Damage Caused by Bacchisa medioviolacea Breuning (Coleoptera: Cerambycidae) in Wild Apple (Docynia indica) Orchards in Northwest Vietnam
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Bacchisa from Orchards
2.2. Identification and Life Cycle
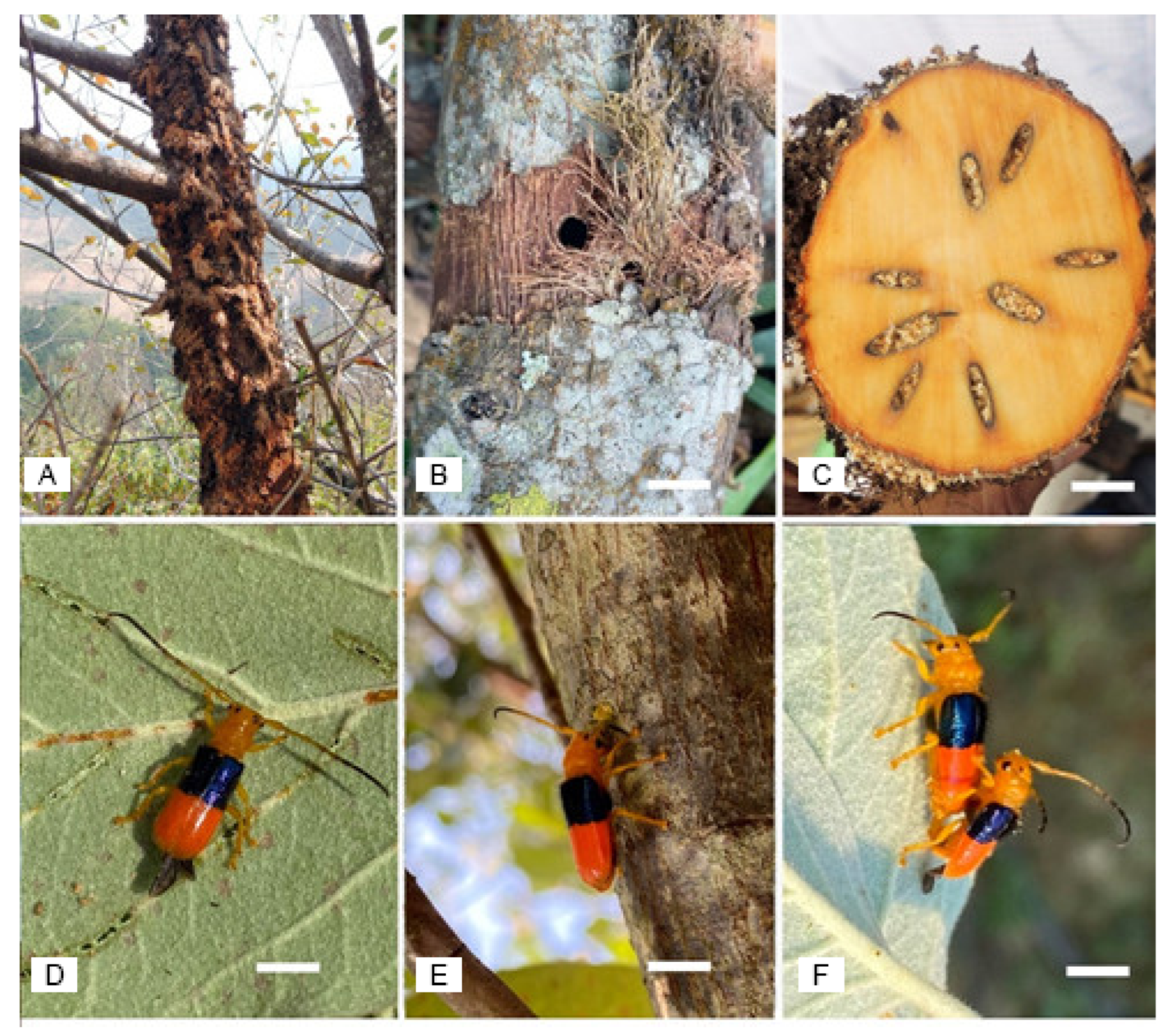
2.3. Assessment of Damage and Severity
2.4. Data Analysis
3. Results
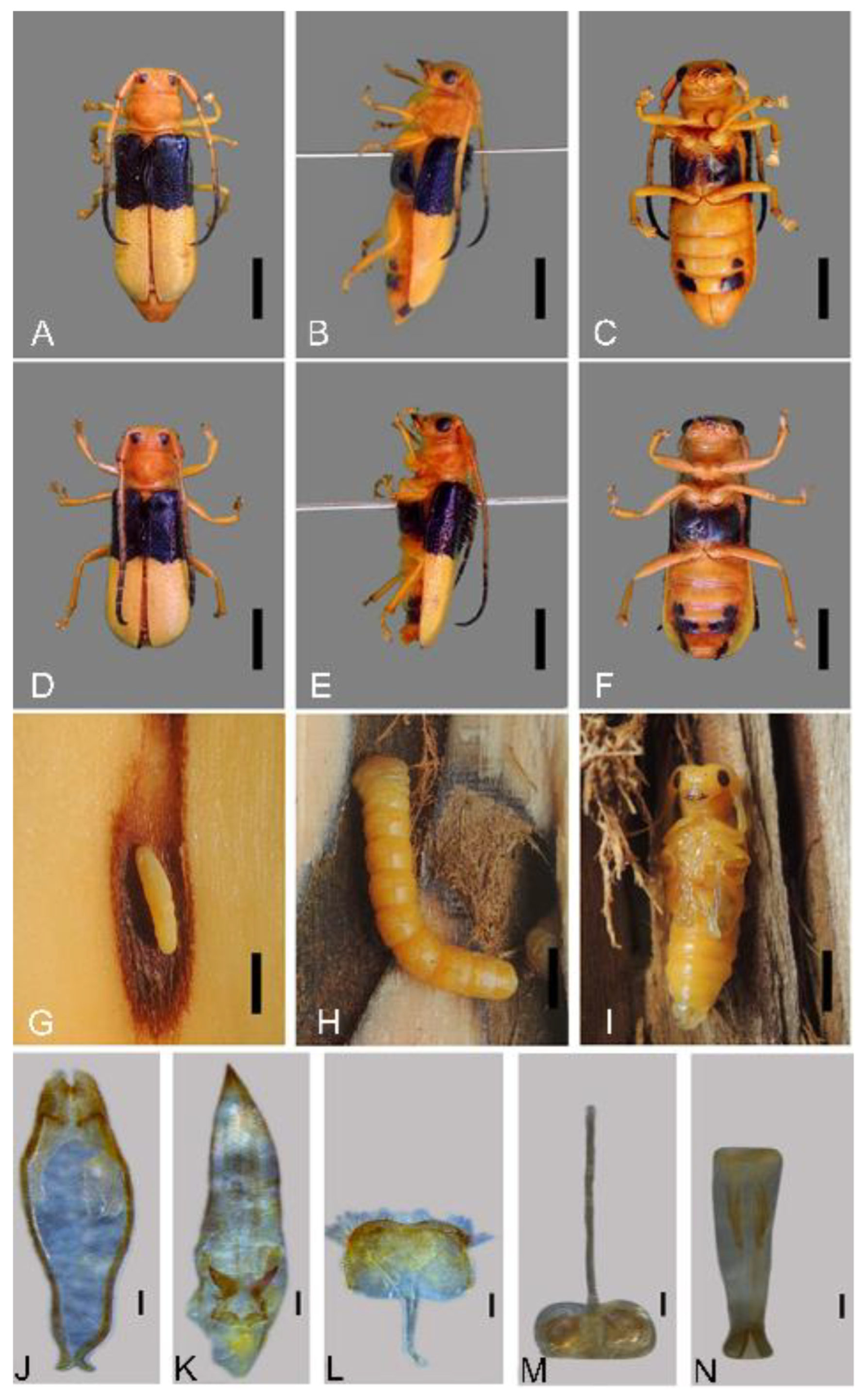
3.1. Identification and Description
3.2. Life Cycle
3.3. Damage Incidence and Severity
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bisecurity Australia. Import of Asian (‘Shandong’) Pear (Pyrus pyrifolia (Burm.) Nakai and P. ussuriensis var. viridis T. Lee) Fruit from Shandong Province in the People’s Republic of China; Bisecurity Australia: Canberra, Australia, 2003. [Google Scholar]
- Do, T.L. The Medicinal Plants and Traditional Medicines in Vietnam; Medical Publishing House: Hanoi, Vietnam, 2004; pp. 355–357. (In Vietnamese) [Google Scholar]
- Vo, V.C. Dictionary of Medicinal Plants in Vietnam; Medical Publishing House: Hanoi, Vietnam, 2012; pp. 1097–1098. (In Vietnamese) [Google Scholar]
- Biswas, J.; Barman, P.; Rahman, M.; Ghosh, R.A. Comprehensive Review on the Endemic Plant Docynia indica: Its Ethnobotanical, Phytochemical, and Ethnomedicinal Perspective. Asian. J. Biol. Life Sci. 2021, 10, 515. [Google Scholar] [CrossRef]
- Rymbai, H.; Roy, A.R.; Deshmukh, N.A.; Jha, A.K.; Shimray, W.; War, G.F.; Ngachan, S.V. Analysis study on potential underutilized edible fruit genetic resources of the foothills track of Eastern Himalayas, India. Genet. Resour. Crop. Evol. 2016, 63, 125–139. [Google Scholar] [CrossRef]
