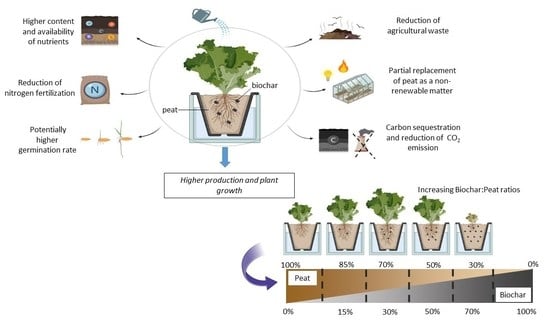
Influence of Biochar Mixed into Peat Substrate on Lettuce Growth and Nutrient Supply
Abstract
1. Introduction
2. Materials and Methods
2.1. Physical and Chemical Analysis of the Substrates
2.2. Determination of Nutrients Content
2.3. Plant Cultivation and Experimental Design
2.4. Leaf SPAD Index and Quantum Yield
2.5. Determination of Plant Biomass, Leaf Parameters and Nitrogen Use Efficiency
2.6. Statistical Analysis
3. Results
3.1. Properties of the Substrate
3.2. Plant Germination
3.3. Nutrient Dynamics in Substrates
3.4. NO3− and NH4+ Content in the Substrates
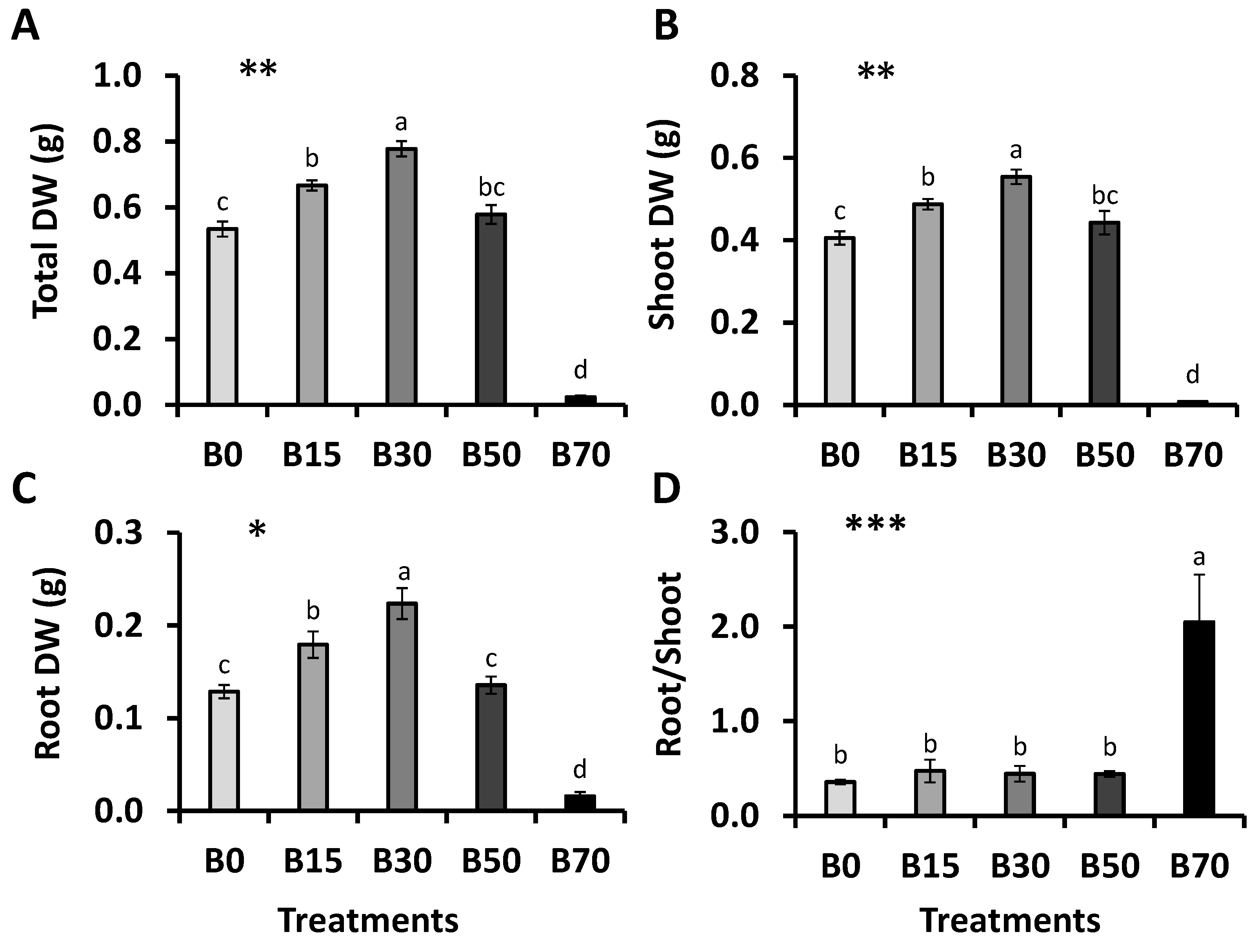
3.5. Plant Growth, Water and Stress Parameters
3.6. Plant Nutritional Status
3.7. Content of Different Nitrogen Forms and NUE
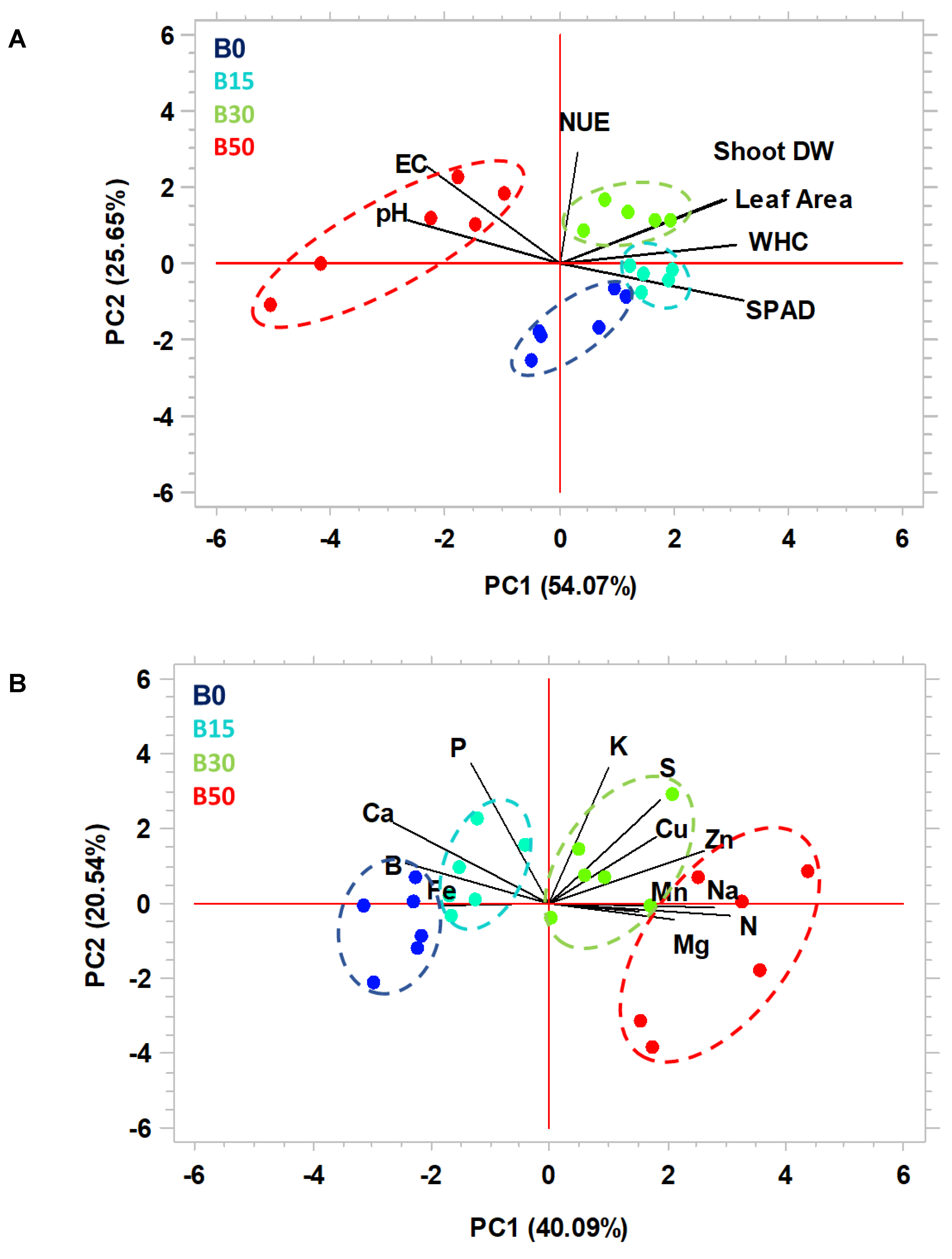
3.8. Principal Component Analysis (PCA)
4. Discussion
4.1. Substrate Characterization
4.2. Plant Physiological Characterization
4.3. Nutrients Dynamic and Effect in the Substrate-Plant System
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Zulfiqar, F.; Younis, A.; Chen, J. Biochar or Biochar-Compost Amendment to a Peat-Based Substrate Improves Growth of Syngonium podophyllum. Agronomy 2019, 9, 460. [Google Scholar] [CrossRef]
- Gruda, N.S. Increasing Sustainability of Growing Media Constituents and Stand-Alone Substrates in Soilless Culture Systems. Agronomy 2020, 9, 298. [Google Scholar] [CrossRef]
- Glaser, B.; Asomah, A.A.A. Plant Growth and Chemical Properties of Commercial Biochar-versus Peat-Based Growing Media. Horticulturae 2022, 8, 339. [Google Scholar] [CrossRef]
- Kern, J.; Tammeorg, P.; Shanskiy, M.; Sakrabani, R.; Knicker, H.; Kammann, C.; Tuhkanen, E.-M.; Smidt, G.; Prasad, M.; Tiilikkala, K.; et al. Synergistic use of peat and charred material in growing media—An option to reduce the pressure on peatlands? J. Environ. Eng. Landsc. Manag. 2017, 25, 160–174. [Google Scholar] [CrossRef]
- Matysek, M.; Leake, J.; Banwart, S.; Johnson, I.; Page, S.; Kaduk, J.; Smalley, A.; Cumming, A.; Zona, D. Optimizing fen peatland water-table depth for romaine lettuce growth to reduce peat wastage under future climate warming. Soil Use Manag. 2021, 38, 341–354. [Google Scholar] [CrossRef]
- Larcher, F.; Scariot, V. Assessment of Partial Peat Substitutes for the Production of Camellia japonica. HortScience 2009, 44, 312–316. [Google Scholar] [CrossRef]
- Nocentini, M.; Panettieri, M.; Barragán, J.M.G.D.C.; Mastrolonardo, G.; Knicker, H. Recycling pyrolyzed organic waste from plant nurseries, rice production and shrimp industry as peat substitute in potting substrates. J. Environ. Manag. 2020, 277, 111436. [Google Scholar] [CrossRef]
- Schmidt, H.P.; Bucheli, T.; Kammann, C.; Glaser, B.; Abiven, S.; Leifeld, J.; Soja, G.; Hagemann, N. Guidelines European Biochar Certificate; Version 10.1; European Biochar Foundation (EBC): Arbaz, Switzerland, 2022; pp. 1–63. [Google Scholar]
