Melatonin-Induced Detoxification of Organic Pollutants and Alleviation of Phytotoxicity in Selected Horticultural Crops
Abstract
1. Introduction
2. Organic Pollutants and Phytotoxicity
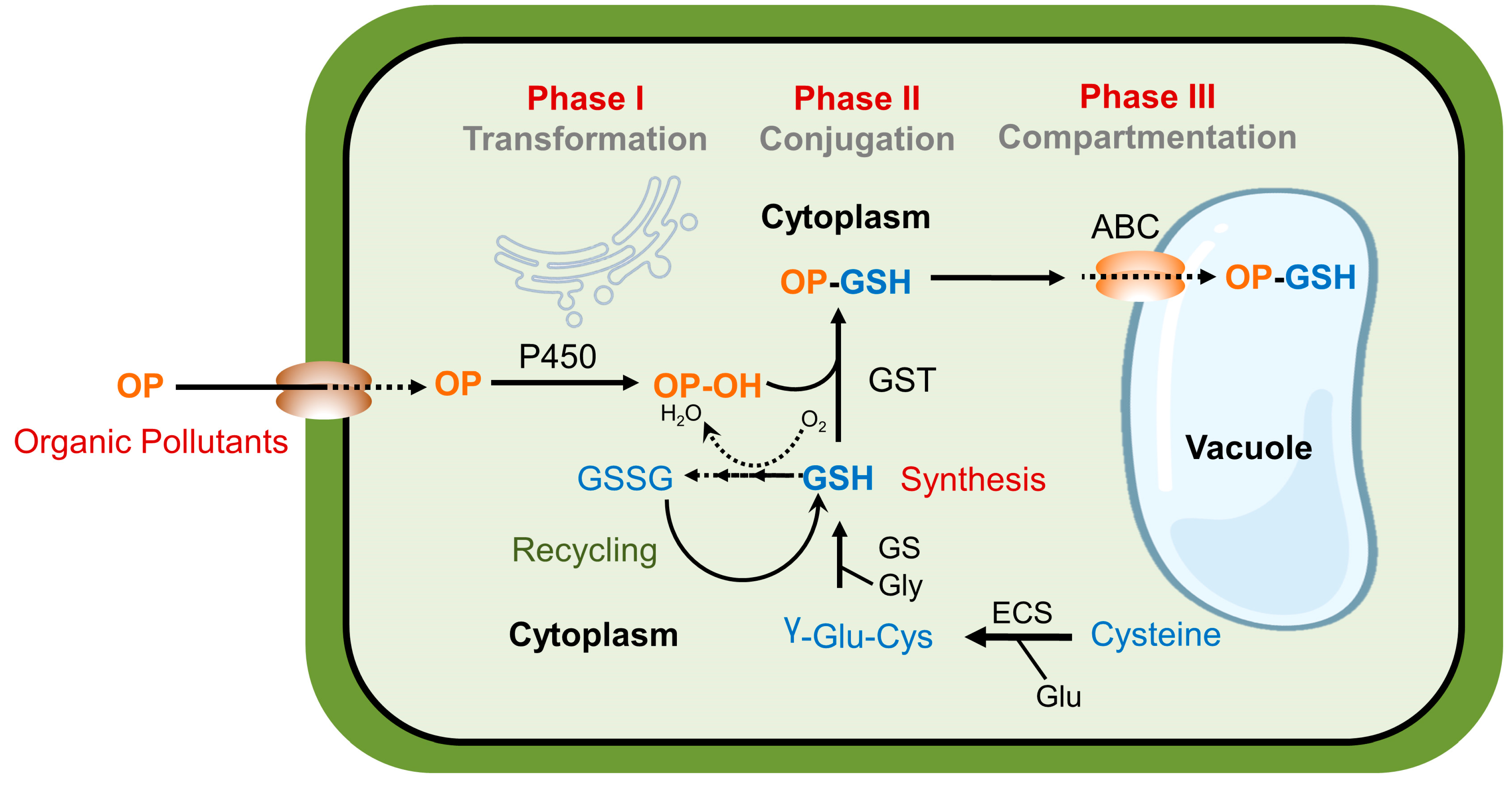
3. Mechanisms of Pollutant Detoxification
4. Melatonin: A Master Growth Regulator of Plant Stress Tolerance
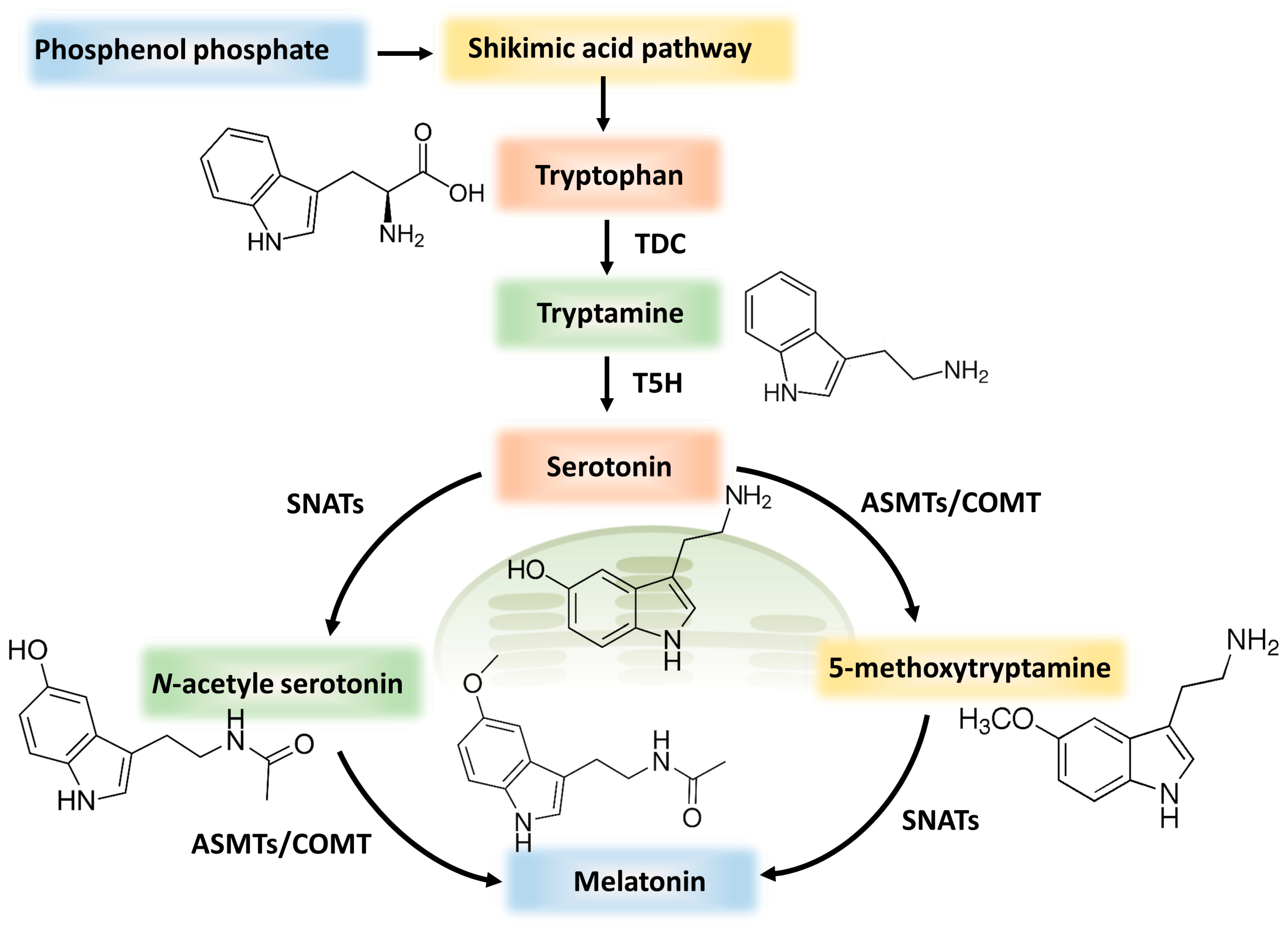
4.1. Melatonin Synthesis and Sources
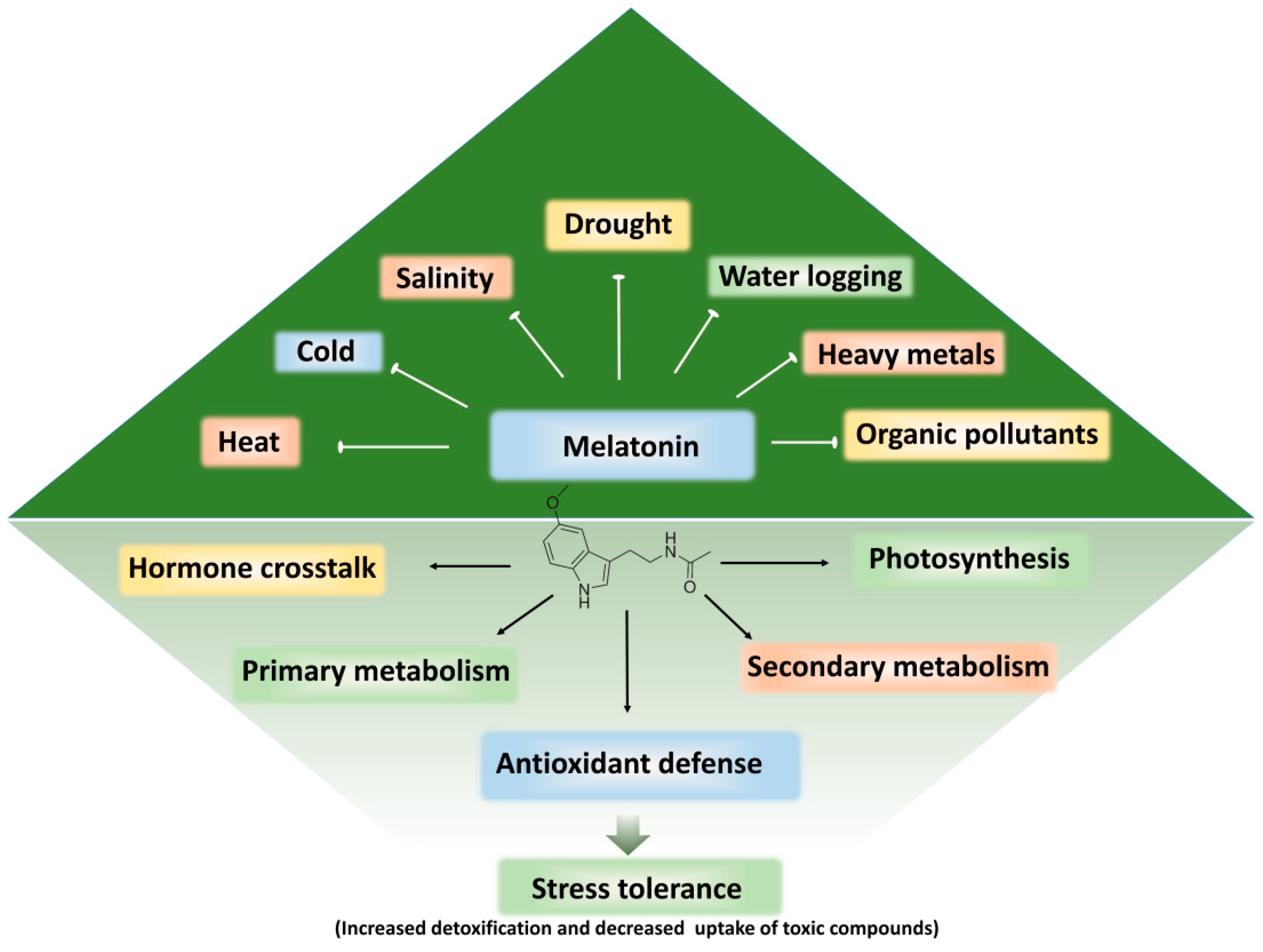
4.2. Melatonin in Plant Physiology, Metabolism, and Abiotic Stress Tolerance
5. Melatonin-Induced Detoxification and Alleviation of Phytotoxicity
