The Influence of Phytohormones on the Efficiency of Callus Formation, Its Morphologically Properties and Content of Bioactive Compounds in In Vitro Cultures of Daucus carota L.
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Cultures of Carrots
2.2. Methods of Material Preparation and Determination of the Selected Components
2.3. Statistical Evaluations
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karuppusamy, A. A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J. Med. Plants Res. 2009, 3, 1222–1239. [Google Scholar] [CrossRef]
- Davies, K.M.; Espley, R.V. Opportunities and challenges for metabolic engineering of secondary metabolite pathways for improved human health characters in fruit and vegetable crops. N. Z. J. Crop Hort. 2013, 41, 154–177. [Google Scholar] [CrossRef]
- Gaosheng, H.; Jingming, J. Production of useful secondary metabolites through regulation of biosynthetic pathway in cell and tissue suspension culture of medicinal plants. In Recent Advances in Plant In Vitro Culture; Leva, A., Ed.; Intechopen: London, UK, 2012; pp. 197–200. ISBN 978-953-51-5331-3. [Google Scholar] [CrossRef]
- Shilpa, K.; Varun, K.; Lakshmi, B.S. An alternative method of natural drug production: Eliciting secondary production using plant cell culture. J. Plant Sci. 2010, 5, 222–247. [Google Scholar] [CrossRef]
- Rao, R.S.; Ravishankar, G.A. Plant tissue cultures: Chemical factories of secondary metabolites. Biotechnol. Adv. 2002, 20, 101–153. [Google Scholar] [CrossRef]
- Mousavizadeh, S.J.; Mashayekhi, K.; Akbarpour, V.; Kalati, H.; Ghasemi, Y. Effect of IAA and 2,4-D on somatic embryogenesis and pigments synthesis of carrot root secondary phloem. Aust. J. Agric. Res. 2010, 1, 126–131. Available online: https://www.sciencej.com/akbarpour_1_4_2010_126_131.pdf (accessed on 1 December 2021).
- Nishi, A.; Kurosaki, F. Daucus Carota L. (Carrot): In Vitro Production of Carotenoids and Phytoalexins; Biotechnology in Agriculture and Forestry, Medicinal and Aromatic Plants V; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1993; Volume 24, pp. 178–191. [Google Scholar] [CrossRef]
- Arafa, N.M.; Mohamed, S.S.; Aly, U.I. In vitro antimicrobial activity of carrot callus extracts as affected by tyrosine and tryptophan precursor. Int. J. PharmTech Res. 2016, 9, 121–129. Available online: https://www.sphinxsai.com/2016/ph_vol9_no9/ph01.htm (accessed on 1 December 2021).
- Keutgen, A.J.; Wszelaczyńska, E.; Pobereżny, J.; Kozera, W.; Knapowski, T.; Mozolewski, W. Health promoting properties of carrot (Daucus carota L.) as a function of cultivar and use of soil fertility enhancer UGmax. Ecol. Technol. 2015, XXIII, 206–210. [Google Scholar]
- Keutgen, A.J.; Wszelaczyńska, E.; Pobereżny, J. Influence of cultivar and UGmax on antioxidative properties of carrot roots (Daucus carota L.) and their stability during freezing process. Environ. Prot. Nat. Resour. 2014, 25, 19–22. [Google Scholar] [CrossRef]
- Cefola, M.; Pace, B.; Renna, M.; Santamaria, P.; Signore, A.; Serio, F. Compositional analysis and antioxidant profile of yellow. orange and purple Polignano carrots. Ital. J. Food Sci. 2012, 24, 284–291. Available online: https://www.researchgate.net/publication/230842053 (accessed on 1 December 2021).