- De la Rosa, J.M.; Rosado, M.; Paneque, M.; Miller, A.Z.; Knicker, H. Effects of aging under field conditions on biochar structure and composition: Implications for biochar stability in soils. Sci. Total Environ. 2018, 613–614, 969–976. [Google Scholar] [CrossRef]
- Hossain, Z.; Bahar, M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.N.; Kirkham, M.B.; Chowdhury, S.; Bolan, N. Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Li, D.; Zhao, R.; Peng, X.; Ma, Z.; Zhao, Y.; Gong, T.; Sun, M.; Jiao, Y.; Yang, T.; Xi, B. Biochar-related studies from 1999 to 2018: A bibliometrics-based review. Environ. Sci. Pollut. Res. 2020, 27, 2898–2908. [Google Scholar] [CrossRef]
- Wu, P.; Ata-Ul-Karim, S.T.; Singh, B.P.; Wang, H.; Wu, T.; Liu, C.; Fang, G.; Zhou, D.; Wang, Y.; Chen, W. A scientometric review of biochar research in the past 20 years (1998–2018). Biochar 2019, 1, 23–43. [Google Scholar] [CrossRef]
- Huang, L.; Gu, M. Effects of Biochar on Container Substrate Properties and Growth of Plants—A Review. Horticulturae 2019, 5, 14. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Schmidt, H.; Kammann, C.; Hagemann, N.; Leifeld, J.; Bucheli, T.D.; Monedero, M.A.S.; Cayuela, M.L. Biochar in agriculture—A systematic review of 26 global meta-analyses. GCB Bioenergy 2021, 13, 1708–1730. [Google Scholar] [CrossRef]
- Alkharabsheh, H.M.; Seleiman, M.F.; Battaglia, M.L.; Shami, A.; Jalal, R.S.; Alhammad, B.A.; Almutairi, K.F.; Al–Saif, A.M. Biochar and Its Broad Impacts in Soil Quality and Fertility, Nutrient Leaching and Crop Productivity: A Review. Agronomy 2021, 11, 993. [Google Scholar] [CrossRef]
- Jindo, K.; Sánchez-Monedero, M.A.; Mastrolonardo, G.; Audette, Y.; Higashikawa, F.S.; Silva, C.A.; Akashi, K.; Mondini, C. Role of biochar in promoting circular economy in the agriculture sector. Part 2: A review of the biochar roles in growing media, composting and as soil amendment. Chem. Biol. Technol. Agric. 2020, 7, 1–10. [Google Scholar] [CrossRef]
- Shackley, S.; Russychaert, G.; Zwart, K.; Glaser, B. (Eds.) Biochar in European Soils and Agriculture: Science and Practice; Routledge: London, UK; New York, NY, USA, 2016; ISBN 9780415711661. [Google Scholar]
- Huang, L.; Gu, M.; Yu, P.; Zhou, C.; Liu, X. Biochar and Vermicompost Amendments Affect Substrate Properties and Plant Growth of Basil and Tomato. Agronomy 2020, 10, 224. [Google Scholar] [CrossRef]
- Carter, S.; Shackley, S.; Sohi, S.; Suy, T.B.; Haefele, S. The Impact of Biochar Application on Soil Properties and Plant Growth of Pot Grown Lettuce (Lactuca sativa) and Cabbage (Brassica chinensis). Agronomy 2013, 3, 404–418. [Google Scholar] [CrossRef]
