Abstract
Dalbergia odorifera, a crucial medicinal and commercial plant, shows great potential for ecological restoration in karst rocky desertification (KRD) areas. However, no studies have examined its adaptation mechanism to barren KRD environments. We focused on the physiological and protein variations of D. odorifera grown under loam substrate (LS), composite substrate (CS, 50% gravel), and gravel substrate (GS, 100% gravel). Results showed that volume, surface area, and dry weight of root in CS were the highest. Proteomic analysis revealed 516 and 443 differentially accumulated proteins (DAPs) in CS compared with LS and GS, respectively. Functional analysis showed that epidermal morphogenesis, organic substrate transport, lipid transport, and detection of abiotic stimulus were enriched in the overlapped DAPs. In addition, compared to LS, specific DAPs in CS were enriched to Gene Ontology (GO) terms such as root hair cell differentiation, ATP, carbohydrate metabolism, and also to pathways including carbohydrate digestion and absorption, starch and sucrose metabolism, HIF-1 signaling, mineral absorption, and lysosome. However, specific DAPs in CS relative to GS were clustered to hydrogen peroxide, fatty acid biosynthesis, and lipid metabolism. Furthermore, a series of transcripts encoding crucial DAPs were confirmed by RT-qPCR. In conclusion, the physiological characteristics and proteomic landscape showed that CS substrate was more favorable to the adaptation of D. odorifera to KRD than LS and GS substrates. The protein evaluation related to substrates in this study provides further evidence for ecological management of D. odorifera in KRD areas.
1. Introduction
The important medicinal plant Dalbergia odorifera T. Chen is a medium-sized semi-deciduous tree belonging to family Fabaceae (Leguminosae) that is indigenous to Hainan, South China [1,2]. Currently, D. odorifera also grows in relatively low numbers in Guangdong, Guangxi, and Fujian by introduction and cultivation, and it has been included in the national secondary protection of endangered plants [3,4]. The rosewood of D. odorifera is extremely valuable for manufacturing furniture, artifacts, and musical instruments, not only for its distinctive color and beautiful patterns, but also for its high density and corrosion resistance [5]. The heartwood of D. odorifera, called “Jiangxiang” in China, has been widely used in traditional Chinese medicine for the treatment of cardiovascular diseases, cancer, diabetes, blood disorders, ischemia, swelling, necrosis, and rheumatic pain due to its analgesic, anti-tumor, anti-coagulation, anti-inflammatory [6,7], anti-oxidative, anti-microbial, coronary artery expansion, blood pressure reduction, blood lipid reduction, and other properties [8,9,10,11]. Besides its great commercial and medicinal value, D. odorifera also improves soil microbial biomass and restores soil quality because its roots can fix nitrogen symbiotically with rhizobia [12,13]. Yue et al. used 18 culture substances filled with different substrate combinations and proportions of red soil, coconut coir powder, deciduous leaf powder, and sand to determine their effects on the growth of D. odorifera [14]. Li et al. used a substrate mixed with spent mushroom residue and peat in different proportions to measure the growth of D. odorifera [15]. However, the mechanism of D. odorifera adaptation to desertified rocky barren environments is unclear. Thus, it is urgent to build healthy desertified rocky barren environments and to investigate mechanisms for the growth of D. odorifera.
Substrate composition determines the crucial physical and chemical characteristics of soil, such as permeability, pH, mineral content, and water-holding capacity, which greatly influence the amount of available nutrients, root growth, microbial community composition, and plant development [16]. Under acidic environments, the substrate may be unfavorable to the growth and development of D. odorifera seedlings [14]. He et al. [17] studied different proportions of coconut bran, peat soil, yellow heart soil, pond mud, calcium superphosphate, and calcium magnesium phosphate fertilizer and found that a ratio of yellow heart soil: coconut bran = 1:2 was the best substrate for D. odorifera seedlings. By comparison, the growth performance of D. odorifera seedlings with peat soil was poor, owing to the partial acidity of peat soil and poor air permeability. However, studies by Li et al. [18] found that peat soil not only has a positive effect on the growth and physiological state of tissue-cultured seedlings of D. odorifera, but also promotes the formation of rhizobia. Cai et al. [19] demonstrated that a ratio of field soil: coconut bran powder: sugarcane bran powder = 1:1:1 and a substrate ratio of compound fertilizer containing 3% nitrogen is conducive to the growth and development of container seedlings of D. odorifera, because coconut bran powder has good pore structure and water-holding capacity as well as strong buffering activity, while coconut bran content that is too high or too low is not conducive to seedling growth [17]. Yue et al. [14] formed 18 culture substances with different substrate combinations and ratios of coconut bran powder, red soil, deciduous leaf powder, and sand in Haikou, tropical Hainan, and found that substrate ratios of red soil: coconut bran = 2:2 and red soil: sand = 2:2 improved the growth and biomass accumulation of D. odorifera [20]. Thus, D. odorifera seedlings can also grow well in acidic substrate, even in karst rocky desertification environments with high temperatures, drought, high calcium and magnesium, and a lack of nutrients, D. odorifera can grow well. In summary, the acidity or alkalinity of the substrate may not be the main factor affecting the growth of D. odorifera; rather, the permeability of the substrate may be the most important factor.
With increasing afforestation, the focus has shifted to drought, alpine, karst rocky desertification, and other difficult sites. In addition, recent de novo sequencing of D. odorifera at the chromosome level by Hong et al. [2] revealed its genomic information, which provides a basis for studying the important economic characteristics of D. odorifera at the molecular level. In this study, the basic properties of the substrate and plant were first studied. Furthermore, protein sequences annotated by genome were used as the database, and a comprehension proteomic analysis based on LC-MS/MS was applied to analyze the protein abundance differences in the root tips of D. odorifera seedlings under different substrate conditions. The workflow of physiological and proteomic studies of D. odorifera in different substrates are shown in Figure 1. The purpose of the study was to discover potential functional proteins related to the root growth of D. odorifera seedlings and to provide a technical reference and theoretical basis for strong seedling cultivation (breeding) and popularization of D. odorifera at the molecular level.