- Sharma, P.B.; Handique, P.J.; Devi, H.S. Antioxidant properties, physico-chemical characteristics and proximate composition of five wild fruits of Manipur, India. J. Food. Sci. Technol. 2015, 52, 894–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shende, K.M.; Singh, N.I.; Negi, P.S. Phytochemical characterization and biological activities of Docynia indica (Wall) fruit extracts. J. Mol. Genet. Med. 2016, 10, 2041–2045. [Google Scholar]
- Deng, X.; Zhao, X.; Lan, Z.; Jiang, J.; Yin, W.; Chen, L. Anti-tumor effects of flavonoids from the ethnic medicine Docynia delavayi (Franch.) Schneid. and its possible mechanism. J. Med. Food 2014, 17, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Shu, G.; Chen, L.; Mi, X.; Mei, Z.; Deng, X.A. Flavonoid component from Docynia delavayi (Franch.) Schneid represses transplanted H22 hepatoma growth and exhibits low toxic effect on tumor-bearing mice. Food. Chem. Toxicol. 2012, 50, 3166–3173. [Google Scholar] [CrossRef] [PubMed]
- Vietdelta. The Top 50 Most Famous Specialty Fruit of Vietnam. Available online: http://vdelta.com.vn/news/59/the-top-50-most-famous-specialty-fruit-of-vietnam (accessed on 30 May 2019).
- MARD Decision No. 4961/QD-BNN-TCLN Dated 17/11/2014 of the Ministry of Agriculture and Rural Development of Vietnam on Promulgating a List of Main Tree Species for Planting Production Forests and a List of Main Tree Species for Afforestation According to Forest Ecological Regions; Ministry of Agriculture and Rural Development of Vietnam: Hanoi, Vietnam, 2014. (In Vietnamese)
- Chinh, N.N.; Chung, C.T.; Can, V.V.; Dung, N.X.; Dao, N.K.; Hop, T.; Oanh, T.T.; Quynh, N.B.; Thin, N.N. Vietnam Forest Trees; Labour and Social Publisher: Hanoi, Vietnam, 2009; pp. 518–555. (In Vietnamese) [Google Scholar]