- Massa, D.; Bonetti, A.; Cacini, S.; Faraloni, C.; Prisa, D.; Tuccio, L.; Petruccelli, R. Soilless tomato grown under nutritional stress increases green biomass but not yield or quality in presence of biochar as growing medium. Hortic. Environ. Biotechnol. 2019, 60, 871–881. [Google Scholar] [CrossRef]
- Campos, P.; Miller, A.Z.; Knicker, H.; Costa-Pereira, M.F.; Merino, A.; De la Rosa, J.M. Chemical, physical and morphological properties of biochars produced from agricultural residues: Implications for their use as soil amendment. Waste Manag. 2020, 105, 256–267. [Google Scholar] [CrossRef]
- Gascó, G.; Cely, P.; Paz-Ferreiro, J.; Plaza, C.; Méndez, A. Relation between biochar properties and effects on seed germination and plant development. Biol. Agric. Hortic. 2016, 32, 237–247. [Google Scholar] [CrossRef]
- Mickan, B.S.; Ren, A.-T.; Buhlmann, C.H.; Ghadouani, A.; Solaiman, Z.M.; Jenkins, S.; Pang, J.; Ryan, M.H. Closing the circle for urban food waste anaerobic digestion: The use of digestate and biochar on plant growth in potting soil. J. Clean. Prod. 2022, 347, 131071. [Google Scholar] [CrossRef]
- Purakayastha, T.; Bera, T.; Bhaduri, D.; Sarkar, B.; Mandal, S.; Wade, P.; Kumari, S.; Biswas, S.; Menon, M.; Pathak, H.; et al. A review on biochar modulated soil condition improvements and nutrient dynamics concerning crop yields: Pathways to climate change mitigation and global food security. Chemosphere 2019, 227, 345–365. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Fang, Z.; Van Zwieten, L.; Bolan, N.; Dong, D.; Quin, B.F.; Meng, J.; Li, F.; Wu, F.; Wang, H.; et al. A critical review of biochar-based nitrogen fertilizers and their effects on crop production and the environment. Biochar 2022, 4, 1–19. [Google Scholar] [CrossRef]
- Liao, J.; Liu, X.; Hu, A.; Song, H.; Chen, X.; Zhang, Z. Effects of biochar-based controlled release nitrogen fertilizer on nitro-gen-use efficiency of oilseed rape (Brassica napus L.). Sci. Rep. 2020, 10, 11063. [Google Scholar] [CrossRef]
- Sashidhar, P.; Kochar, M.; Singh, B.; Gupta, M.; Cahill, D.; Adholeya, A.; Dubey, M. Biochar for delivery of agri-inputs: Current status and future perspectives. Sci. Total Environ. 2019, 703, 134892. [Google Scholar] [CrossRef]
- de la Rosa, J.M.; Paneque, M.; Miller, A.Z.; Knicker, H. Relating physical and chemical properties of four different biochars and their application rate to biomass production of Lolium perenne on a Calcic Cambisol during a pot experiment of 79days. Sci. Total Environ. 2014, 499, 175–184. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Communications in Soil Science and Plant Analysis Rapid Colorimetric Determination of Nitrate in Plant Tissue by Nitration of Salicylic Acid. Commun. Soil Sci. Plant Anal. 1975, 37–41. [Google Scholar] [CrossRef]
- Greweling, T.; Peech, M. Chemical soil tests. Cornell Univ. Agric. Exp. Sta. Bull. 1960, 960. [Google Scholar]
