Leaf Removal Impacted Jasmonic Acid Metabolism and AsA-GSH in the Roots of Malus baccata (L.) Borkh. under Suboptimal Low Root-Zone Temperatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
2.2. Superoxide Radical (O2−), Hydrogen Peroxide (H2O2), and Malondialdehyde (MDA) Analyses
2.3. Antioxidant Enzyme Activity
2.4. Jasmonic Acid (JA), Methyl Jasmonate (MeJA), and Jasmonate Isoleucine (JA-Ile) Levels
2.5. Allene Oxide Synthase (AOS), Jasmonic Acid Carboxyl Methyltransferase (JMT), and Jasmonate-Resistant 1 (JAR) Activities
2.6. Total RNA Extraction and Gene Transcript Measurement
2.7. Statistical Analysis
3. Results
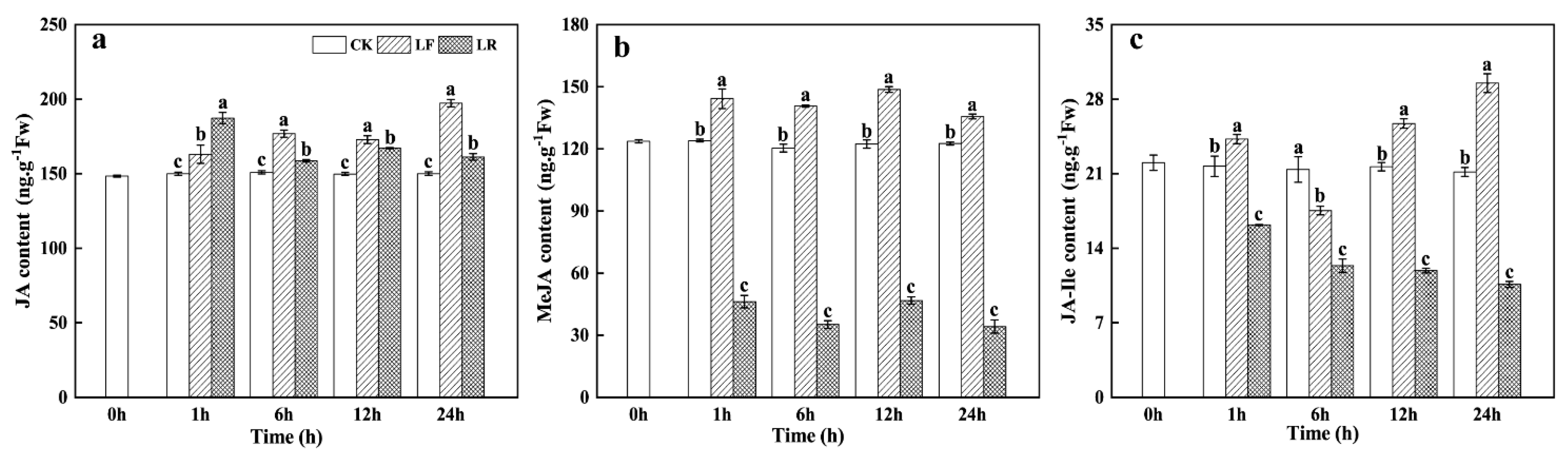
3.1. Endogenous Jasmonates (JAs) Contents
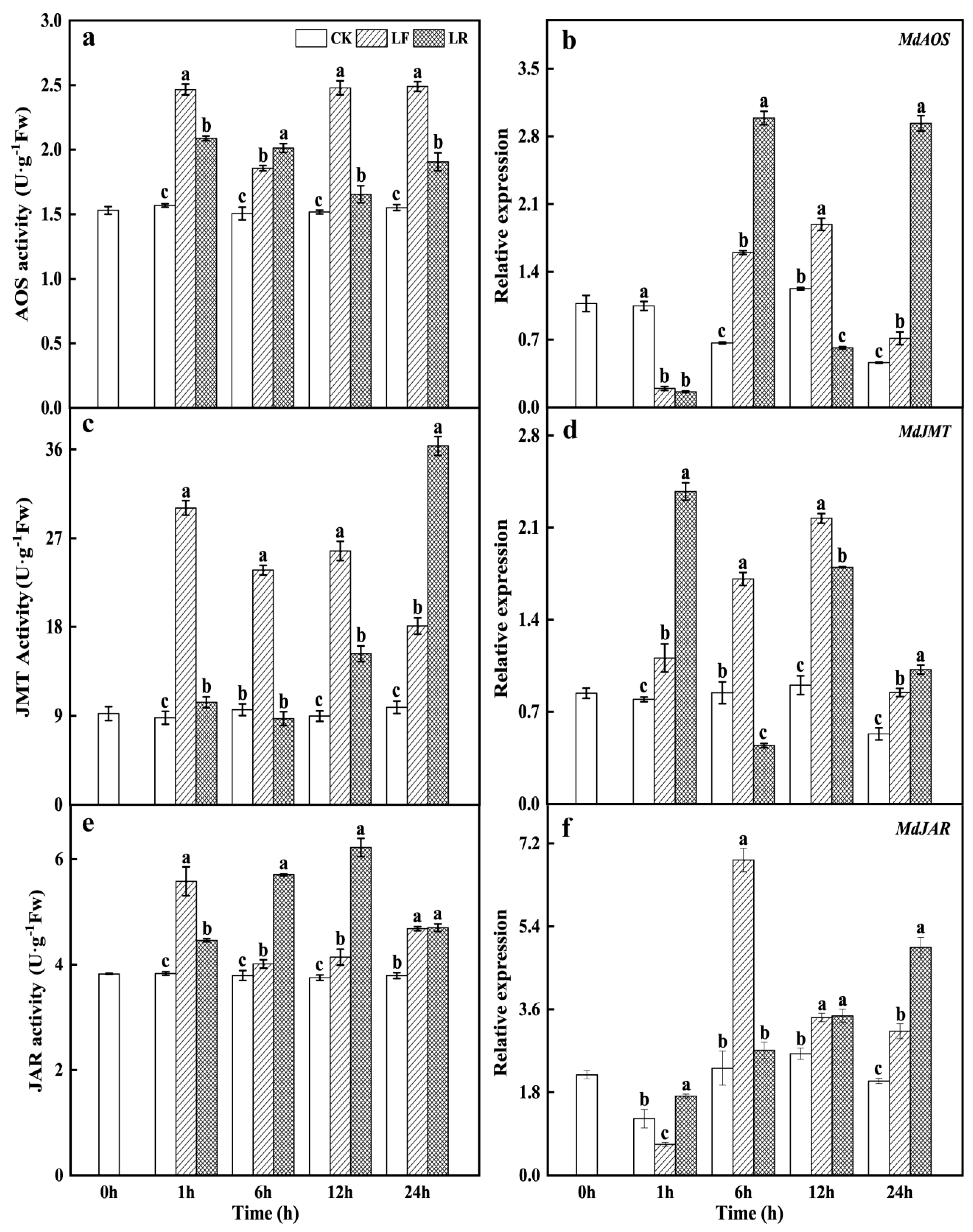
3.2. Activities and Transcription Levels of Key Enzymes in the Jasmonate Biosynthesis Pathway
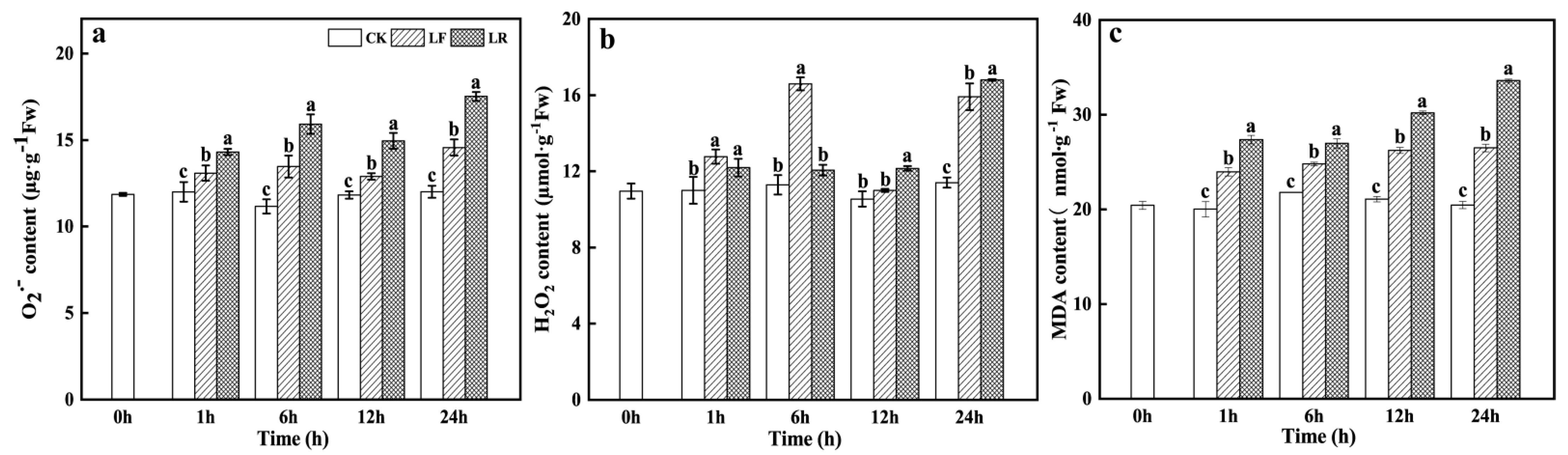
3.3. Superoxide Radical (O2−), Hydrogen Peroxide (H2O2), and Malondialdehyde (MDA) Contents
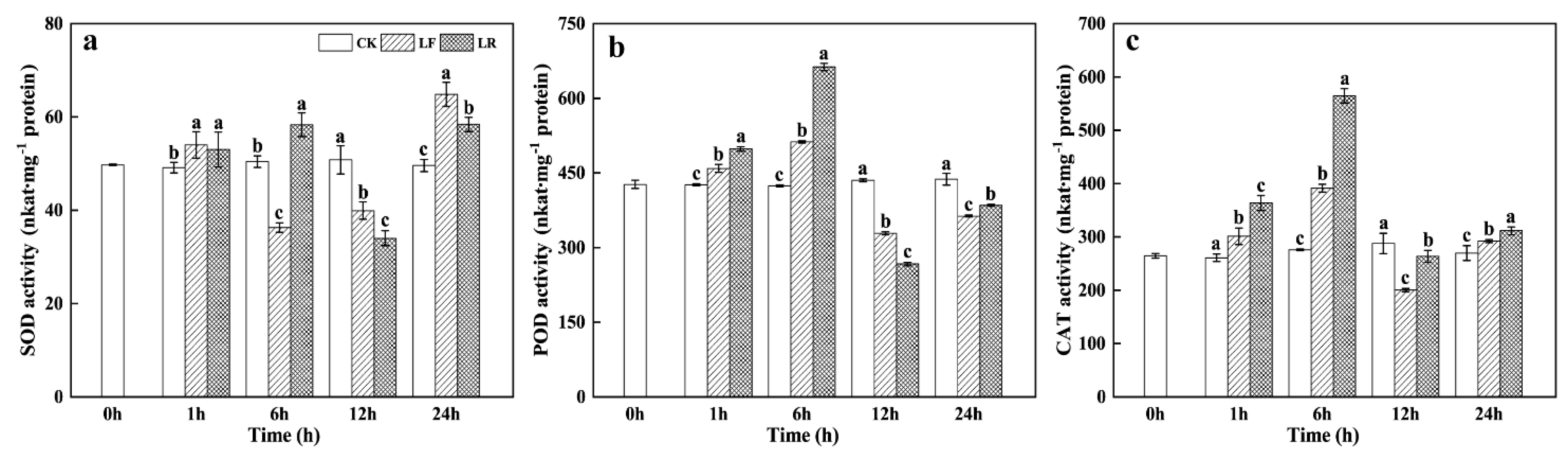
3.4. Superoxide Dismutase (SOD), Peroxidase (POD), and Catalase (CAT) Activities
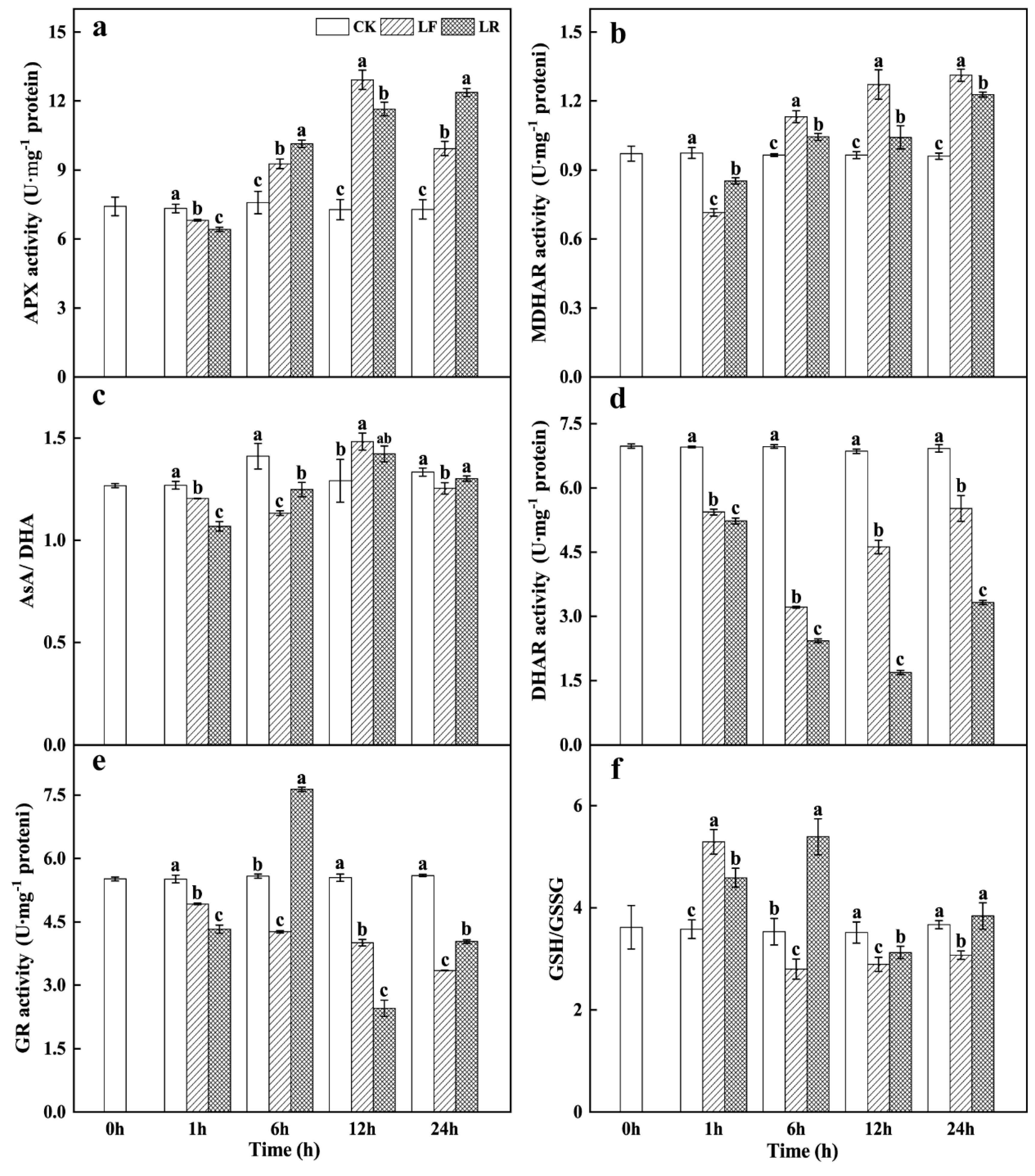
3.5. Ascorbate–Glutathione (AsA–GSH) Cycle Activity
3.6. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanghera, G.S.; Wani, S.H.; Hussain, W.; Singh, N.B. Engineering cold stress tolerance in crop plants. Curr. Genom. 2011, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.; Fujita, M. Exogenous spermidine alleviates low temperature injury in mung bean (Vigna radiata L.) seedlings by modulating ascorbate-glutathione and glyoxalase pathway. Int. J. Mol. Sci. 2015, 16, 30117–30132. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Sui, N. Overexpression of maize MYB-IF35 increases chilling tolerance in Arabidopsis. Plant Physiol. Biochem. 2019, 135, 167–173. [Google Scholar] [CrossRef]
- García-Limones, C.; Hervás, A.; Navas-Cortés, J.A.; Jimenez-Díaz, R.; Tena, M. Induction of an antioxidant enzyme system and other oxidative stress markers as-sociated with compatible and incompatible interactions between chickpea (Cicer arietinum L.) and fusarium oxysporum f. sp. ciceris. Physiol. Mol. Plant Pathol. 2002, 61, 325–337. [Google Scholar] [CrossRef]
- Baek, K.H.; Skinner, D.Z. Alteration of antioxidant enzyme gene expression during cold acclimation of near-isogenic wheat lines. Plant Sci. 2003, 165, 1221–1227. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Parihar, P.; Mishra, R.K.; Tripathi, D.K.; Singh, V.P.; Chauhan, D.K.; Prasad, S.M. Reactive oxygen species (ROS): Beneficial companions of plants’ developmental processes. Front. Plant Sci. 2016, 7, 1299. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Li, Z.; Xu, J.G.; Gao, Y.; Wang, C.; Guo, G.Y.; Luo, Y.; Huang, Y.T.; Hu, W.M.; Sheteiwy, M.S.; Guan, Y.J.; et al. The synergistic priming effect of exogenous salicylic acid and H2O2 on chilling tolerance enhancement during maize (Zea mays L.) seed germination. Front. Plant Sci. 2017, 8, 1153. [Google Scholar] [CrossRef]