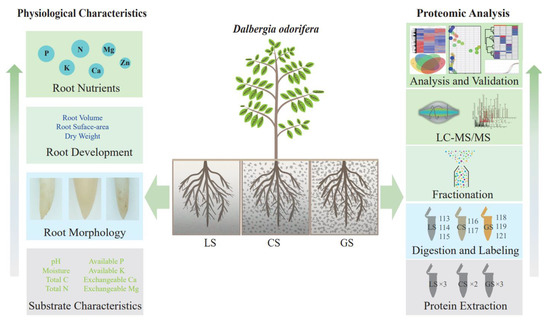
Figure 1.
The workflow of physiological and proteomic studies of D. odorifera in loam and gravel substrates. LS: loam substrate, CS: composite substrate (50% gravel), and GS: gravel substrate (100% gravel).
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
In this study, the gravel content was divided into three substrates: loam substrate (LS), composite substrate (CS, 50% gravel), and gravel substrate (GS, 100% gravel). The rock used in this study was collected from the karst area of Nanning, Guangxi. After cleaning and crushing, gravel particles of about 0.5 cm3 were mixed with screened loam (pH = 6.21; bulk density of 1.26 g/cm3, maximum field water capacity of 24.48%, total nitrogen content of 0.82%, available potassium content of 0.006 g/kg, and available phosphorus content of 0.014 g/kg). Different levels of gravel content were used to simulate different levels of rocky desertification. The cultivation substrates were put into plastic buckets (35 cm × 30 cm) with a 2 cm diameter hole at the bottom. The planting diameter of each barrel was 0.7–0.9 cm. Nine 1-year-old seedlings of D. odorifera with a height of 54–56 cm were washed and planted for each treatment group. The cultivation experiment was conducted in the greenhouse of Forestry College of Guangxi University. During the experiment, consistent field management was adopted, each experimental group was sprayed once in the morning and evening, and weeds were regularly removed from the buckets.
2.2. Collection of Experimental Materials
The fresh weight of the plant, fresh weight of root, and length of root were examined. The fresh root sample was dried to constant weight, crushed, and screened for element determination. For proteomic analysis, five root tips were collected and mixed in each substrate as one replicate.
2.3. Analysis of Basic Properties and Mineral Element Content of the Substrate and Plant
For each sample, 0.25 g of root powder was digested with 5 mL sulfuric acid overnight. Next, it was heated for digestion at 80–100 °C for 1–2 h and digested to a clear solution at 170 °C. During digestion, hydrogen peroxide was added to accelerate the decomposition. After digestion, the solution was diluted to 1 L with deionized water. The nitrogen, phosphorus, and potassium levels were determined using the industrial standard in plants implemented by the Ministry of Agriculture of the People’s Republic of China on 1 December 2011. The content of Ca, Mg and Zn was detected by flame atomic absorption spectrophotometry.
2.4. Protein Preparation of Root Tip for Proteomic Analysis
For each sample, 0.1 g of root tips was collected and ground into powder with liquid nitrogen. The powder was transferred into precooled centrifuge tubes and 1 mL lysis buffer (8 M urea, 2 mM EDTA, 10 mM DTT, and 1% PMSF) was added immediately. Protein was promoted to dissolution under reversing, shaking, and ultrasonic treatment. The extraction was centrifuged at 12,000× g for 15 min. The protein supernatant was collected and quantified using a modified Bradford Kit (Beyotime Biotechnology). A total of 100 µg protein of each sample was used for proteomic analysis. The protein was firstly purified by precipitation with 3 × volume of precooled acetone at −20 °C for 3 h. The protein pellet obtained by centrifugation was redissolved with 20 µL resolution buffer (8 M urea, 100 mM TEAB). Then, the reduction, alkylation, and enzymolysis processes were carried out according to Lin et al. [21].
2.5. iTRAQ-Based Differential Proteomic Analysis by LC-MS/MS
After digestion, the peptide production was desalted using a C18 solid phase extraction column (Oasis HLB cartridge, Waters, Milford, MA, USA) and dried into powder by vacuum centrifugation. Peptide powder was rehydrated with 500 mM TEAB and labeled using an iTRAQ 8-plex Kit (AB Sciex, Framingham, MA, USA). Three replicates of root tip in the LS substrate were labeled with iTRAQ-113, 114, and 115; three replicates in the GS substrate were labeled with iTRAQ-118, 119, and 121; and two replicates in the CS substrate were labeled with iTRAQ-116 and 117. The labeled peptide of all substrates was pooled for drying and redissolved with HPLC solution A (2% ACN, pH 10.0) for fraction separation using a Bridge Peptide BEH C18 column (130 Å, 3.5 μm, 4.6 × 250 mm) under a Waters 2695/2998 HPLC instrument. A total of 22 peptide fractions were collected and desalted using Ziptip C18.
For each peptide fraction, a 78 min gradient of peptide elution was applied on an EASY-nLC 1000 UPLC system combined with Full-MS/ddMS2 analysis by a Q Exactive series (Thermo Scientific, CA, USA) instrument. A reversed-phase pre-column (Acclaim PepMap®100 C18, 3 μm, 100 Å, 75 μm × 2 cm) was used for pre-separation at a flow rate of 5 μL/min, and a reversed-phase analytical column (Acclaim PepMap® RSLC C18, 2 μm, 100 Å, 50 μm × 15 cm) was used for depth separation of peptide ions at a flow rate of 300 nL/min. The eluted peptide ions were selected by quadrupole and analyzed by Orbitrap at a resolution of 60,000. For parent ions, the full-scan range was set at 350–2000 m/z. The parent ions of the top 20 highest intensities were selected for fragmentation at 32% NCE in HCD collision mode. Subsequently, the fragment ions were analyzed by Orbitrap at a resolution of 15,000. The dynamic exclusion time was set at 20 s.
2.6. Data Processing and Bioinformatics Analysis
The resulting mass raw data were matched with the genome-annotated protein database of D. odorifera (30,310 sequences, http://gigadb.org/dataset/100760/ (accessed on 14 March 2021)) by SEQUEST software integrated with the Proteome Discoverer (version 2.1, Thermo Scientific) platform. Typsin/P was set as the lysis enzyme and a max miscleavage of 2 was allowed. Carbamidomethyl© was set as the fixed modification, and iTRAQ 8plex (N-term), iTRAQ 8-plex (K, Y, N-terminal), and oxidation (M) were set as the variable modifications. A mass error of 10 ppm precursor ions was allowed, and fragment ions were allowed at 0.02 Da. The peptide confidence FDR was set as 0.01.