- Arscott, S.A.; Tanumihardjo, S.A. Carrots of many colors provide basic nutrition and bioavailable phytochemicals acting as a functional food. Compr. Rev. Food Sci. Food Saf. 2010, 9, 223–239. [Google Scholar] [CrossRef]
- Yau, Y.-Y.; Santos, K.; Simon, P. Molecular tagging and selection for sugar type in carrot roots using co-dominant. PCR-based markers. Mol. Breed. 2005, 16, 1–10. [Google Scholar] [CrossRef]
- Simon, P.W.; Wolff, X.Y.; Peterson, C.E.; Kammerlohr, D.S.; Rubatzky, V.E.; Strandberg, J.O.; Basset, M.J.; White, J.M. High carotene mass carrot population. HortScience 1989, 24, 174–175. [Google Scholar]
- Wszelaczyńska, E.; Pobereżny, J.; Keutgen, A.J.; Szczepanek, M.; Idaszewska, N.; Brewka, J. Qualitative changes in carrot preserves depending on foliar fertilization on plants with magnesium and on selected technological processes. Food Sci. Technol. Qual. 2015, 5, 182–197. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Al-Mallah, M.K. Determination of β-carotene in Carrot (Daucus carota L.) plants regenerated from stems callus. Rafidain J. Sci. 2013, 24, 27–36. Available online: https://www.iasj.net/iasj?func=fulltext&aId=74507 (accessed on 1 December 2021). [CrossRef]
- Bohm, V.; Putpitasari-Nienaber, N.L.; Ferruzzi, M.G.; Schwartz, S.J. Trolox equivalent antioxidant capacity of different geometrical isomers of α-carotene, β-carotene, lycopene and zeaxanthin. J. Agric. Food Chem. 2002, 50, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Steward, F.C. Growth and development of cultivated cells. III. Interpretations of the growth from free cells to carrot plants. Am. J. Bot. 1958, 45, 709–713. [Google Scholar] [CrossRef]