- Tiep, H.V.; Thuong, P.H.; Nguyen, L.; Lua, H.T.; Thuan, V.V.; Kieu, L.T.; Carsan, S.; Degrande, A.; Catacutan, D.; Harwood, C. Domestication of Docynia indica in Vietnam. For. Trees. Livelihoods 2018, 27, 230–242. [Google Scholar] [CrossRef]
- Lua, H.T.; Degrande, A.; Catacutan, D.; Hoa, N.T.; Cuong, V.K. Son Tra (Docynia indica) Value Chain and Market Analysis; World Agroforestry Centre: Hanoi, Vietnam, 2013. [Google Scholar]
- Chen, H.; Dong, L.; Zhou, C.; Liu, X.; Lian, F. Studies on the biology and control Bacchisa atritarsis Pic. Jiangxi Plant Protect. 2002, 25, 65–67. [Google Scholar]
- Li, L.Y.; Wang, R.; Waterhouse, D.F. The Distribution and Importance of Arthropod Pests and Weeds of Agriculture and Forestry Plantations in Southern China; Monographs, Australian Centre for International Agricultural Research: Canberra, Australia, 1997. [Google Scholar]
- Wang, Q. Cerambycid pests in agricultural and horticultural crops. In Cerambycidae of the World: Biology Pest Management; Wang, Q., Ed.; CRC Press/Taylor Francis: Boca Raton, FL, USA, 2017; pp. 471–472. [Google Scholar]
- Xu, C.; Yang, Z.D.; He, Y.Q. Observation for biological characteristics and control of Bacchisa fortunei. J. Northwest For. Uni. 2007, 22, 109–110. (In Chinese) [Google Scholar]
- Yang, B.L.; Yan, N.S.; Chen, G.; Tao, M. Behavior and control of longhorned beetle Bacchisa gueryi (Coleoptera: Cerambycidae). China Fruits. 1994, 5, 9–11. (In Chinese) [Google Scholar]
- Breuning, S. Révision des “Astathini”. Longicornia 1956, 3, 417–519. [Google Scholar]
- McMaugh, T. Guidelines for Surveillance for Plant Pests in Asia and the Pacific; Union Offset: Canberra, Australia, 2005. [Google Scholar]