Differentially accumulated proteins (DAPs) were identified using a two-tailed Fisher’s exact test with the threshold of |log2FC| > 1, and p < 0.05. The matched proteins were annotated to clusters of orthologous genes (COG) functions by sequence blast against the COG database and annotated to gene ontology (GO) functions by sequence blast against the Uniprot-GOA database. The online tool KAAS (https://www.genome.jp/tools/kaas/ (accessed on 17 March 2021)) was used for Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway annotation through sequence blast. For functional enrichment, including GO and KEGG, the enrichment-based clustered heatmap and bubble plot were visualized by the “pheatmap” and “ggplot2” packages in R software.
2.7. Quantitative Reverse Transcription PCR (RT-qPCR)
A series of potential functional genes in crucial KEGG pathways was validated by RT-qPCR following the MIQE guidelines [22]. Primers were designed based on the gene sequence and synthesized by Qinke (Guangzhou, China) (Supplementary Table S1). Total RNA was isolated from the powdered root tips using Trizol reagent (Thermo Scientific) according to the manufacturer’s instructions. RNA samples were quantified by NanoDrop spectrophotometry (Thermo Scientific). The cDNA was carried out using HiScript II Q RT SuperMix for qPCR (+gDNA wiper) (Vazyme, Nanjin, China) according to the manufacturer’s instructions. Briefly, genomic DNA was deleted from 0.5 µg RNA sample and then reverse transcribed into cDNA with this procedure: 50 °C for 15 min and 85 °C for 5 s. Next, the then 2× ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjin, China) to amplify cDNA with a qTOWERE3 Real-Time PCR System (AnalytikJena, Germany) according to the manufacturer’s instructions. The qPCR procedure was as follows: 95 °C for 30 s, 40 cycles for 95 °C for 10 s and 60 °C for 30 s, 95 °C for 15 s, 60 °C for 1 min, 95 °C for 15 s. Quantification of the HIS2 gene served as an endogenous control [23], and the gene expression was determined by the 2−ΔΔCt method [24].
2.8. Statistical Analysis
Data were presented as the mean values and standard deviation (mean ± SD). Statistical differences among the three substrates were performed by one way ANOVA and LSD multiple comparisons tests using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). A p value < 0.05 was considered statistically significant.
3. Results
3.1. Comparison of Basic Physicochemical Characteristics of Three Growth Substrates
Basic physical and chemical properties including pH, moisture, total C, total N, available P, available K, exchangeable Ca, and exchangeable Mg of the three substrates were analyzed (Table 1). The content of moisture, total N, available P, available K, and exchangeable Mg in LS reached 33.50 ± 0.72%, 1.84 ± 1.53 g/kg, 3.72 ± 0.13 mg/kg, 55.33 ± 3.38 mg/kg and 3.79 ± 0.26 cmol/kg respectively, which was significantly higher than those of GS. In contrast, the pH and exchangeable calcium content in LS was much lower than that of GS.

Table 1.
Basic physicochemical characteristics of three growth substrates.
3.2. Root Tip Development of D. odorifera
The root tip phenotypes of plants grown in the three growth substrates are shown in Figure 2A. The average root diameter was largest in CS, followed by LS and GS. In the whole root system, the total root volume and root surface area of plants grown in CS were significantly larger compared to those grown in LS and GS (p < 0.05) (Figure 2B,C). The dry weight of the primary root, secondary root, and tertiary root of plants grown in GS were significantly lower than those of CS and LS. There was no significant difference between the CS and LS substrates (Figure 2D). Thus, compared with LS and GS, the CS substrate promoted root tip development in D. odorifera.
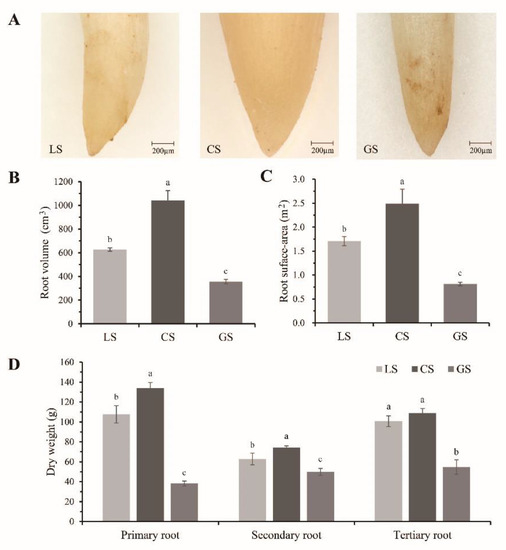
Figure 2.
Root tip development of D. odorifera under three growth substrates: (A) the representative morphology of root tip; (B) total root volume of plants; (C) total root surface area of plants; and (D) dry weight of primary root, secondary root, and tertiary root of plants. LS: loam substrate, CS: composite substrate (50% gravel), and GS: gravel substrate (100% gravel). Error bars represent the SD of the mean. Different lowercase letters indicate a significant difference with the p value < 0.05.
3.3. Substrate Containing Gravel Changes Root Element Content
To evaluate the effects on essential nutrients under different levels of substrate containing gravel, the roots content of macroelements (N, P, K, Ca, Mg) and microelement (Zn) were detected (Table 2). The N content of roots was significantly higher in the CS substrate compared to the LS and GS substrates s, there was no significance between the LS and the GS substrates. The P and K contents of the roots decreased significantly under the CS substrate compared to the LS and GS substrates. The Ca, Mg, and Zn contents in the roots also changed significantly with gravel content. With increasing gravel ratio, the contents of Ca and Zn in the roots showed an upward trend in the GS substrate, respectively. The content of Mg was also highest in the GS substrate. Therefore, substrate containing gravel could change root element levels.

Table 2.
Contents of essential roots nutrients of three growth substrates.
3.4. Differential Proteomics in Root Tips
To investigate the protein abundance difference in root tips of D. odorifera in substrate containing gravel, a high-throughput proteomic strategy of iTRAQ stable isotope labeling combined with LC-MS/MS was employed. The distribution of peptide mass error showed that the mass error of most peptides was within ± 5 ppm (Figure 3A), which indicated that the mass axis of mass spectrometry was accurate. A total of 57,063 peptide-spectrum matches (PSM) were obtained, corresponding to 17,414 peptides, and matching 4081 identified and 3876 quantified protein groups (PGs) (Figure 3B and Table S2). The cluster heatmap of all quantitative protein abundance levels showed that the distance within each substrate was short, and there was great variation between substrate s (Figure 3C). This indicated good repeatability of the experiment. The distribution of DAPs in each comparison substrate was visualized by a volcanic map. A total of 173 upregulated proteins (URPs) and 343 downregulated proteins (DRPs) were identified in the LS vs. CS comparison group (Figure 3D). In the GS vs. CS comparison group, 174 URPs and 269 DRPs were identified (Figure 3E). In addition, a Venn diagram analysis shown that 154 DRPs and 77 URPs overlapped between the two comparison groups, 96 URPs and 188 DRPs were specific DAPs in the LS vs. CS comparison group, and 96 URPs and 115 DRPs were specific DAPs in the GS vs. CS comparison group (Figure 3F). The details of protein identification and quantification are provided in Table S2.