- Liu, J.; Li, F.; Liang, L.; Jiang, Y.; Chen, J. Fibroin delays chilling injury of postharvest banana fruit via enhanced antioxidant capability during cold storage. Metabolites 2019, 9, 152. [Google Scholar] [CrossRef]
- Aidoo, M.K.; Sherman, T.; Lazarovitch, N.; Fait, A.; Rachmilevitch, S. A bell pepper cultivar tolerant to chilling enhanced nitrogen allocation and stress-related metabolite accumulation in the roots in response to low root-zone temperature. Physiol. Plant 2017, 161, 196–210. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Jia, F.F.; Zhang, X.M.; Qiao, Y.X.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Temperature effects on the reactive oxygen species formation and antioxidant defence in roots of two cucurbit species with contrasting root zone temperature optima. Acta Physiol. Plant 2012, 34, 713–720. [Google Scholar] [CrossRef]
- Yong, H.E.; Jing, Y.; Biao, Z.H.U.; Zhu, Z.J. Low root zone temperature exacerbates the ion imbalance and photosynthesis inhibition and induces antioxidant responses in tomato plants under salinity. J. Integr. Agric. 2014, 13, 89–99. [Google Scholar]
- Anwar, A.; Di, Q.H.; Yan, Y.; He, C.X.; Li, Y.S.; Yu, X.C. Exogenous 24-epibrassinolide alleviates the detrimental effects of suboptimal root zone temperature in cucumber seedlings. Arch. Agron. Soil Sci. 2019, 65, 1927–1940. [Google Scholar] [CrossRef]
- Panchuk, I.I.; Volkov, R.A.; Schoffl, F. Heat stress-and heat shock transcription factor-dependent expression and activity of ascorbate peroxidase in Arabidopsis. Plant Physiol. 2002, 129, 838–853. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Dhawan, M.; Sharma, I.; Pati, P.K. Interdependency of reactive oxygen species generating and scavenging system in salt sensitive and salt tolerant cultivars of rice. BMC. Plant Biol. 2016, 16, 131. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Verma, P.C.; Singh, K.; Upadhyay, S.K. Molecular characterization of ascorbate peroxidase (APX) and APX-related (APX-R) genes in Triticum aestivum L. Genomics 2020, 112, 4208–4223. [Google Scholar] [CrossRef]
- Yao, M.; Ge, W.; Zhou, Q.; Zhou, X.; Luo, M.; Zhao, Y.; Ji, S. Exogenous glutathione alleviates chilling injury in postharvest bell pepper by modulating the ascorbate-glutathione (AsA–GSH) cycle. Food Chem. 2021, 352, 129458. [Google Scholar] [CrossRef]