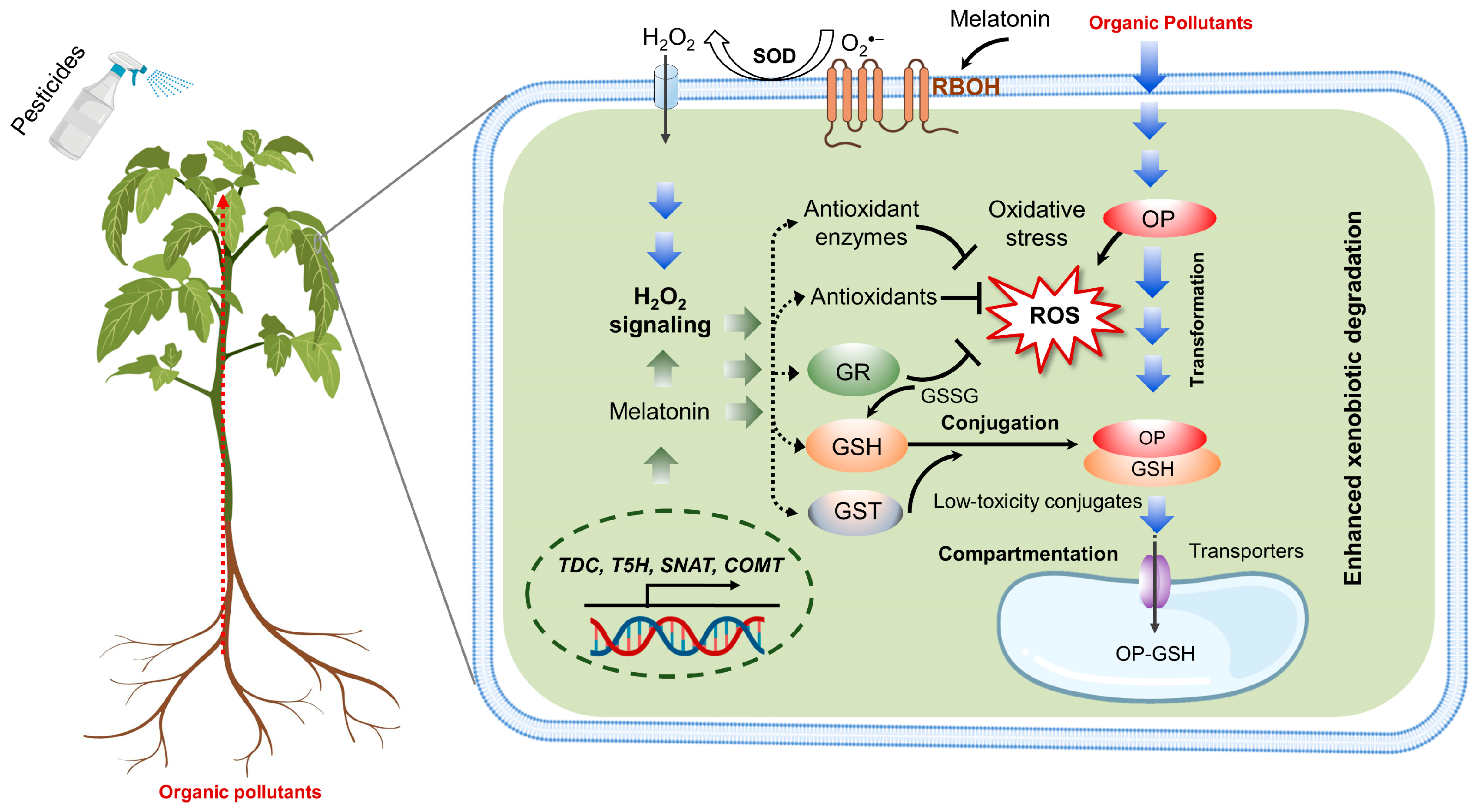
5.1. Exogenous Melatonin Alleviates Organic Pollutant-Induced Stress
5.2. Potential Mechanisms of Melatonin-Induced Xenobiotic Detoxification
5.3. Melatonin-Induced Reduction in Pesticide Residue in Postharvest Horticultural Management
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdul Mutalib, A.A.; Jaafar, N.F. ZnO photocatalysts applications in abating the organic pollutant contamination: A mini review. Total Environ. Res. Themes 2022, 3–4, 100013. [Google Scholar] [CrossRef]