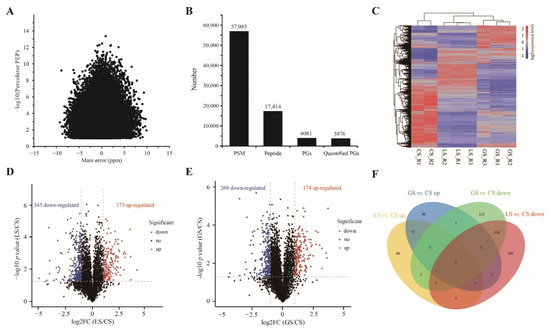
Figure 3.
Summary statistics of the differential proteomics: (A) distribution of peptide mass error; (B) distribution of total protein. PSM: peptide-spectrum matches, PGs: protein groups; (C) the cluster heatmap of all quantitative protein abundance levels. Blue represents an abundance level below the mean, and red represents an expression level above the mean; (D) the volcanic plot of the LS vs. CS comparison group; (E) the volcanic plot of the GS vs. CS comparison group (Blue: downregulated; Red: upregulated; Black: no difference); and (F) Venn diagram of the two comparison groups.
3.5. Functional Analysis of the Overlapped DAPs Regulated by LS and GS Substrates
In order to clarify the potential function of the overlapped DAPs in the comparison of GS vs. CS and LS vs. CS, COG and GO functional annotation and enrichment of these proteins was performed. The statistical results of KOG annotation demonstrated that the 77 overlapped URPs and 155 overlapped DRPs in CS were mainly annotated to the posttranslational modification, protein turnover, chaperones (O), carbohydrate transport and metabolism (G), and transcription (K) functional categories, along with the general function prediction alone (R) (Figure 4A). The bubble diagram of the GO_BP enrichment analysis showed that these overlapped URPs were significantly enriched in biological processes including cell proliferation, plant epidermal morphogenesis, bud system morphogenesis, stomatal development, organic substrate transport, chromatin assembly, and lipid transport (Figure 4B). The overlapped DRPs were significantly enriched in biological processes, including gene expression regulation, cell cycle regulation, response to light, and detection of abiotic stimuli (Figure 4C). The details of protein annotation are listed in Table S2.
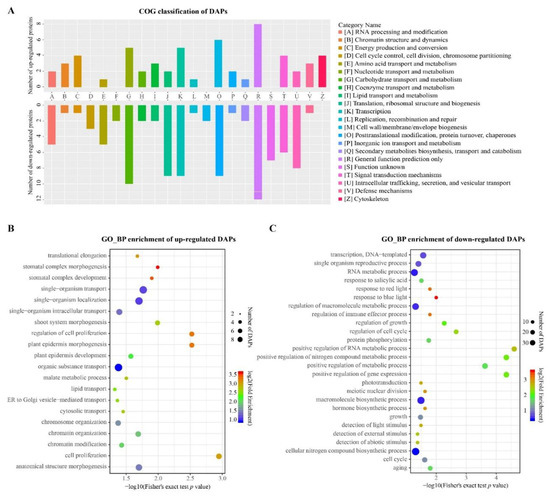
Figure 4.
Functional annotation and enrichment analysis of the overlapped differentially accumulated proteins (DAPs): (A) clusters of orthologous genes (COG) classification of overlapped DAPs; (B) gene ontology_biological process (GO_BP) enrichment analysis of overlapped upregulated proteins (URPs); and (C) GO_BP enrichment analysis of overlapped downregulated proteins (DRPs).
3.6. Functional Analysis of DAPs Specifically Regulated by LS and GS Substrates
In order to analyze the variation in characteristics and potential functions of the DAPs under different substrate conditions, we performed cluster analysis of these proteins based on enrichment of the GO and KEGG functions. Cluster analysis of enriched gene ontology_biological process (GO_BP) functional terms showed that specific URPs in the comparison of LS vs. CS were mainly enriched in process clusters including organic substrate metabolism, ATP metabolism, carbohydrate metabolism, root hair cell differentiation, endogenous metabolism, and response to metal ions. The specific DRPs in the LS substrate relative to CS were mainly concentrated in process clusters such as regulation of RNA metabolism and gene expression, response to stimulation, and signal transduction. However, the enriched processes of specific URPs in the comparison of GS vs. CS were mainly clustered to hydrogen peroxide metabolism, fatty acid biosynthesis, lipid metabolism, and seed development. The enriched processes of specific DRPs were mainly clustered to lipid transport, response to multiple sugars, cell wall biosynthesis, and biological processes such as DNA modification (Figure 5A). Specifically, the abundance of a large number of cell wall-related proteins, such as expansins and endoglucanases, appeared in the GO term of cell wall biosynthesis. Cluster analysis of enriched gene ontology_molecular function (GO_MF) functional terms showed that the specific URPs were mainly clustered in amylase, oxidoreductase activity, catalytic activity, and GAPDH activity, and that the specific DRPs were mainly clustered in kinase activity nucleic acid binding, ATP binding, and ubiquitin molecular binding in the comparison of LS vs. CS. In contrast, the enriched biological processes of specific URPs were mainly clustered to malate dehydrogenase and coenzyme A carboxylase, and the enriched molecular functions of specific DRPs were mainly clustered to transferase activity and phosphatase activity in the comparison of GS vs. CS (Figure 5B). Furthermore, enrichment-based cluster analysis of the KEGG pathway indicated that the specific URPs in the comparison of LS vs. CS were gathered in the metabolic pathway, carbohydrate digestion and absorption, starch and sucrose metabolism, anthocyanin metabolism, and HIF-1 signaling pathway, while specific DRPs were enriched in base excision repair, mineral absorption, lysosome, and other KEGG pathways. In comparison, the enriched pathways were clustered in fatty acid metabolism, pyruvate metabolism, biotin metabolism, and secondary metabolite synthesis of specific URPs, and the enriched pathways were clustered in fatty acid elongation, the calcium signaling pathway, and the phosphatidylinositol signaling pathway of specific DRPs in GS vs. CS (Figure 5C).