- Song, L.; Wang, J.; Shafi, M.; Liu, Y.; Wang, J.; Wu, J.; Wu, A. Hypobaric treatment effects on chilling injury, mitochondrial dysfunction, and the ascorbate-glutathione (AsA-GSH) cycle in postharvest peach fruit. J. Agric. Food Chem. 2016, 64, 4665–4674. [Google Scholar] [CrossRef]
- Liu, T.; Hu, X.; Zhang, J.; Zhang, J.; Du, Q.; Li, J. H2O2 mediates ALA-induced glutathione and ascorbate accumulation in the perception and resistance to oxidative stress in Solanum lycopersicum at low temperatures. BMC Plant Biol. 2018, 18, 34. [Google Scholar] [CrossRef]
- Li, J.; Arkorful, E.; Cheng, S.; Zhou, Q.; Li, H.; Chen, X.; Li, X. Alleviation of cold damage by exogenous application of melatonin in vegetatively propagated tea plant (Camellia sinensis (L.) Kuntze). Sci. Hortic. 2018, 238, 356–362. [Google Scholar] [CrossRef]
- Zhao, H.; Ye, L.; Wang, Y.; Zhou, X.; Yang, J.; Wang, J.; Zou, Z. Melatonin increases the chilling tolerance of chloroplast in cucumber seedlings by regulating photosynthetic electron flux and the ascorbate-glutathione cycle. Front. Plant Sci. 2016, 7, 1814. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lu, X.; Ma, H.; Lyu, D. Jasmonic acid regulates the ascorbate–glutathione cycle in Malus baccata Borkh. roots under low root-zone temperature. Acta Physiol. Plant 2017, 39, 174. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Zahid, Z.; Mubarik, M.S.; Javed, R.; Siddiqui, M.H.; Hasanuzzaman, M. Jasmonic acid: A key frontier in conferring abiotic stress tolerance in plants. Plant Cell Rep. 2020, 40, 1513–1541. [Google Scholar] [CrossRef]
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and mechanism of jasmonic acid in plant responses to abiotic and biotic stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef] [PubMed]
- Pedranzani, H.; Racagni, G.; Alemano, S.; Miersch, O.; Ramírez, I.; Peña-Cortés, H.; Abdala, G. Salt tolerant tomato plants show increased levels of jasmonic acid. Plant Growth Regul. 2003, 41, 149–158. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, L.; Wang, F.; Yu, D. Jasmonate regulates the inducer of CBF expression-c-repeat binding factor/DRE binding factor1 cascade and freezing tolerance in Arabidopsis. Plant Cell 2013, 25, 2907–2924. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Guo, J.; Zhu, A.; Zhang, L.; Zhang, M. Exogenous jasmonic acid can enhance tolerance of wheat seedlings to salt stress. Ecotox. Environ. Safe. 2014, 104, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wu, H.; Ma, S.; Xiang, D.; Liu, R.; Xiong, L. OsJAZ1 attenuates drought resistance by regulating JA and ABA signaling in rice. Front. Plant Sci. 2017, 8, 2108. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, S.; Hong, H.; Zhou, Y. Transcriptomic profiling of the flower scent biosynthesis pathway of Cymbidium faberi Rolfe and functional characterization of its jasmonic acid carboxyl methyltransferase gene. BMC Genom. 2019, 20, 125. [Google Scholar] [CrossRef]
