babota, M.; Gheldiu, A.-M.; Tomuta, I.; Popa, D.-S. Enhanced Recovery of Phenolic and Tocopherolic Compounds from Walnut (Juglans Regia L.) Male Flowers Based on Process Optimization of Ultrasonic Assisted-Extraction: Phytochemical Profile and Biological Activities. Antioxidants 2021, 10, 607. [Google Scholar] [CrossRef]
- Sulaiman, S.F.; Sajak, A.A.B.; Ooi, K.L.; Seow, E.M. Effect of solvents in extracting polyphenols and antioxidants of selected raw vegetables. J. Food Compos. Anal. 2011, 24, 506–515. [Google Scholar] [CrossRef]
- Kessler, M.; Ubeaud, G.; Jung, L. Anti-and pro-oxidant activity of rutin and quercetin derivatives. J. Pharm. Pharmacol. 2003, 55, 131–142. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Dehpour, A.A.; Ebrahimzadeh, M.A.; Fazel, N.S.; Mohammad, N.S. Antioxidant activity of the methanol extract of Ferula assafoetida and its essential oil composition. Grasas Y Aceites 2009, 60, 405–412. [Google Scholar]
- Onwuka, G.I. Food Analysis and Instrumentation: Theory and Practice; Naphthalic prints: Surulere, Lagos, Nigeria, 2005; pp. 219–230. [Google Scholar]
- Jones, M.M. Chemistry and Society; Saunders College Publishing: Rochester, NY, USA, 1987. [Google Scholar]
- World Health Organization. Quality Control Methods for Medicinal Plant Materials; World Health Organization: Geneva, Switzerland, 1998. [Google Scholar]
- Hotz, C.; Brown, K.H. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr. Bull. 2004, 25, 194–195. [Google Scholar]
- Chaturvedi, U.C.; Shrivastava, R.; Upreti, R.K. Viral infections and trace elements: A complex interaction. Curr. Sci. 2004, 87, 1536–1554. [Google Scholar]
- Serdar, M.; Bakir, F.; Hasimi, A.; Celik, T.; Akin, O.; Kenar, L.; Yildirimkaya, M. Trace and toxic element patterns in nonsmoker patients with noninsulin-dependent diabetes mellitus, impaired glucose tolerance, and fasting glucose. Int. J. Diabetes Dev. Ctries. 2009, 29, 35. [Google Scholar] [CrossRef] [Green Version]
- Annan, K.; Kojo, A.I.; Cindy, A.; Samuel, A.N.; Tunkumgnen, B.M. Profile of heavy metals in some medicinal plants from Ghana commonly used as components of herbal formulations. Pharmacogn. Res. 2010, 2, 41. [Google Scholar] [CrossRef] [Green Version]
- Agomuo, E.N. Proximate, phytochemical, and mineral element analysis of the sclerotium of Pleurotus tuber-regium. Int. Sci. Res. J. 2011, 3, 104–107. [Google Scholar]
- Yazdanparast, R.; Bahramikia, S.; Ardestani, A. Nasturtium officinale reduces oxidative stress and enhances antioxidant capacity in hypercholesterolaemic rats. Chem.-Biol. Interact. 2008, 172, 176–184. [Google Scholar] [CrossRef]
- Ak, T.; Gülçin, İ. Antioxidant and radical scavenging properties of curcumin. Chem.-Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Alnajar, Z.A.A.; Abdulla, M.A.; Ali, H.M.; Alshawsh, M.A.; Hadi, A.H.A. Acute toxicity evaluation, antibacterial, antioxidant and immunomodulatory effects of Melastoma malabathricum. Molecules 2012, 17, 3547–3559. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.D.; Herdeiro, R.S.; Mathias, C.J.; Panek, A.D.; Silveira, C.S.; Rodrigues, V.P.; Nogueira, F.L.P. Evaluation of antioxidant activity of Brazilian plants. Pharmacol. Res. 2005, 52, 229–233. [Google Scholar] [CrossRef]
- Bhuiyan, M.A.R.; Hoque, M.Z.; Hossain, S.J. Free radical scavenging activities of Zizyphus mauritiana. World J. Agric. Sci. 2009, 5, 318–322. [Google Scholar]
- Lee, K.W.; Kim, Y.J.; Lee, H.J.; Lee, C.Y. Cocoa has more phenolic phytochemicals and a higher antioxidant capacity than teas and red wine. J. Agric. Food Chem. 2003, 51, 7292–7295. [Google Scholar] [CrossRef]
- Lipinski, B. Hydroxyl radical and its scavengers in health and disease. Oxidative Med. Cell. Longev. 2011, 2011, 809696. [Google Scholar] [CrossRef] [Green Version]
- Joseph, B.; Priya, M. Review on nutritional, medicinal and pharmacological properties of guava (Psidium guajava Linn.). Int. J. Pharma Bio. Sci. 2011, 2, 53–69. [Google Scholar]
- Choi, C.W.; Kim, S.C.; Hwang, S.S.; Choi, B.K.; Ahn, H.J.; Lee, M.Y.; Kim, S.K. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci. 2002, 163, 1161–1168. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free. Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagetia, G.C.; Baliga, M.S. The evaluation of nitric oxide scavenging activity of certain Indian medicinal plants in vitro: A preliminary study. J. Med. Food 2004, 7, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Lajis, N.H.; Israf, D.A.; Hamzah, A.S.; Khozirah, S.; Khamis, S.; Syahida, A. Evaluation of antioxidant and nitric oxide inhibitory activities of selected Malaysian medicinal plants. J. Ethnopharmacol. 2004, 92, 263–267. [Google Scholar] [CrossRef]
- López-Alarcón, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).