- Harborne, J.B.; Mayer, A.M.; Bar-Nun, N. Identification of the major anthocyanin of carrot cells in tissue culture as cyanidin 3-(sinapoylxylosylglucosylgalactoside). Z. Naturforsch. 1983, 38, 1055–1056. [Google Scholar] [CrossRef]
- Butt, S.J.; Varis, S.; Nasir, I.A.; Sheraz, S.; Shahid, A.; Ali, Q. Micropropagation in advanced vegetable production: A review. Adv. Life Sci. 2015, 2, 48–57. Available online: https://www.als-journal.com/articles/vol2issue2/222.15/pdf.pdf (accessed on 1 December 2021).
- Kiszczak, W.; Kowalska, U.; Kapuścińska, A.; Burian, M.; Górecka, K. Effect of low temperature on in vitro androgenesis of carrot (Daucus carota L.). In Vitro Cell Dev. Biol. Plant 2015, 51, 135–142. [Google Scholar] [CrossRef]
- Ojha, A.; Kumar, S.; Singh, R. Plant regeneration via somatic embryogenesis from root explants of Daucus carota L., subsp. Halophilus. Med. Plant 2012, 4, 65–70. [Google Scholar] [CrossRef]
- Rabiei, K.; Polyakov, A.; Khodambashi, M.; Sharafova, O.; Kalashnikva, E.; Hooshmand, S.; Omidi, M. Carrot (Daucus carota L.) in vitro regeneration. Veg. Crop. Res. Bull. 2010, 73, 13–22. [Google Scholar] [CrossRef]
- Pant, B.; Manandhar, S. In vitro propagation of carrot (Daucus carota L.). Sci. World 2007, 5, 51–53. [Google Scholar] [CrossRef]
- Teruaki, S.; Kenji, K. Improvement of synchronization on carrot somatic embryo culture by controlling dissolved oxygen concentration. environment control in Biology. Adv. Life Sci. 1999, 37, 179–184. [Google Scholar]
- Jay, V.; Genestier, S.; Courduroux, J.C. Bioreactor studies of the effect of medium pH on carrot (Daucus carota L.) somatic embryogenesis. Plant Cell Tissue Organ Cult. 1994, 36, 205–209. [Google Scholar] [CrossRef]