- Siboza, X.I.; Bertling, I.; Odindo, A.O. Enzymatic antioxidants in response to methyl jasmonate and salicylic acid and their effect on chilling tolerance in lemon fruit [Citrus limon (L.) Burm. F.]. Sci. Hortic. 2017, 225, 659–667. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Yu, R.; Wu, C.; Fan, G.; Li, T. Effects of postharvest application of methyl jasmonate on physicochemical characteristics and antioxidant system of the blueberry fruit. Sci. Hortic. 2019, 258, 108785. [Google Scholar] [CrossRef]
- Serna-Escolano, V.; Martínez-Romero, D.; Giménez, M.J.; Serrano, M.; García-Martínez, S.; Valero, D.; Zapata, P.J. Enhancing antioxidant systems by preharvest treatments with methyl jasmonate and salicylic acid leads to maintain lemon quality during cold storage. Food Chem. 2021, 338, 128044. [Google Scholar] [CrossRef]
- Liu, W.; Wang, H.; Chen, Y.; Zhu, S.; Chen, M.; Lan, X.; Chen, G.; Liao, Z. Cold stress improves the production of artemisinin depending on the increase in endogenous jasmonate. Biotechnol. Appl. Biochem. 2017, 64, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Yang, H. Physiological Basis of Jasmonic Acids in Malus baccata Borkh. In Responding to the Stress of Suboptimal Root-Zone Temperature. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2020. [Google Scholar]
- Ren, J.; Li, X.; Mao, J.; Zuo, C.; Zhao, X.; Chen, B. Physiological and quantitative phosphoproteome analyses of drought stress-induced mechanisms in Malus baccata (L.) Borkh. Biochem. Syst. Ecol. 2017, 72, 47–55. [Google Scholar] [CrossRef]
- Han, D.; Han, J.; Yang, G.; Wang, S.; Xu, T.; Li, W. An ERF transcription factor gene from Malus baccata (L.) Borkh, MbERF11, affects cold and salt stress tolerance in Arabidopsi. Forests 2020, 11, 514. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Baldwin, I.T. Transport of [2-14C] jasmonic acid from leaves to roots mimics wound-induced changes in endogenous jasmonic acid pools in Nicotiana sylvestris. Planta 1997, 203, 436–441. [Google Scholar]
- Huanpu, M.; Zhimin, L.; Hongjie, Z. The transport and distribution of JA and ABA in M. micromalus Makino and their relation to water in soil. Acta Phytophysiol. Sin. 1998, 24, 253–258. [Google Scholar]
- Xin, L.; Shuqiu, Z.; Fengyi, Y. Effects of localized wounding on the transport and distribution of exogenous JA in wheat seedling. Acta Phytophysiol. Sin. 2001, 27, 156–160. [Google Scholar]