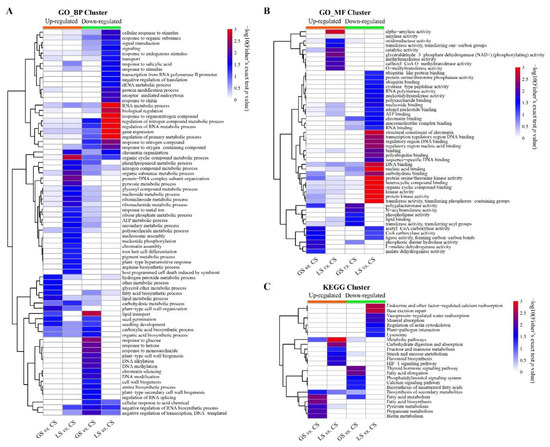
Figure 5.
Functional annotation and enrichment analysis of DAPs specifically regulated by LS and GS substrates: (A) cluster analysis of enriched gene ontology_biological process (GO_BP) functional terms; (B) cluster analysis of enriched gene ontology_molecular function (GO_MF) functional terms; and (C) enrichment-based cluster analysis of KEGG pathway.
3.7. Effects of Substrate on Proteins Related to Air Permeability, Glucose Metabolism, and Heat Conduction
Based on the proteomic results, proteins related to air permeability, glucose metabolism, and heat conduction were significantly regulated by substrate containing gravel. Three proteins (pyruvate dehydrogenase E1 component subunit alpha (PDHA), glyceraldehyde-3-phosphate dehydrogenase (GAPC2), and enolase (ENO1)) annotated to the HIF-1 signaling pathway, five proteins (probable fructokinase-6 (PFK6), β-glucosidase 12 (BGLU12), endoglucanase 17 (GUN17), α-amylase (AMY1), and β-amylase (BMY1)) annotated to starch and sucrose metabolism, and three proteins (putative 3,4-dihydroxy-2-butanone kinase (DHBK), putative GDP-L-fucose synthase 2 (GER2), and PFK6) annotated to fructose and mannose metabolism were downregulated in the CS and GS substrates as compared to the LS substrate, while two proteins including copper transporter 6 (COPT6) and copper transport protein (CCH)) associated with mineral absorption and three proteins including α-N-acetylglucosaminidase (NAGLU), V-ATPase subunit H (VAH-H) and thiol protease aleurain-like (ALEUL) associated with lysosome were upregulated in the CS substrate as compared to the LS substrate (Figure 6A). Eight of these proteins were verified by RT-qPCR analysis. Except for BGLU12, the mRNA expression trends of the remaining seven genes (PDHA, GAPC2, PKF6, AMY1, COPT6, CCH and VHA-H) were consistent with the proteomic results (Figure 6B). These results indicated that substrate containing gravel was related to hypoxic stress. Multiple processes in glucose metabolism were restrained, but the process of mineral absorption and lysosome may have been promoted.
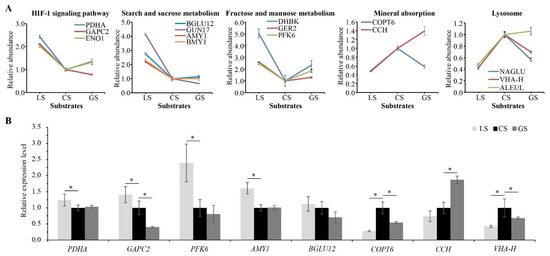
Figure 6.
Effects of substrate containing gravel on proteins related to air permeability, glucose metabolism, and heat conduction. PFK6 shown in fructose and mannose metabolism was also annotated to starch and sucrose metabolism: (A) related protein abundance levels; and (B) RT-qPCR analysis of eight related proteins. Error bars represent the SD of the quantitative mean value of proteomic and RT-qPCR results. * represents significant difference with the p value < 0.05.
3.8. Effects of Substrate on Proteins Related to Nutrient Metabolism and Secondary Metabolism
Based on the proteomic results, we also found that proteins related to nutrient metabolism and secondary metabolism were significantly regulated by substrate containing gravel. Four proteins (3-hydroxyacyl-[acyl-carrier-protein] dehydratase (FABZ), acetyl-CoA acetyltransferase (AAT1), Acetyl-CoA carboxylase 1 (ACC1), biotin carboxyl carrier protein of acetyl-CoA carboxylase (ACCB-1)) annotated to fatty acid metabolism, five proteins (AAT1, ACC1, ACCB-1 and two malate dehydrogenases (MMDH, MMDHI) annotated to pyruvate metabolism, and another seven proteins (histidinol-phosphate aminotransferase (HPA), peroxidase 15 (POD), peroxidase 55 (PER55), tryptophan synthase alpha chain (TRPA1), NAD(P)H dehydrogenase (FQR1), 5′-nucleotidase (SurE) and cationic peroxidase 1 (PNC1)) annotated to biosynthesis of secondary metabolism were downregulated in the CS substrate as compared to the GS substrate. Two proteins including very-long-chain enoyl-CoA reductase (ECR) and very-long-chain 3-oxoacyl-CoA reductase 1 (KCR1) associated with fatty acid elongation and two proteins including calmodulin (CAL1) and phosphoinositide phospholipase C 2 (PLC2) associated with the calcium signaling pathway were upregulated in the CS substrate as compared to the LS and GS substrates (Figure 7A). Six of these proteins were verified by qRT-PCR analysis. The expression trend of four proteins was consistent with the proteomic results (Figure 7B). These results indicated that the substrate containing gravel is related to nutrition stress. Multiple processes in fatty acid metabolism, pyruvate metabolism, and biosynthesis of secondary metabolites were restrained, but the process of fatty acid elongation and the calcium signaling pathway might been promoted in CS substrate.
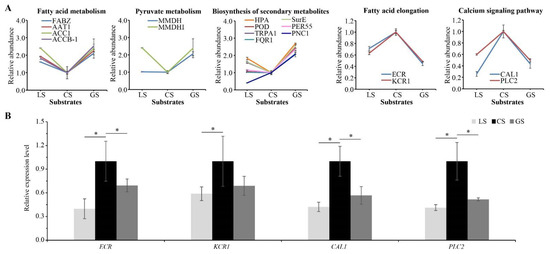
Figure 7.