- Bradstreet, R.B. Kjeldahl Method for Organic Nitrogen. Anal. Chem. 1954, 26, 185–187. [Google Scholar] [CrossRef]
- Franco-Navarro, J.D.; Díaz-Rueda, P.; Rivero-Núñez, C.M.; Brumós, J.; E Rubio-Casal, A.; de Cires, A.; Colmenero-Flores, J.M.; A Rosales, M. Chloride nutrition improves drought resistance by enhancing water deficit avoidance and tolerance mechanisms. J. Exp. Bot. 2021, 72, 5246–5261. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; Dezonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Barrs, H.D.; Weatherley, P.E. A Re-Examination of the Relative Turgidity Technique for Estimating Water Deficits in Leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Marcelis, L.; Heuvelink, E.; Goudriaan, J. Modelling biomass production and yield of horticultural crops: A review. Sci. Hortic. 1998, 74, 83–111. [Google Scholar] [CrossRef]
- Longstreth, D.J.; Nobel, P.S. Salinity Effects on Leaf Anatomy. Plant Physiol. 1979, 63, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Congreves, K.A.; Otchere, O.; Ferland, D.; Farzadfar, S.; Williams, S.; Arcand, M.M. Nitrogen Use Efficiency Definitions of Today and Tomorrow. Front. Plant Sci. 2021, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Joseph, S. (Eds.) Biochar for Environmental Management: Science, Technology and Implementation; Routledge: London, UK, 2015; 448p. [Google Scholar] [CrossRef]
- Prasad, M.; Chrysargyris, A.; McDaniel, N.; Kavanagh, A.; Gruda, N.S.; Tzortzakis, N. Plant Nutrient Availability and pH of Biochars and Their Fractions, with the Possible Use as a Component in a Growing Media. Agronomy 2020, 10, 10. [Google Scholar] [CrossRef]
- Wortman, S.E. Crop physiological response to nutrient solution electrical conductivity and pH in an ebb-and-flow hydroponic system. Sci. Hortic. 2015, 194, 34–42. [Google Scholar] [CrossRef]
- Gezahegn, S.; Sain, M.; Thomas, S.C. Variation in Feedstock Wood Chemistry Strongly Influences Biochar Liming Potential. Soil Syst. 2019, 3, 26. [Google Scholar] [CrossRef]
- Hailegnaw, N.S.; Mercl, F.; Pračke, K.; Száková, J.; Tlustoš, P. Mutual relationships of biochar and soil pH, CEC, and exchangeable base cations in a model laboratory experiment. J. Soils Sediments 2019, 19, 2405–2416. [Google Scholar] [CrossRef]
- Asao, T. Hydroponics—A Standard Methodology for Plant Biological Researches; Intechopen: London, UK, 2012; ISBN 9789535103868. [Google Scholar]
- Nieto, A.; Gascó, G.; Paz-Ferreiro, J.; Fernández, J.; Plaza, C.; Méndez, A. The effect of pruning waste and biochar addition on brown peat based growing media properties. Sci. Hortic. 2016, 199, 142–148. [Google Scholar] [CrossRef]
- George, M. Unravelling the impact of potentially toxic elements and biochar on soil: A review. Environ. Chall. 2022, 8, 100540. [Google Scholar] [CrossRef]