- Ruan, J.; Zhou, Y.; Zhou, M.; Yan, J.; Khurshid, M.; Weng, W.; Zhang, K. Jasmonic acid signaling pathway in plants. Int. J. Mol. Sci. 2019, 20, 2479. [Google Scholar] [CrossRef]
- Li, M.; Yu, G.; Ma, J.; Liu, P. Interactions of importers in long-distance transmission of wound-induced jasmonate. Plant Signal. Behav. 2021, 16, 1886490. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, S.; Xu, X.; Korpelainen, H.; Li, C. Changes in antioxidant enzyme activities and isozyme profiles in leaves of male and female Populus cathayana infected with Melampsora larici-populina. Tree Physiol. 2010, 30, 116–128. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Qin, J.; Long, L.; Ma, Y.; Li, H.; Li, K.; Luo, Z.B. Net cadmium flux and accumulation reveal tissue-specific oxidative stress and detoxification in Populus × canescens. Physiol. Plant 2011, 143, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Han, Y.; Jiang, H.; Korpelainen, H.; Li, C. Nitrogen nutrient status induces sexual differences in responses to cadmium in Populus yunnanensis. J. Exp. Bot. 2011, 62, 5037–5050. [Google Scholar] [CrossRef]
- Luo, Z.B.; Calfapietra, C.; Scarascia-Mugnozza, G.; Liberlo, M.; Polle, A. Carbon-based secondary metabolites and internal nitrogen pools in Populus nigra under free air CO2 enrichment (FACE) and nitrogen fertilisation. Plant Soil 2008, 304, 45–57. [Google Scholar] [CrossRef]
- Polle, A.; Chakrabarti, K.; Schurmann, W.; Renneberg, H. Composition and properties of hydrogen peroxide decomposing systems in extracellular and total extracts from needles of Norway Spruce (Picea abies L., Karst.). Plant Physiol. 1990, 94, 312–319. [Google Scholar] [CrossRef]
- Chew, O.; Whelan, J.; Millar, A.H. Molecular definition of the ascorbate-glutathione cycle in Arabidopsis mitochondria reveals dual targeting of antioxidant defenses in plants. J. Biol. Chem. 2003, 278, 46869–46877. [Google Scholar] [CrossRef]
- Edwards, E.A.; Rawsthorne, S.; Mullineaux, P.M. Subcellular distribution of multiple forms of glutathione reductase in leaves of pea (Pisum sativum L.). Planta 1990, 180, 278–284. [Google Scholar] [CrossRef]
- Engelberth, J.; Schmelz, E.A.; Alborn, H.T.; Cardoza, Y.J.; Huang, J.; Tumlinson, J.H. Simultaneous quantification of jasmonic acid and salicylic acid in plants by vapor-phase extraction and gas chromatography-chemical ionization-mass spectrometry. Anal. Biochem. 2003, 312, 242–250. [Google Scholar] [CrossRef]
- Pan, X.; Welti, R.; Wang, X. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nat. Protoc. 2010, 5, 986–992. [Google Scholar] [CrossRef]