Effects of substrates on proteins related to nutrient metabolism and secondary metabolism: (A) related protein abundance levels; and (B) RT-qPCR analysis of four related proteins. Error bars represent the SD of the quantitative mean value of proteomic and RT-qPCR results. * represents a significant difference with the p value < 0.05.
4. Discussion
In this study, we compared properties including permeability, pH, mineral content, and water-holding capacity under CS, LS, and GS substrates. The results showed that the LS cultivation condition had the advantages of sufficient nutrients, good water-holding capacity, and thermal stability, but had less air permeability and acid pH stress compared to GS substrate. By contrast, the GS cultivation condition had good air permeability and high mineral ion content, but caused various stresses including nutrient deficiency, drought, low thermal stability, and alkaline pH. The advantages and disadvantages of CS cultivation condition were between those of LS and GS substrates. Due to the high throughput results, wide application, and high efficiency, proteomic technology is widely used to study the molecular response and functional mechanism of plants under biotic and abiotic stresses. Here, a comprehensive proteomic analysis was carried out to clarify the potential mechanism of root tip protein response to various stresses in substrate containing gravel.
4.1. Effects of Substrates on Respiration of Root Tips
Hypoxia caused by waterlogging is the main stress factor affecting plant roots. Plant roots show a deficient energy supply, redox imbalance, and weak photosynthesis reaction under hypoxic condition, which seriously inhibits plant growth and metabolism [25,26]. Due to the low permeability and high water-holding capability of LS substrate, D. odorifera grown under LS substrate for a long time may suffer hypoxic stress. The present study found that compared to LS substrate, the HIF-1 signaling pathway in the root tips of D. odorifera was significantly downregulated in substrates containing gravel. These proteins include pyruvate dehydrogenase E1 component subunit alpha (PDHA1), glycoraldehyde-3-phosphate dehydrogenase GAPC2 (GAPC2), and enolase (ENO1). PHDA1 is one of the important enzymatic components in the pyrovate dehydrogenase complex, which catalyzes the overall conversion of pyruvate to acetyl-CoA and CO2. Consistent with a recent study by Luis et al. [27], multiple PDH proteins in mitochondria were upregulated under hypoxic stress. Enolase plays an important role in the synthesis of pyruvate from D-glyceraldehyde 3-phosphate. Hypoxia can induce an increase in enolase transcription and enzyme activity [28,29]. The differential expression of these proteins indicated that glucose metabolism was regulated under hypoxic stress in substrate containing gravel. In addition, multiple proteins in the metabolic pathways of starch and sucrose, fructose, and mannose were also significantly reduced [30]. Taken together, these results indicate that D. odorifera could adapt to the environment of substrates containing gravel by changing the HIF-1 signaling pathway.
4.2. Effects of Substrates on Fatty Acid Metabolism of Root Tips
Fatty acids have crucial nutritional and physiological significance. Polyunsaturated fatty acids, in particular, have large carbon chains and rich double bonds or their direct precursors. In our proteomic results, a group of proteins associated with fatty acid synthesis was significantly downregulated in the CS substrate, including 3-hydroxyacyl-[acyl-carrier-protein] dehydratase (FABZ), acetyl-CoA acetyltransferase (AAT1), acetyl-CoA carboxylase 1 (ACC1), and biotin carboxyl carrier protein of acetyl-CoA carboxylase (ACCB-1). However, two enzymes (very-long-chain enoyl-CoA reductase (ECR) and very-long-chain 3-oxoacyl-CoA reductase 1 (KCR1)) participating in unsaturated fatty acid synthesis were significantly increased in the CS substrate. Zhou et al. showed that the expression of FABZ involved in the accumulation of C16 and C18 fatty acids was upregulated [31]. Satoh et al. found that fatty acid production could be enhanced by increasing coenzyme A (CoA) biosynthesis and malonyl-CoA supply by introducing exogenous pantothenate kinase (CoaA) and acetyl-CoA carboxylase (ACC) in Escherichia coli [32]. The differential abundance of these proteins indicated that fatty acid metabolism was regulated under nutrition stress in CS substrate. In addition, multiple proteins in pyruvate metabolism and biosynthesis of secondary metabolism were also significantly reduced. Together, these data indicated that D. odorifera may adapt to the living environment of CS substrate by changing its fatty acid metabolism.
4.3. Effects of Substrates on Cell Wall Remodeling of Root Tips
The organization and development of the cell wall is very important in the response to a variety of abiotic stresses, including drought, cold, heat, heavy metals, waterlogging, and others. Cell wall-related proteins are crucial in the expansion of the cell wall, including various celluloses and hemicelluloses related to enzymes, pectin-modifying enzymes, and lignin biosynthesis enzymes [33]. The abundance of 10 proteins related to cell walls was regulated over two-fold in our proteomic study of the LS vs. CS comparison, including five upregulated proteins: peroxidase 3 (PER3), endochitinase (CHI4), two expansins (EXPA3, EXPA4), and endoglucanase 17 (GUN17), and five downregulated proteins: endochitinase (CHI4), endoglucanase 24 (GUN24), glucan endo-1,3-beta-glucosidase 14 (E1314), Dof zinc finger protein DOF3.4 (DOF34), and glucomannan 4-beta-mannosyltransferase 9 (CSLA9). In the comparison of GS vs. CS, 13 proteins related to the cell wall were changed more than two-fold, including four upregulated proteins: glycerophosphodiester phosphodiesterase (GPDL4), expansin-A3 (EXPA3), expansin-A8 (EXPA8), and beta-galactosidase 6 (BGAL6), and nine downregulated proteins: fasciclin-like arabinogalactan protein 11 (FLA11), endochitinase PR4 (CHI4), Omega-hydroxypalmitate O-feruloyl transferase (HHT1), fasciclin-like arabinogalactan protein 11 (FLA11), polygalacturonase (PGLR4), endoglucanase 24 (GUN24), arabinosyltransferase RRA3 (RRA3), Dof zinc finger protein DOF3.4 (DOF34), and glucomannan 4-beta-mannosyltransferase 9 (CSLA9). Interestingly, the abundance levels of all identified cell expansin proteins were increased, while the abundance levels of enzymes associated with cell wall degradation were decreased in CS compared to LS and GS. Expansin proteins are a cluster of cell wall proteins that participate in cell wall extension. The increased expansin proteins and xyloglucan endotransglucosylase/hydrolase (XTH) might maintain the accumulation of rhamnogalacturonan I branching that maintains cell wall plasticity [33,34]. Class III peroxidases are plant-specific cell wall proteins associated with multiple abiotic stresses. They are an important executor in the cell wall remodeling process that regulates the ROS level and the cross-link glycoproteins and phenolic compounds [33,35]. Both of these substrates could induce the cell wall remodeling of root tips. The plants adapted to the respective stresses in LS and GS substrates by increasing cell wall expansion and reducing cell wall degradation. Previous studies have suggested that the formation of the plant secondary cell wall and the deposition of cellulose and lignin are closely related to salt tolerance [36].