- Awad, Y.M.; Lee, S.-E.; Ahmed, M.B.M.; Vu, N.T.; Farooq, M.; Kim, I.S.; Kim, H.S.; Vithanage, M.; Usman, A.R.A.; Al-Wabel, M.; et al. Biochar, a potential hydroponic growth substrate, enhances the nutritional status and growth of leafy vegetables. J. Clean. Prod. 2017, 156, 581–588. [Google Scholar] [CrossRef]
- Prasad, M.; Tzortzakis, N.; McDaniel, N. Chemical characterization of biochar and assessment of the nutrient dynamics by means of preliminary plant growth tests. J. Environ. Manag. 2018, 216, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Regulation EU Regulation of the European Parliament and of the Council Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003. Off. J. Eur. Union 2019, 2019, 114.
- Brdar-Jokanović, M. Boron Toxicity and Deficiency in Agricultural Plants. Int. J. Mol. Sci. 2020, 21, 1424. [Google Scholar] [CrossRef]
- Yu, H.; Zou, W.; Chen, J.; Chen, H.; Yu, Z.; Huang, J.; Tang, H.; Wei, X.; Gao, B. Biochar amendment improves crop production in problem soils: A review. J. Environ. Manag. 2018, 232, 8–21. [Google Scholar] [CrossRef]
- Buss, W.; Graham, M.C.; Shepherd, J.G.; Mašek, O. Risks and benefits of marginal biomass-derived biochars for plant growth. Sci. Total Environ. 2016, 569–570, 496–506. [Google Scholar] [CrossRef]
- Atzori, G.; Pane, C.; Zaccardelli, M.; Cacini, S.; Massa, D. The Role of Peat-Free Organic Substrates in the Sustainable Management of Soilless Cultivations. Agronomy 2021, 11, 1236. [Google Scholar] [CrossRef]
- Wang, C.; Luo, D.; Zhang, X.; Huang, R.; Cao, Y.; Liu, G.; Zhang, Y.; Wang, H. Biochar-based slow-release of fertilizers for sustainable agriculture: A mini review. Environ. Sci. Ecotechnol. 2022, 10, 100167. [Google Scholar] [CrossRef]
- Nelissen, V.; Rütting, T.; Huygens, D.; Staelens, J.; Ruysschaert, G.; Boeckx, P. Maize biochars accelerate short-term soil nitrogen dynamics in a loamy sand soil. Soil Biol. Biochem. 2012, 55, 20–27. [Google Scholar] [CrossRef]
- Zhang, M.; Song, G.; Gelardi, D.L.; Huang, L.; Khan, E.; Mašek, O.; Parikh, S.J.; Ok, Y.S. Evaluating biochar and its modifications for the removal of ammonium, nitrate, and phosphate in water. Water Res. 2020, 186, 116303. [Google Scholar] [CrossRef] [PubMed]
- Fidel, R.B.; Laird, D.A.; Spokas, K.A. Sorption of ammonium and nitrate to biochars is electrostatic and pH-dependent. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dan, X.; Meng, L.; He, M.; Chen, S.; He, X.; Zhao, C.; Li, X.; Cai, Z.; Zhang, J.; Müller, C. Gross N transformations and plant N use efficiency in intensive vegetable production soils. Soil Biol. Biochem. 2022, 174, 108817. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, H.; Shen, F.; Yang, G.; Zhang, Y.; Zeng, Y.; Wang, L.; Xiao, H.; Deng, S. Biochar produced from oak sawdust by Lanthanum (La)-involved pyrolysis for adsorption of ammonium (NH4+), nitrate (NO3−), and phosphate (PO43−). Chemosphere 2015, 119, 646–653. [Google Scholar] [CrossRef]