- Axelrod, B.; Cheesbrough, T.M.; Laakso, S. Lipoxygenase from soybeans: EC1.13.11.12 linoleate: Oxygen oxidoreductase. Methods Enzymol. 1981, 71, 441–451. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, L.; Sun, Y.; Zheng, S.; Wang, J.; Zhang, T. Hydrogen peroxide is involved in strigolactone induced low temperature stress tolerance in rape seedlings (Brassica rapa L.). Plant Physiol. Biochem. 2020, 157, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lu, X.; Ma, H.; Lyu, D. Comparative proteomic analysis reveals the roots response to low root-zone temperature in Malus baccata. J. Plant Res. 2018, 131, 865–878. [Google Scholar] [CrossRef]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef]
- Ahmad, P.; Rasool, S.; Gul, A.; Sheikh, S.A.; Akram, N.A.; Ashraf, M.; Gucel, S. Jasmonates: Multifunctional roles in stress tolerance. Front. Plant Sci. 2016, 7, 813. [Google Scholar] [CrossRef]
- Ku, Y.S.; Sintaha, M.; Cheung, M.Y.; Lam, H.M. Plant hormone signaling crosstalks between biotic and abiotic stress responses. Int. J. Mol. Sci. 2018, 19, 3206. [Google Scholar] [CrossRef]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef]
- Jang, G.; Shim, J.S.; Jung, C.; Song, J.T.; Lee, H.Y.; Chung, P.J.; Choi, Y.D. Volatile methyl jasmonate is a transmissible form of jasmonate and its biosynthesis is involved in systemic jasmonate response in wounding. Plant Biotechnol. Rep. 2014, 8, 409–419. [Google Scholar] [CrossRef]
- Sheard, L.B.; Tan, X.; Mao, H.; Withers, J.; Ben-Nissan, G.; Hinds, T.R.; Zheng, N. Jasmonate perception by inositol-phosphate-potentiated COI1–JAZ co-receptor. Nature 2010, 468, 400–405. [Google Scholar] [CrossRef]
- Santino, A.; Taurino, M.; De Domenico, S.; Bonsegna, S.; Poltronieri, P.; Pastor, V.; Flors, V. Jasmonate signaling in plant development and defense response to multiple (a) biotic stresses. Plant Cell Rep. 2013, 32, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- Dumin, W.; Rostas, M.; Winefield, C. Identification and functional characterisation of an allene oxide synthase from grapevine (Vitis vinifera L. Sauvignon blanc). Mol. Biol. Rep. 2018, 45, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.B.; Lee, H.Y.; Seo, J.S.; Jung, C.; Jeon, J.H.; Kim, J.H.; Do Choi, Y. Overexpression of jasmonic acid carboxyl methyltransferase increases tuber yield and size in transgenic potato. Plant Biotechnol. Rep. 2011, 5, 27–34. [Google Scholar] [CrossRef]
- Schaller, A.; Stintzi, A. Enzymes in jasmonate biosynthesis-structure, function, regulation. Phytochemistry 2009, 70, 1532–1538. [Google Scholar] [CrossRef]
- Li, Q.; Lei, S.; Du, K.; Li, L.; Pang, X.; Wang, Z.; Wei, M.; Fu, S.; Hu, L.; Xu, L. RNA-seq based transcriptomic analysis uncovers α-linolenic acid and jasmonic acid biosynthesis pathways respond to cold acclimation in Camellia japonica. Sci. Rep. 2016, 6, 36463. [Google Scholar] [CrossRef]