- Fujimura, T.; Komamine, A. Involvement of endogenous auxin in somatic embryogenesis in a carrot cell suspension culture. Z. Pflanzenphysiol. 1979, 95, 13–19. [Google Scholar] [CrossRef]
- Chen, C.H.; Holden, D.J. Differential morphogenetic responses of carrot callus to naphthaleneacetic acid, 2, 4-Dichlorophenoxyacetic acid, and Tordon in vitro. Proc. South Dak. Acad. Sci. 1973, 52, 66–71. [Google Scholar]
- Tavares, A.C.; Salgueiro, L.R.; Canhoto, J.M. In vitro propagation of the wild carrot Daucus carota L. subsp. halophilus (Brot.) A. Pujadas for conservation purposes. Vitr. Cell. Dev. Biol. Plant 2010, 46, 47–56. [Google Scholar] [CrossRef][Green Version]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assay with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Yau, Y.-Y.; Wang, K.Y. Increased regeneration ability of transgenic callus of carrot (Daucus carota L.) on B5-based regeneration medium. J. Appl. Hortic. 2012, 14, 152–156. [Google Scholar] [CrossRef]
- Pobereżny, J.; Wszelaczyńska, E.; Keutgen, A.J. Yield and chemical content of carrot storage roots depending on foliar fertilization with magnesium and duration of storage. J. Elem. 2012, 3, 479–494. [Google Scholar] [CrossRef]
- Michalik, B. Hodowla marchwi. In Hodowla Roślin Warzywnych; Niemirowicz-Szczytt, K., Ed.; Wyd. SGGW: Warszawa, Poland, 1993; pp. 55–77. [Google Scholar]
- Keutgen, A.J.; Pawelzik, E. Quality and nutritional value of strawberry fruit under long term salt stress. Food Chem. 2008, 107, 1413–1420. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidative power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Keutgen, A. Modification of spinach quality by selected pre- and postharvest treatment. Beitr. Zu Agrarwiss. 2000, 24, 126. [Google Scholar]