- Duan, P.; Zhou, J.; Feng, L.; Jansen-Willems, A.B.; Xiong, Z. Pathways and controls of N2O production in greenhouse vegetable production soils. Biol. Fertil. Soils 2019, 55, 285–297. [Google Scholar] [CrossRef]
- Wang, Z.; Zong, H.; Zheng, H.; Liu, G.; Chen, L.; Xing, B. Reduced nitrification and abundance of ammonia-oxidizing bacteria in acidic soil amended with biochar. Chemosphere 2015, 138, 576–583. [Google Scholar] [CrossRef]
- Xiao, Z.; Rasmann, S.; Yue, L.; Lian, F.; Zou, H.; Wang, Z. The effect of biochar amendment on N-cycling genes in soils: A meta-analysis. Sci. Total. Environ. 2019, 696, 133984. [Google Scholar] [CrossRef]
- Pereira, E.I.P.; Conz, R.F.; Six, J. Nitrogen utilization and environmental losses in organic greenhouse lettuce amended with two distinct biochars. Sci. Total Environ. 2017, 598, 1169–1176. [Google Scholar] [CrossRef]

| Treatment | TC (mg g−1) | TN (mg g−1) | C:N | pH | EC (µS cm−1) | BD (g cm−3) | WHC (% w:w) |
|---|---|---|---|---|---|---|---|
| Biochar | 408.9 ± 0.5 | 0.5 ± 0.0 | 817.8 | 10.4 ± 0.0 | 1530 ± 17 | 0.3 ± 0.0 | 164 ± 24 |
| Peat | 510.0 ± 11.3 | 19.9 ± 0.2 | 25.6 | 5.7 ± 0.0 | 460 ± 5 | 0.4 ± 0.0 | 118 ± 29 |
| Treatment | Biochar (v:v %) | Peat (v:v %) | Vermiculite (v:v %) | pH | EC (µS cm−1) | WHC (% w:w) | |
|---|---|---|---|---|---|---|---|
| 0 DAS a | 31 DAS | ||||||
| B0 B15 B30 B50 B70 | 0 15 30 50 70 | 70 55 40 20 0 | 30 30 30 30 30 | 7.9 ± 0.1 bc 7.6 ± 0.1 c 7.7 ± 0.0 bc 8.1 ± 0.1 b 9.4 ± 0.1 a | 117 ± 9 d 215 ± 3 cd 371 ± 17 c 642 ± 29 b 1129 ± 45 a | 211 ± 49 119 ± 16 107 ± 19 96 ± 10 108 ± 13 | 269 ± 4 ab 272 ± 6 ab 289 ± 6 a 242 ± 10 bc 223 ± 6 c |
| p | ** | * | ns b | *** | |||
| Treatment | N (mg g−1 DW a) | P (mg g−1 DW) | S (mg g−1 DW) | Mg (mg g−1 DW) | Ca (mg g−1 DW) | K (mg g−1 DW) | |
|---|---|---|---|---|---|---|---|
| Pure Biochar | 0.50 | 4.40 | 0.75 | 6.01 | 45.2 | 14.4 | |
| 0 DAS b | B0 B15 B30 B50 B70 | 13.62 ± 0.18 a 10.73 ± 0.48 b 7.70 ± 0.16 c 4.49 ± 0.19 d 0.38 ± 0.02 e | 0.45 ± 0.02 c 1.39 ± 0.11 bc 1.29 ± 0.01 bc 2.34 ± 0.37 ab 3.43 ± 0.32 a | 1.68 ± 0.02 a 1.23 ± 0.20 ab 1.05 ± 0.12 ab 0.68 ± 0.11 b 0.54 ± 0.04 b | 32.42 ± 4.08 35.60 ± 1.50 32.68 ± 5.73 29.94 ± 3.24 32.16 ± 2.35 | 9.35 ± 0.28 c 19.66 ± 5.58 bc 15.98 ± 0.32 bc 28.09 ± 4.74 ab 41.88 ± 1.09 a | 1.04 ± 0.07 c 3.11 ± 0.37 bc 4.81 ± 0.40 bc 7.33 ± 0.73 ab 11.11 ± 0.89 a |
| p | *** | ** | ** | ns | * | *** | |
| 31 DAS | B0 B15 B30 B50 B70 | 13.92 ± 0.33 c 18.41 ± 1.60 bc 24.19 ± 1.30 b 36.26 ± 0.35 a 36.64 ± 0.12 a | 0.64 ± 0.04 d 1.41 ± 0.15 c 1.78 ± 0.06 c 2.76 ± 0.10 b 3.85 ± 0.18 a | 2.36 ± 0.05 a 1.98 ± 0.13 a 2.14 ± 0.13 a 1.36 ± 0.09 b 0.65 ± 0.03 c | 44.43 ± 3.05 a 48.27 ± 3.38 a 35.84 ± 3.12 ab 36.93 ± 3.23 ab 31.47 ± 2.53 b | 12.50 ± 0.22 d 19.45 ± 1.43 cd 27.47 ± 1.59 c 39.44 ± 2.36 b 57.34 ± 4.90 a | 1.90 ± 0.27 e 4.70 ± 0.34 d 7.05 ± 0.18 c 10.40 ± 0.27 b 12.20 ± 0.45 a |