- Fukumoto, K.; Alamgir, K.M.; Yamashita, Y.; Mori, I.C.; Matsuura, H.; Galis, I. Response of rice to insect elicitors and the role of OsJAR1 in wound and herbivory-induced JA-Ile accumulation. J. Integr. Plant Biol. 2013, 55, 775–784. [Google Scholar] [CrossRef]
- Svyatyna, K.; Jikumarum, Y.; Brendel, R.; Reichelt, M.; Mitöfer, A.; Takano, M.; Kamiya, Y.; Nick, P.; Riemann, M. Light induces jasmonate-isoleucine conjugation via OsJAR1-dependent and independent pathways in rice. Plant Cell Environ. 2014, 37, 827–839. [Google Scholar] [CrossRef]
- Chen, H.J.; Fu, T.Y.; Yang, S.L.; Hsieh, H.L. FIN219/JAR1 and cryptochrome1 antagonize each other to modulate photomorphogenesis under blue light in Arabidopsis. PLoS Genet. 2018, 14, e1007248. [Google Scholar]
- Li, Q.; Zheng, J.; Li, S.; Huang, G.; Skilling, S.J.; Wang, L.; Liu, P. Transporter-mediated nuclear entry of jasmonoyl-isoleucine is essential for jasmonate signaling. Mol. Plant 2017, 10, 695–708. [Google Scholar] [CrossRef]
- Prajapati, R.; Mittal, D.; Meena, M.K.; Vadassery, J. Jasmonic acid (JA) induced-calcium elevation in Arabidopsis is highly variable due to time of day and conversion to JA-Ile. J. Plant Biochem. Biotechnol. 2020, 29, 816–823. [Google Scholar] [CrossRef]
- Wakuta, S.; Suzuki, E.; Saburi, W.; Matsuura, H.; Nabeta, K.; Imai, R.; Matsui, H. OsJAR1 and OsJAR2 are jasmonyl-L-isoleucine synthases involved in wound-and pathogen-induced jasmonic acid signalling. Biochem. Biophys. Res. Commun. 2011, 409, 634–639. [Google Scholar] [CrossRef]
- Wang, B.; Wang, S.; Wang, Z. Genome-wide comprehensive analysis the molecular phylogenetic evaluation and tissue-specific expression of SABATH gene family in Salvia miltiorrhiza. Genes 2017, 8, 365. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Niu, J.; Li, B.; Huang, Y.; Han, L.; Liu, Y.; Zhou, W.; Hu, S.; Li, L.; Wang, D.; et al. Molecular characterization and overexpression of SmJMT increases the production of phenolic acids in Salvia miltiorrhiza. Int. J. Mol. Sci. 2018, 19, 3788. [Google Scholar] [CrossRef]
- Seo, H.S.; Song, J.T.; Cheong, J.J.; Lee, Y.H.; Lee, Y.W.; Hwang, I.; Choi, Y.D. Jasmonic acid carboxyl methyltransferase: A key enzyme for jasmonate-regulated plant responses. Proc. Natl. Acad. Sci. USA 2001, 98, 4788–4793. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Kim, Y.S.; Park, S.H.; Koo, Y.J.; Choi, Y.D.; Chung, Y.Y.; Kim, J.K. Methyl jasmonate reduces grain yield by mediating stress signals to alter spikelet development in rice. Plant Physiol. 2009, 149, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Hou, Y.; Li, Z.; Zheng, Y.; Jin, P. Effects of CaCl2 Treatment alleviates chilling injury of loquat fruit (Eribotrya japonica) by modulating ROS homeostasis. Foods 2021, 10, 1662. [Google Scholar] [CrossRef]