- Nguyen, B.T.; Dao, X.T. Pests and Diseases and Plant Protection Measures; Agricultural Publisher: Hanoi, Vietnam, 2004. (In Vietnamese) [Google Scholar]
- Saito, A. Cerambycid beetles (Coleoptera, Cerambycidae) from Northern Vietnam II. A new species of the genus Bacchisa (Lamiinae, Astathini). Elytra 1999, 27, 36–41. [Google Scholar]
- Thomson, M.J. Systema cerambycidarum eu exposé de tous les genres compris dans la famille des Cerambycides et familles limitrophes. Mém. Soc. R. Sci. Liége. 1866, 19, 1–560. [Google Scholar]
- Guan, Y.Q. Occurrence and control of Chreonoma fortunei. Jiangxi Fruit Trees 1999, 1, 36. (In Chinese) [Google Scholar]
- She, D.; Fu, F.; Chen, F.; Zhou, C.; Ma, G. The occurrence of diseases and pests on the trunk and branches of Yunhexueli pear cultivar and their control. South China Fruits 2005, 4, 66–68. (In Chinese) [Google Scholar]
- Sato, T.; Sato, N. Development of New Insecticide “Robinhood”; Sumitomo Kagaku Co., Ltd.: Tokyo, Japan, 2017. [Google Scholar]
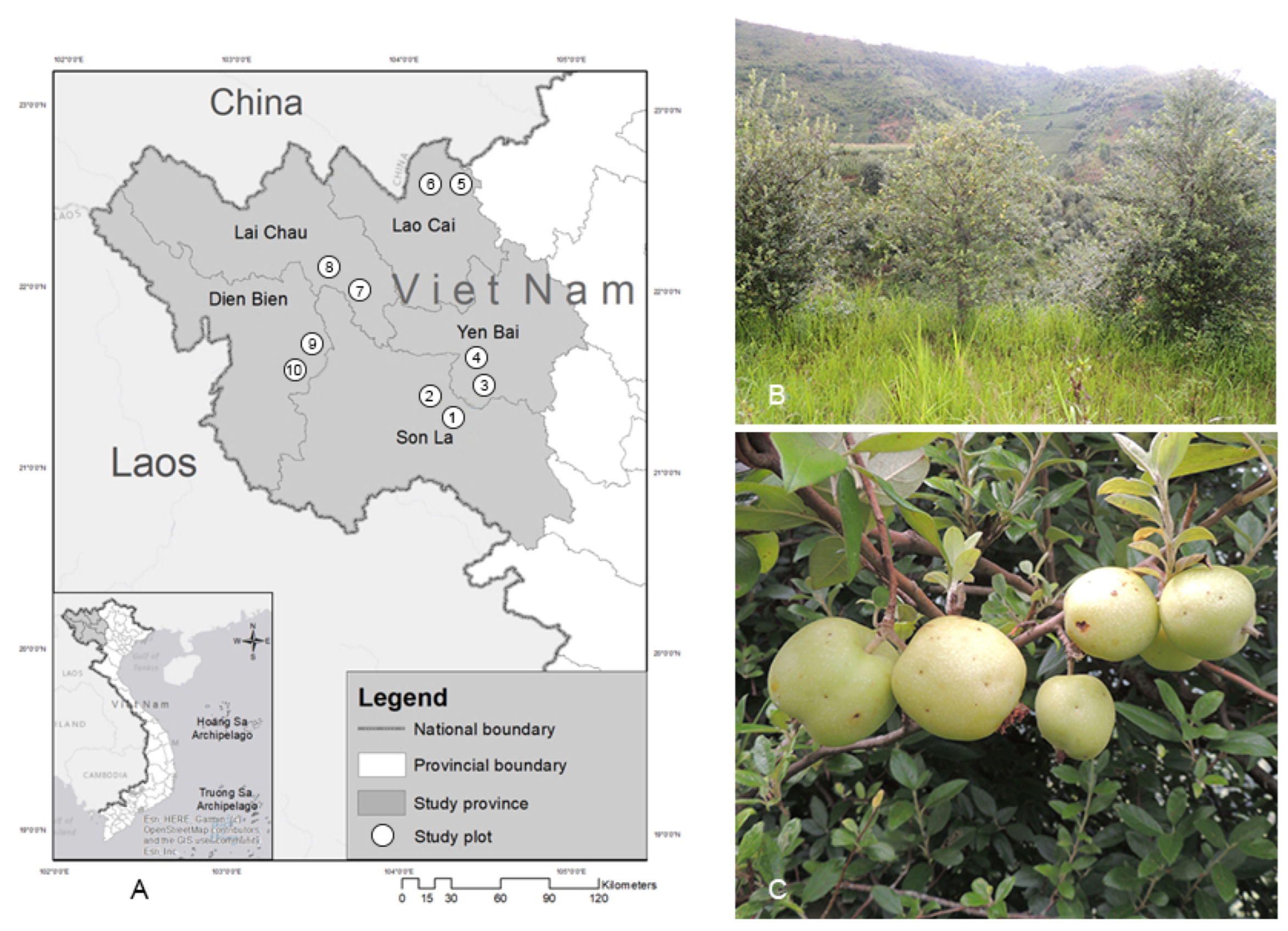
| Province | Map Number (Co ordinates) | 10–15-Year-Old Trees | 16–20-Year-Old Trees | ||
|---|---|---|---|---|---|
| P% | DI | P% | DI | ||
| Son La | 1 (21°24′75.7′′ N; 103°33′87.0′′ E) 2 (21°24′81.5′′ N; 103°33′93.5′ E) | 57.5 ± 4.11 | 1.23 ± 0.07 | 72.1 ± 5.05 | 1.77 ± 0.26 |
| Yen Bai | 3 (21°41′46.0′ N; 104°13′74.2′′ E) 4 (21°41′65.5′′ N; 104°13′87.8′′ E) | 53.8 ± 3.18 | 1.08 ± 0.09 | 70.5 ± 3.24 | 1.75 ± 0.17 |
| Lao Cao | 5 (22°33′00.8′′ N; 104°20′24.6′′ E) 6 (22°31′90.8′′ N; 104°18′16.1′′ E) | 40.9 ± 0.46 | 0.59 ± 0.04 | 65.7 ± 4.71 | 1.63 ± 0.17 |
| Lai Chau | 7 (22°12′47.9′′ N; 103°34′52.6′′ E) 8 (22°12′53.6′′ N; 103°34′57.8′′ E) | 40.3 ± 2.56 | 0.61 ± 0.04 | 57.5 ± 3.72 | 1.30 ± 0.11 |