| p | ** | * | * | * | * | * | |
| T c HT d TxHT e | *** *** *** | *** ** ns | *** *** * | ns ** ns | *** *** ns | *** *** ns |
| Treatment | Fe (mg g−1 DW a) | Mn (µg g−1 DW) | Cu (µg g−1 DW) | Zn (µg g−1 DW) | B (µg g−1 DW) | Na (µg g−1 DW) | |
|---|---|---|---|---|---|---|---|
| Pure Biochar | 2.42 | 250 | 147 | 169 | 47.9 | 735 | |
| 0 DAS b | B0 B15 B30 B50 B70 | 16.15 ± 2.01 18.32 ± 1.54 16.19 ± 3.74 18.45 ± 5.86 15.35 ± 1.19 | 315.65 ± 43.21 352.11 ± 18.95 301.34 ± 37.83 472.61 ± 113.00 487.23 ± 43.72 | 74.74 ± 11.87 b 107.33 ± 14.70 b 92.42 ± 13.32 b 140.51 ± 25.32 ab 196.73 ± 6.63 a | 48.45 ± 3.40 c 68.51 ± 4.60 bc 76.70 ± 10.06 abc 173.39 ± 50.19 ab 179.85 ± 10.36 a | 1.12 ± 0.09 c 9.18 ± 1.50 bc 12.58 ± 0.50 bc 23.40 ± 4.11 ab 37.58 ± 1.92 a | 145.95 ± 9.60 c 188.43 ± 19.26 bc 228.10 ± 9.10 bc 370.67 ± 34.04 ab 486.23 ± 27.75 a |
| p | ns | ns | * | * | *** | *** | |
| 31 DAS | B0 B15 B30 B50 B70 | 22.31 ± 0.97 22.30 ± 1.28 19.70 ± 1.08 21.46 ± 2.43 20.24 ± 1.11 | 427.14 ± 18.19 b 493.56 ± 21.88 ab 546.61 ± 19.26 ab 606.89 ± 57.49 a 629.08 ± 37.08 a | 86.55 ± 6.11 c 127.29 ± 8.69 bc 142.44 ± 6.31 bc 186.92 ± 12.92 b 253.07 ± 14.20 a | 98.69 ± 3.60 d 117.23 ± 5.30 cd 189.04 ± 24.06 bc 212.28 ± 12.16 ab 283.02 ± 12.63 a | 11.83 ± 0.81 d 20.54 ± 1.22 cd 31.98 ± 2.42 bc 43.93 ± 1.41 ab 51.41 ± 3.70 a | 135.50 ± 14.80 d 239.99 ± 21.99 c 410.52 ± 18.62 b 501.37 ± 25.97 ab 524.52 ± 22.07 a |
| p | ns | ** | *** | *** | *** | ** | |
| T c HT d TxHT e | ns ** ns | *** *** ns | *** *** ns | *** *** ns | *** *** ns | *** *** ** |
| NO3− (mg g−1 DW a) | NH4+ (mg g−1 DW) | |||
|---|---|---|---|---|
| Treatment | 0 DAS b | 31 DAS | 0 DAS | 31 DAS |
| Biochar | Bd c | bd | ||
| B0 B15 B30 B50 B70 | 0.26 ± 0.01 a 0.24 ± 0.01 ab 0.22 ± 0.01 b 0.19 ± 0.01 c 0.17 ± 0.01 d | 0.55 ± 0.07 c 0.67 ± 0.09 bc 1.13 ± 0.10 ab 1.27 ± 0.11 a 1.42 ± 0.21 a | 0.24 ± 0.01 a 0.19 ± 0.01 b 0.14 ± 0.01 c 0.08 ± 0.01 d 0.01 ± 0.01 e | 0.05 ± 0.01 a 0.03 ± 0.01 ab 0.02 ± 0.01 b 0.02 ± 0.01 b 0.01 ± 0.01 b |
| p | ** | * | ** | * |
| T d HT e TxHT f | *** *** *** | *** *** *** | ||
| Treatment | Water Content (%) | Leaf Area (cm2) | Specific Leaf Area (cm2 g−1) | Succulence (mg H2O cm −2) | SPAD Index | Quantum Yield (F’m F’v−1) |
|---|---|---|---|---|---|---|
| B0 B15 B30 B50 | 95.48 ± 0.13 95.83 ± 0.13 95.85 ± 0.12 95.63 ± 0.27 | 259.19 ± 16.16 b 319.98 ± 12.72 ab 371.80 ± 30.40 a 324.99 ± 19.72 ab | 74.83 ± 5.81 68.09 ± 2.42 63.14 ± 5.31 67.82 ± 2.01 | 31.68 ± 1.72 35.31 ± 1.84 35.53 ± 3.39 33.37 ± 2.19 | 16.70 ± 0.30 a 16.92 ± 1.17 a 16.87 ± 0.58 a 13.17 ± 0.59 b | 0.74 ± 0.01 0.74 ± 0.01 0.75 ± 0.01 0.74 ± 0.01 |
| p | ns | * | ns | ns | * | ns |
| Treatment | N (mg g−1 DW a) | P (mg g−1 DW) | S (mg g−1 DW) | Mg (mg g−1 DW) | Ca (mg g−1 DW) | K (mg g−1 DW) |