- Keutgen, A.J.; Wszelaczyńska, E.; Pobereżny, J.; Przewodowska, A.; Przewodowski, W.; Milczarek, W.; Tatarowska, D.; Flis, B.; Keutgen, N. Antioxidant properties of potato tubers (Solanum tuberosum L.) as a consequence of genetic potential and growing conditions. PLoS ONE 2019, 14, e0222976. [Google Scholar] [CrossRef] [PubMed]
- Mikołajczyk, S.; Wojciechowski, A. Ocena zdolności regeneracyjnych in vitro wybranych genotypów rzepaku (Brassica napus L.) przy zastosowaniu różnych barw światła i regulatorów wzrostu. Rośl. Oleiste 1999, 20, 81–92. [Google Scholar]
- Gatz, A.; Tomaszewska-Sowa, M.; Figas, A. Changes accompanying proliferative capacity and morphology of Nicotiana tabacum L. callus in response to 2, 4-D. Acta Agrobot. 2017, 70, 4. [Google Scholar] [CrossRef]
- Oggema, J.N.; Kinyua, M.G.; Ouma, J.P.; Owuoche, J.O. Agronomic performance of locally adapted sweet potato (Ipomoea batatas (L) Lam.) cultivars derived from tissue culture regenerated plants. Afr. J. Biotechnol. 2007, 6, 1418–1425. [Google Scholar] [CrossRef]
- Silvertand, B.; van Rooyen, A.; Lavrijsen, P.; van Harten, A.M.; Jacobsen, E. Plant regeneration via organogenesis and somatic embryogenesis in callus cultures derived from mature zygotic embryos of leek (Allium ampeloprasum L.). Euphytica 1996, 91, 261–270. [Google Scholar] [CrossRef]
- Gajewski, M.; Szymczak, P.; Elkner, K.; Dąbrowska, A.; Kret, A.; Danilcenko, H. Some aspects of nutritive and biological value of carrot cultivars with orange, yellow and purple-coloured roots. Veg. Crop. Res. Bull. 2007, 67, 149–161. [Google Scholar] [CrossRef]