| Dien Bien | 9 (21°35′18.0′′ N; 103°30′80.6′′ E) 10 (21°35′26.8′′ N; 103°30′73.6′′ E) | 28.8 ± 1.82 | 0.48 ± 0.07 | 31.1 ± 1.53 | 0.74 ± 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quang, D.N.; Thu, P.Q.; Binh, L.V.; Pham, D.L.; Thang, T.V.; Tong, T.A.; Thu, N.H.; Thanh, N.V.; Thong, N.Q.; Tiep, B.Q.; et al. Damage Caused by Bacchisa medioviolacea Breuning (Coleoptera: Cerambycidae) in Wild Apple (Docynia indica) Orchards in Northwest Vietnam. Horticulturae 2022, 8, 1219. https://doi.org/10.3390/horticulturae8121219
Quang DN, Thu PQ, Binh LV, Pham DL, Thang TV, Tong TA, Thu NH, Thanh NV, Thong NQ, Tiep BQ, et al. Damage Caused by Bacchisa medioviolacea Breuning (Coleoptera: Cerambycidae) in Wild Apple (Docynia indica) Orchards in Northwest Vietnam. Horticulturae. 2022; 8(12):1219. https://doi.org/10.3390/horticulturae8121219
Chicago/Turabian StyleQuang, Dao Ngoc, Pham Quang Thu, Le Van Binh, Duy Long Pham, Tran Viet Thang, Trang A. Tong, Nguyen Hoai Thu, Nguyen Van Thanh, Nguyen Quoc Thong, Bui Quang Tiep, and et al. 2022. "Damage Caused by Bacchisa medioviolacea Breuning (Coleoptera: Cerambycidae) in Wild Apple (Docynia indica) Orchards in Northwest Vietnam" Horticulturae 8, no. 12: 1219. https://doi.org/10.3390/horticulturae8121219
APA StyleQuang, D. N., Thu, P. Q., Binh, L. V., Pham, D. L., Thang, T. V., Tong, T. A., Thu, N. H., Thanh, N. V., Thong, N. Q., Tiep, B. Q., & Dell, B. (2022). Damage Caused by Bacchisa medioviolacea Breuning (Coleoptera: Cerambycidae) in Wild Apple (Docynia indica) Orchards in Northwest Vietnam. Horticulturae, 8(12), 1219. https://doi.org/10.3390/horticulturae8121219