|---|---|---|---|---|---|---|
| B0 B15 B30 B50 | 41.51 ± 0.46 bc 39.39 ± 1.61 c 50.08 ± 2.00 ab 55.32 ± 2.88 a | 8.54 ± 0.30 a 8.72 ± 0.30 a 8.59 ± 0.25 a 7.05 ± 0.47 b | 3.56 ± 0.16 3.90 ± 0.13 4.38 ± 0.10 4.07 ± 0.36 | 4.59 ± 0.21 b 3.91 ± 0.12 b 4.23 ± 0.11 b 5.67 ± 0.30 a | 12.95 ± 0.43 a 11.78 ± 0.51 a 9.84 ± 0.37 b 7.87 ± 0.56 c | 71.09 ± 2.09 b 83.21 ± 2.47 a 83.60 ± 1.50 a 76.65 ± 3.16 ab |
| p | * | ** | ns | *** | *** | ** |
| Treatment | Fe (µg g−1 DW a) | Mn (µg g−1 DW) | Cu (µg g−1 DW) | Zn (µg g−1 DW) | B (µg g−1 DW) | Na (mg g−1 DW) |
|---|---|---|---|---|---|---|
| B0 B15 B30 B50 | 266.85 ± 48.93 a 160.24 ± 12.20 b 115.97 ± 5.08 b 132.10 ± 17.66 b | 156.90 ± 11.64 a 89.06 ± 5.59 b 140.39 ± 3.84 ab 190.81 ± 24.27 a | 6.77 ± 0.43 b 10.69 ± 0.98 ab 15.96 ± 4.46 a 13.02 ± 0.53 ab | 64.43 ± 3.63 b 96.05 ± 8.07 ab 97.93 ± 5.86 a 112.70 ± 12.01 a | 36.11 ± 1.26 a 28.57 ± 1.07 b 26.62 ± 1.36 b 24.03 ± 1.58 b | 1.16 ± 0.18 b 1.15 ± 0.08 b 1.48 ± 0.11 ab 1.84 ± 0.09 a |
| p | ** | *** | * | ** | *** | ** |
| Treatment | Organic N (mg g−1 DW a) | NO3− (mg g−1 DW) | NH4+ (µg g−1 DW) | TNA (mg N) | NUE (g DW g−1 N) |
|---|---|---|---|---|---|
| B0 B15 B30 B50 | 33.32 ± 1.36 b 33.83 ± 0.70 b 38.04 ± 0.59 a 37.17 ± 0.26 a | 7.62 ± 0.19 bc 5.17 ± 0.42 c 13.90 ± 2.09 ab 18.01 ± 2.63 a | 0.07 ± 0.01 0.09 ± 0.01 0.09 ± 0.01 0.10 ± 0.01 | 30.32 ± 3.85 b 29.21 ± 2.29 b 45.18 ± 2.13 a 32.07 ± 0.78 ab | 0.91 ± 0.04 c 1.46 ± 0.03 b 2.23 ± 0.12 a 1.53 ± 0.16 b |
| p | * | * | ns | * | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Rodríguez, Á.F.; Moreno-Racero, F.J.; García de Castro Barragán, J.M.; Colmenero-Flores, J.M.; Greggio, N.; Knicker, H.; Rosales, M.A. Influence of Biochar Mixed into Peat Substrate on Lettuce Growth and Nutrient Supply. Horticulturae 2022, 8, 1214. https://doi.org/10.3390/horticulturae8121214
García-Rodríguez ÁF, Moreno-Racero FJ, García de Castro Barragán JM, Colmenero-Flores JM, Greggio N, Knicker H, Rosales MA. Influence of Biochar Mixed into Peat Substrate on Lettuce Growth and Nutrient Supply. Horticulturae. 2022; 8(12):1214. https://doi.org/10.3390/horticulturae8121214
Chicago/Turabian StyleGarcía-Rodríguez, Álvaro F., Francisco J. Moreno-Racero, José M. García de Castro Barragán, José M. Colmenero-Flores, Nicolas Greggio, Heike Knicker, and Miguel A. Rosales. 2022. "Influence of Biochar Mixed into Peat Substrate on Lettuce Growth and Nutrient Supply" Horticulturae 8, no. 12: 1214. https://doi.org/10.3390/horticulturae8121214
APA StyleGarcía-Rodríguez, Á. F., Moreno-Racero, F. J., García de Castro Barragán, J. M., Colmenero-Flores, J. M., Greggio, N., Knicker, H., & Rosales, M. A. (2022). Influence of Biochar Mixed into Peat Substrate on Lettuce Growth and Nutrient Supply. Horticulturae, 8(12), 1214. https://doi.org/10.3390/horticulturae8121214