5. Conclusions
Our results revealed that compared to the LS or GS substrate, CS substrate promoted growth of root tip of D. odorifera from the perspective of total root volume, root surface area, dry weight of primary, secondary roots, and tertiary roots, and macroelements content. Moreover, we profiled the proteome of D. odorifera under three substrate conditions and found that overlapping DAPs are involved in multiple regulatory pathways of plant growth, and CS-specific regulated DAPs are involved in regulating air permeability, glucose metabolism, and heat conduction, nutrient metabolism and secondary metabolism. These results conclude that compared with LS and GS substrates, CS substrate is a more suitable rocky desertification barren environment for D. odorifera growth.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae8121154/s1, Table S1: Primers details of RT-qPCR analysis; Table S2. Combine information of protein quantification and annotation.
Author Contributions
Conceptualization, S.Y. and Z.Y; methodology, S.Y. and Z.Y.; funding acquisition, S.Y.; proteomic analysis and bioinformatic analysis, K.Y.; writing—original draft preparation, K.Y.; writing—review and editing, S.Y. and K.Y.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number No. 31760058.
Data Availability Statement
The original proteomic data (PXD038246) can by fully accessed from the ProteomeXchange consortium via the PRIDE partner repository.
Acknowledgments
We acknowledge the State Key Laboratory for Conservation and Utilization of Subtropical Agro-bioresources for the support of LC-MS/MS platform.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sun, S.; Zeng, X.; Zhang, D.; Guo, S. Diverse fungi associated with partial irregular heartwood of Dalbergia odorifera. Sci. Rep. 2015, 5, 8464. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Li, J.; Liu, X.; Lian, J.; Zhang, N.; Yang, Z.; Niu, Y.; Cui, Z.; Xu, D. The chromosome-level draft genome of Dalbergia odorifera. Gigascience 2020, 9, giaa084. [Google Scholar] [CrossRef] [PubMed]
- Hui, M.; Caixiang, X.; Yun, Y.; Jianhe, W.; Jindong, F.; Shilin, C. Suitable producing areas of Dalbergia odorifera T. Chen. Lishizhen Med. Mater. Med. Res. 2010, 21, 2304–2306. [Google Scholar] [CrossRef]
- Meng, X.W.; Wang, D.Q.; Chen, L.Y.; Shao, F.; Zhang, P.Z.; Hu, S.J.; Zhu, Q.; Liu, R.H. Study on neoflavonoids from heartwood of Dalbergia latifolia. Zhongguo Zhong Yao Za Zhi 2019, 44, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Wariss, H.M.; Yi, T.-S.; Wang, H.; Zhang, R. Characterization of the complete chloroplast genome of Dalbergia odorifera (Leguminosae), a rare and critically endangered legume endemic to China. Conserv. Genet. Resour. 2017, 10, 527–530. [Google Scholar] [CrossRef]
- Lee, D.S.; Li, B.; Keo, S.; Kim, K.S.; Jeong, G.S.; Oh, H.; Kim, Y.C. Inhibitory effect of 9-hydroxy-6,7-dimethoxydalbergiquinol from Dalbergia odorifera on the NF-kappaB-related neuroinflammatory response in lipopolysaccharide-stimulated mouse BV2 microglial cells is mediated by heme oxygenase-1. Int. Immunopharmacol. 2013, 17, 828–835. [Google Scholar] [CrossRef]
- Li, X.; Wu, L.; Liu, W.; Jin, Y.; Chen, Q.; Wang, L.; Fan, X.; Li, Z.; Cheng, Y. A network pharmacology study of Chinese medicine QiShenYiQi to reveal its underlying multi-compound, multi-target, multi-pathway mode of action. PLoS ONE 2014, 9, e95004. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, C.; Meng, H.; Yu, Z.; Yang, M.; Wei, J. Dalbergia odorifera: A review of its traditional uses, phytochemistry, pharmacology, and quality control. J. Ethnopharmacol. 2020, 248, 112328. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, Y. Bioactive sesquiterpenes isolated from the essential oil of Dalbergia odorifera T. Chen. Fitoterapia 2010, 81, 393–396. [Google Scholar] [CrossRef]
- Sugiyama, A.; Zhu, B.M.; Takahara, A.; Satoh, Y.; Hashimoto, K. Cardiac effects of salvia miltiorrhiza/Dalbergia odorifera mixture, an intravenously applicable Chinese medicine widely used for patients with ischemic heart disease in China. Circ. J. 2002, 66, 182–184. [Google Scholar] [CrossRef]
- Chan, S.C.; Chang, Y.S.; Wang, J.P.; Chen, S.C.; Kuo, S.C. Three new flavonoids and antiallergic, anti-inflammatory constituents from the heartwood of Dalbergia odorifera. Planta Med. 1998, 64, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhang, Q.; Zhou, H.; Nong, Z.; Ye, S.; Deng, Q. Introduction of Dalbergia odorifera enhances nitrogen absorption on Eucalyptus through stimulating microbially mediated soil nitrogen-cycling. For. Ecosyst. 2021, 8, 12. [Google Scholar] [CrossRef]
- Lu, J.; Yang, F.; Wang, S.; Ma, H.; Liang, J.; Chen, Y. Co-existence of Rhizobia and Diverse Non-rhizobial Bacteria in the Rhizosphere and Nodules of Dalbergia odorifera Seedlings Inoculated with Bradyrhizobium elkanii, Rhizobium multihospitium-Like and Burkholderia pyrrocinia-Like Strains. Front. Microbiol. 2017, 8, 2255. [Google Scholar] [CrossRef]