- Alasalvar, C.; Grigor, J.M.; Zhang, D.; Quantick, P.C.; Shahidi, F. Comparison of volatiles. phenolics. sugars. antioxidant vitamins. and sensory quality of different colored carrot varieties. J. Agric. Food Chem. 2001, 49, 1410–1416. [Google Scholar] [CrossRef]
- Ismail, A.; Marjan, Z.M.; Foong, C. Total antioxidant activity and phenolic content in selected vegetables. Food Chem. 2004, 87, 581–586. [Google Scholar] [CrossRef]
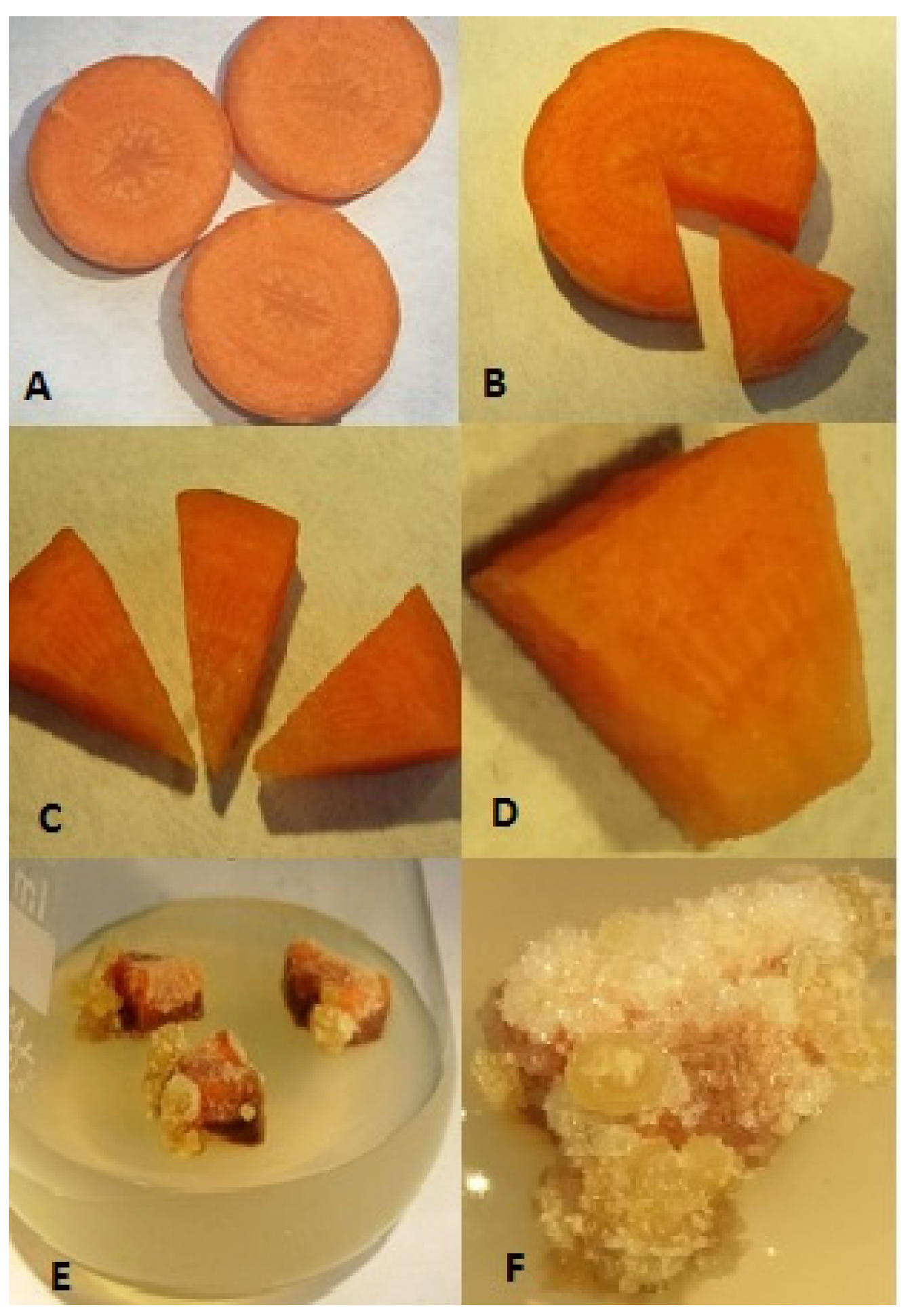
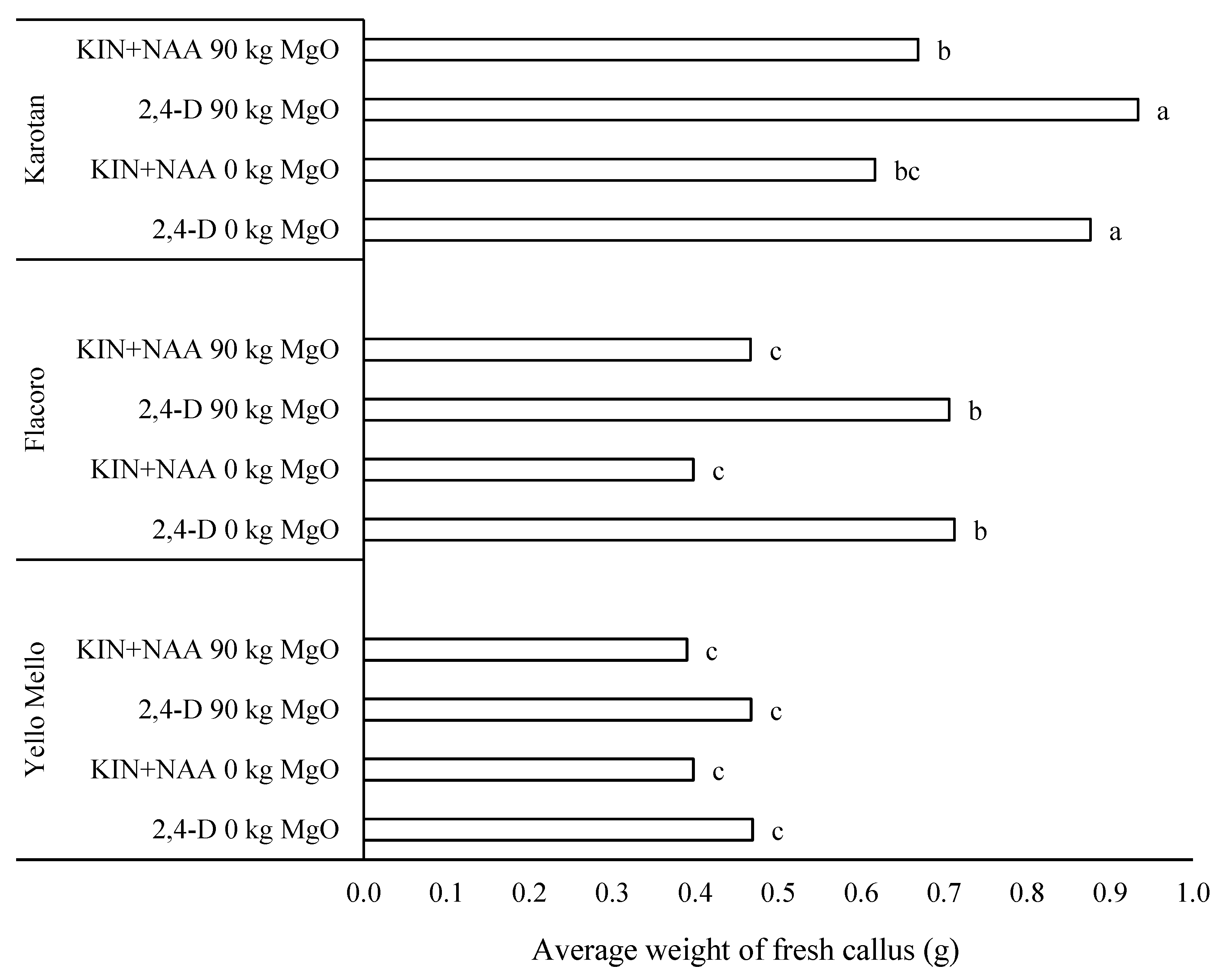

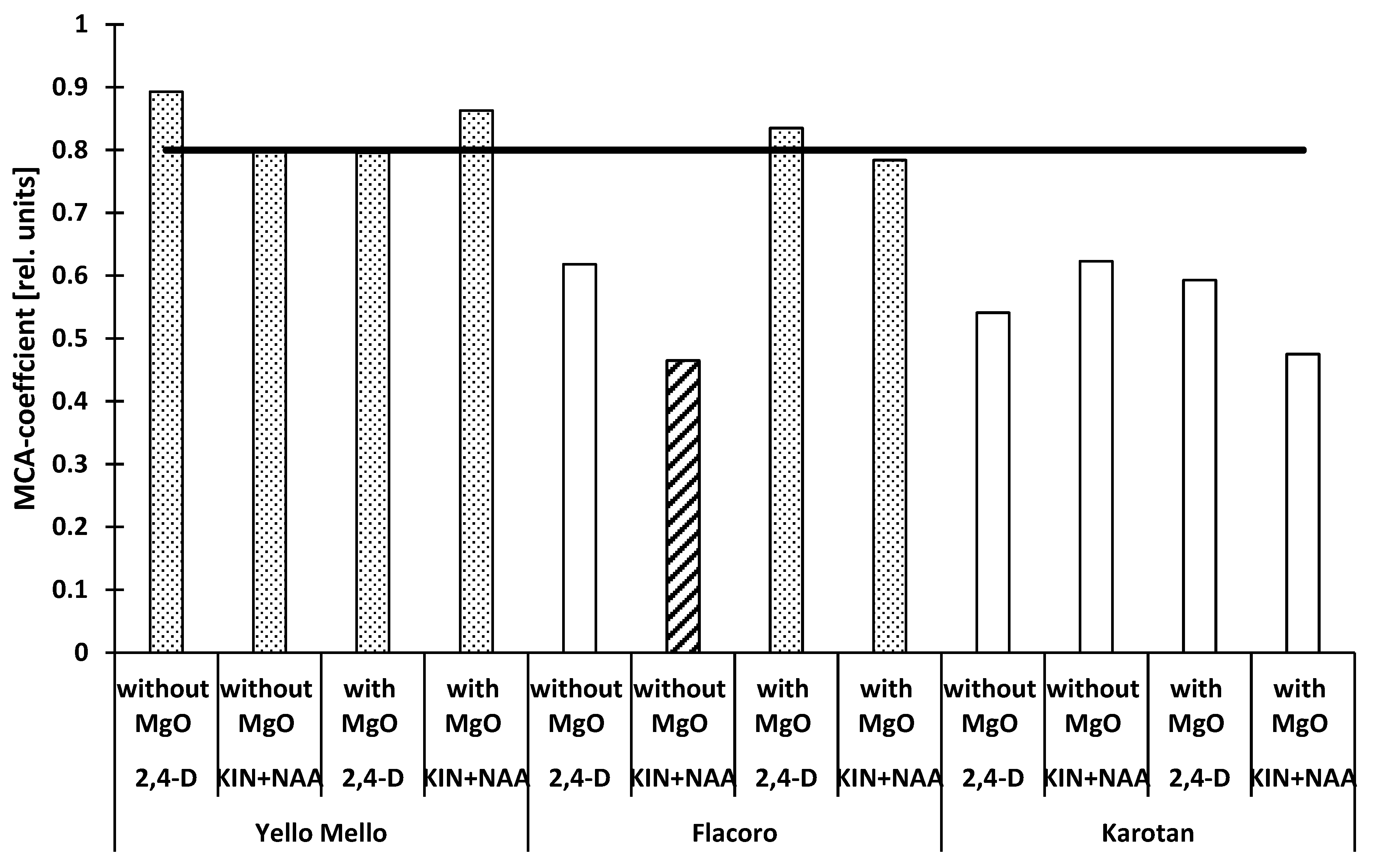
| Variety | Phytohormones in the Medium | MgO Fertilization (kg ha−1) | |
|---|---|---|---|
| 0 | 90 | ||
| Antioxidative potential (FRAP) (mmol Fe2+ kg−1 of fresh callus weight) | |||
| Karotan | 2,4–D | 0.254 ± 0.008 abc | 0.243 ± 0.030 abcd |
| KIN + NAA | 0.259 ± 0.091 abc | 0.207 ± 0.051 bcd | |
| Flacoro | 2,4–D | 0.265 ± 0.004 ab | 0.217 ± 0.018 bcd |
| KIN + NAA | 0.348 ± 0.039 a | 0.175 ± 0.020 bcd | |
| Yello Mello | 2,4–D | 0.138 ± 0.015 d | 0.157 ± 0.033 bcd |
| KIN + NAA | 0.235 ± 0.015 abcd | 0.149 ± 0.049 cd | |
| Polyphenols (mg kg−1 of fresh callus weight) | |||
| Karotan | 2,4–D | 797.92 ± 0.25 abc | 823.89 ± 52.10 abc |
| KIN + NAA | 837.84 ± 213.07 abc | 878.13 ± 156.16 ab | |
| Flacoro | 2,4–D | 899.02 ± 29.12 ab | 654.35 ± 120.00 bc |
| KIN + NAA | 1049.15 ± 124.48 a | 685.15 ± 46.43 bc | |
| Yello Mello | 2,4–D | 653.00 ± 21.77 bc | 581.38 ± 45.20 c |
| KIN + NAA | 724.02 ± 32.34 bc | 645.13 ± 2.44 bc | |
| Carotenoids (mg kg−1 of fresh callus weight) | |||
| Karotan | 2,4–D | 2.41 ± 0.81 ab | 2.44 ± 0.25 ab |