- Kenichi, O. Glutathione-associated regulation of plant growth and stress responses. Antioxid. Redox. Signal. 2005, 7, 973–981. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Luo, Y.; Tang, H.R.; Zhang, Y. Effect of low temperature stress on activities of SOD and enzymes of ascorbate-glutathione cycle. Acta Hortic. Sin. 2007, 34, 1405. [Google Scholar]
- Ramazan, S.; Qazi, H.A.; Dar, Z.A.; John, R. Low temperature elicits differential biochemical and antioxidant responses in maize (Zea mays) genotypes with different susceptibility to low temperature stress. Physiol. Mol. Biol. Plants 2021, 27, 1395–1412. [Google Scholar] [CrossRef] [PubMed]

| Compound | Scan Mode | Precursor Ion (m z−1) | Product Ion (m z−1) | Cone Voltage (V) | Collision Energy (eV) |
|---|---|---|---|---|---|
| JA | − | 209.1 | 59.0 | 32.0 | 14.0 |
| MeJA | + | 225.1 | 155.1 | 35.0 | 12.0 |
| JA-Ile | − | 322.2 | 130.2 | 45.0 | 18.0 |
| Gene and Accession No. | Annealing Temperatures (°C) | Primer Sequences (5′–3′) |
|---|---|---|
| AOS (XM_008379143.2) | 56 | F: CCCTCCTCCTCTTCTGTTTCA R: CCGTTGACTGGTATTTCTGGA |
| JMT NM_101820.4 | 58 | F: AACTGAAGGAAGAAAAAGGTG R: TTGAGAGAGCCAATGAAGACT |
| JAR1 HF10212-RA | 58 | F: GTGCCGACTTTTTCCTACTTT R: CCACTTCCACCACATCTCCTA |
| β-Actin | -- | F: TGGTGAGGCTCTATTCCAAC R: TGGCATATACTCTGGAGGCT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, P.; Zhai, M.; Li, L.; Yang, H.; Ma, H.; Lyu, D. Leaf Removal Impacted Jasmonic Acid Metabolism and AsA-GSH in the Roots of Malus baccata (L.) Borkh. under Suboptimal Low Root-Zone Temperatures. Horticulturae 2022, 8, 1205. https://doi.org/10.3390/horticulturae8121205
Dai P, Zhai M, Li L, Yang H, Ma H, Lyu D. Leaf Removal Impacted Jasmonic Acid Metabolism and AsA-GSH in the Roots of Malus baccata (L.) Borkh. under Suboptimal Low Root-Zone Temperatures. Horticulturae. 2022; 8(12):1205. https://doi.org/10.3390/horticulturae8121205
Chicago/Turabian StyleDai, Ping, Meiling Zhai, Lijie Li, Huan Yang, Huaiyu Ma, and Deguo Lyu. 2022. "Leaf Removal Impacted Jasmonic Acid Metabolism and AsA-GSH in the Roots of Malus baccata (L.) Borkh. under Suboptimal Low Root-Zone Temperatures" Horticulturae 8, no. 12: 1205. https://doi.org/10.3390/horticulturae8121205
APA StyleDai, P., Zhai, M., Li, L., Yang, H., Ma, H., & Lyu, D. (2022). Leaf Removal Impacted Jasmonic Acid Metabolism and AsA-GSH in the Roots of Malus baccata (L.) Borkh. under Suboptimal Low Root-Zone Temperatures. Horticulturae, 8(12), 1205. https://doi.org/10.3390/horticulturae8121205