- Yue, X.H.; Miao, L.F.; Yang, F.; Nawaz, M. Morphological and physiological responses of Dalbergia odorifera T. Chen seedlings to different culture substances. PLoS ONE 2020, 15, e0232051. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xia, H.; Wang, J.; Chen, Q. Nutrient uptake and assimilation in fragrant rosewood (Dalbergia odorifera T.C. Chen) seedlings in growing media with un-composted spent mushroom residue. PLoS ONE 2021, 16, e0249534. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.B.; Heuberger, A.L.; Broeckling, C.D.; Jahn, C.E. Non-Targeted Metabolomics Reveals Sorghum Rhizosphere-Associated Exudates are Influenced by the Belowground Interaction of Substrate and Sorghum Genotype. Int. J. Mol. Sci. 2019, 20, 431. [Google Scholar] [CrossRef]
- He, Q.F.; Peng, Y.H.; Cao, Y.Y.; Jiang, Y.; Lu, G.D. Experiment on substrates for container seedling of Dalbergia odorifera. J. For. Eng. 2012, 26, 92–95. [Google Scholar] [CrossRef]
- Li, X.Y.; Zeng, B.S.; Xu, D.P. Effects of Different Transplant Substrate on Survival Ratio and Growth of Dalbergia odorifera T. Chen Tissue-cultured Plantlets. Seed 2019, 38, 16–20. [Google Scholar] [CrossRef]
- Cao, K.L.; Liu, Z.J.; Chen, Y.L.; Liu, C.H. Study on the Container Seedlings of Dalbergia odorifera. J. Anhui Agric. Sci. 2014, 42, 5082–5083, 5087. [Google Scholar] [CrossRef]
- Sun, C.X.; Feng, M.L.; Liu, L.Y.; Chen, W.J.; Chen, H.; Zhang, M.Y.; Li, X.J. Study on the main Physico-chemical Properties of Coir Culture Medium in Hainan. Chin. J. Trop. Crops 2011, 32, 407–411. [Google Scholar] [CrossRef]
- Lin, Y.; Xiong, W.; Xiao, S.; Li, F.; Lu, Z.; Yan, J.; Fang, X.; Cui, X.; Wen, Y.; Liang, J.; et al. Pharmacoproteomics reveals the mechanism of Chinese dragon’s blood in regulating the RSK/TSC2/mTOR/ribosome pathway in alleviation of DSS-induced acute ulcerative colitis. J. Ethnopharmacol. 2020, 263, 113221. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time pcr experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Meng, H.; Yang, Y.; Gao, Z.H.; Wei, J.H. Selection and Validation of Reference Genes for Gene Expression Studies by RT-PCR in Dalbergia odorifera. Sci. Rep. 2019, 9, 3341. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Luan, H.; Shen, H.; Pan, Y.; Guo, B.; Lv, C.; Xu, R. Elucidating the hypoxic stress response in barley (Hordeum vulgare L.) during waterlogging: A proteomics approach. Sci. Rep. 2018, 8, 9655. [Google Scholar] [CrossRef]
- Pan, R.; He, D.; Xu, L.; Zhou, M.; Li, C.; Wu, C.; Xu, Y.; Zhang, W. Proteomic analysis reveals response of differential wheat (Triticum aestivum L.) genotypes to oxygen deficiency stress. BMC Genom. 2019, 20, 60. [Google Scholar] [CrossRef]
- Boaretto, L.F.; Labate, M.T.V.; Franceschini, L.M.; Cataldi, T.R.; Budzinski, I.G.F.; de Moraes, F.E.; Labate, C.A. Proteomics Reveals an Increase in the Abundance of Glycolytic and Ethanolic Fermentation Enzymes in Developing Sugarcane Culms During Sucrose Accumulation. Front. Plant Sci. 2021, 12, 716964. [Google Scholar] [CrossRef]
- Fox, T.C.; Mujer, C.V.; Andrews, D.L.; Williams, A.S.; Cobb, B.G.; Kennedy, R.A.; Rumpho, M.E. Identification and gene expression of anaerobically induced enolase in Echinochloa phyllopogon and Echinochloa cruspavonis. Plant Physiol. 1995, 109, 433–443. [Google Scholar] [CrossRef][Green Version]
- Albrecht, G.; Mustroph, A.; Fox, T.C. Sugar and fructan accumulation during metabolic adjustment between respiration and fermentation under low oxygen conditions in wheat roots. Physiol. Plant. 2004, 120, 93–105. [Google Scholar] [CrossRef]
- Hwang, J.H.; Yu, S.I.; Lee, B.H.; Lee, D.H. Modulation of Energy Metabolism Is Important for Low-Oxygen Stress Adaptation in Brassicaceae Species. Int. J. Mol. Sci. 2020, 21, 1787. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Z.; Zhu, C.; Yue, J.; Yang, H.; Li, J.; Gao, J.; Xu, R.; Deng, X.; Cheng, Y. Variations of membrane fatty acids and epicuticular wax metabolism in response to oleocellosis in lemon fruit. Food Chem. 2021, 338, 127684. [Google Scholar] [CrossRef]
- Satoh, S.; Ozaki, M.; Matsumoto, S.; Nabatame, T.; Kaku, M.; Shudo, T.; Asayama, M.; Chohnan, S. Enhancement of fatty acid biosynthesis by exogenous acetyl-CoA carboxylase and pantothenate kinase in Escherichia coli. Biotechnol. Lett. 2020, 42, 2595–2605. [Google Scholar] [CrossRef]
- Le Gall, H.; Philippe, F.; Domon, J.M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell Wall Metabolism in Response to Abiotic Stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2014, 5, 771. [Google Scholar] [CrossRef]
- Francoz, E.; Ranocha, P.; Nguyen-Kim, H.; Jamet, E.; Burlat, V.; Dunand, C. Roles of cell wall peroxidases in plant development. Phytochemistry 2015, 112, 15–21. [Google Scholar] [CrossRef]
- Oliveira, D.M.; Mota, T.R.; Salatta, F.V.; Sinzker, R.C.; Koncitikova, R.; Kopecny, D.; Simister, R.; Silva, M.; Goeminne, G.; Morreel, K.; et al. Cell wall remodeling under salt stress: Insights into changes in polysaccharides, feruloylation, lignification, and phenolic metabolism in maize. Plant Cell Environ. 2020, 43, 2172–2191. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).