| KIN + NAA | 2.74 ± 0.58 ab | 3.95 ± 1.42 a | |
| Flacoro | 2,4–D | 1.65 ± 0.24 b | 1.72 ± 0.17 b |
| KIN + NAA | 2.92 ± 0.27 ab | 2.76 ± 0.26 ab | |
| Yello Mello | 2,4–D | 2.31 ± 0.29 ab | 2.76 ± 0.27 ab |
| KIN + NAA | 1.61 ± 0.14 c | 2.90 ± 0.36 ab | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keutgen, A.J.; Tomaszewska-Sowa, M.; Bomberski, A.; Keutgen, N. The Influence of Phytohormones on the Efficiency of Callus Formation, Its Morphologically Properties and Content of Bioactive Compounds in In Vitro Cultures of Daucus carota L. Horticulturae 2022, 8, 100. https://doi.org/10.3390/horticulturae8020100
Keutgen AJ, Tomaszewska-Sowa M, Bomberski A, Keutgen N. The Influence of Phytohormones on the Efficiency of Callus Formation, Its Morphologically Properties and Content of Bioactive Compounds in In Vitro Cultures of Daucus carota L. Horticulturae. 2022; 8(2):100. https://doi.org/10.3390/horticulturae8020100
Chicago/Turabian StyleKeutgen, Anna Jadwiga, Magdalena Tomaszewska-Sowa, Aleksander Bomberski, and Norbert Keutgen. 2022. "The Influence of Phytohormones on the Efficiency of Callus Formation, Its Morphologically Properties and Content of Bioactive Compounds in In Vitro Cultures of Daucus carota L." Horticulturae 8, no. 2: 100. https://doi.org/10.3390/horticulturae8020100
APA StyleKeutgen, A. J., Tomaszewska-Sowa, M., Bomberski, A., & Keutgen, N. (2022). The Influence of Phytohormones on the Efficiency of Callus Formation, Its Morphologically Properties and Content of Bioactive Compounds in In Vitro Cultures of Daucus carota L. Horticulturae, 8(2), 100. https://doi.org/10.3390/horticulturae8020100