- Zhao, W.; Teng, M.; Zhang, J.; Wang, K.; Zhang, J.; Xu, Y.; Wang, C. Insights into the mechanisms of organic pollutant toxicity to earthworms: Advances and perspectives. Environ. Pollut. 2022, 303, 119120. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Y.; He, X.; Lu, Q. Degradation or humification: Rethinking strategies to attenuate organic pollutants. Trends Biotechnol. 2022, 40, 1061–1072. [Google Scholar] [CrossRef]
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S.; et al. Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review. Front. Microbiol. 2022, 13, 962619. [Google Scholar] [CrossRef] [PubMed]
- Tounsadi, H.; Metarfi, Y.; Taleb, M.; El Rhazi, K.; Rais, Z. Impact of chemical substances used in textile industry on the employee’s health: Epidemiological study. Ecotoxicol. Environ. Saf. 2020, 197, 110594. [Google Scholar] [CrossRef]
- Fujita, K.; Inui, H. How does the Cucurbitaceae family take up organic pollutants (POPs, PAHs, and PPCPs)? Rev. Environ. Sci. Bio/Technol. 2021, 20, 751–779. [Google Scholar] [CrossRef]
- Kanwar, M.K.; Xie, D.; Yang, C.; Ahammed, G.J.; Qi, Z.; Hasan, M.K.; Reiter, R.J.; Yu, J.Q.; Zhou, J. Melatonin promotes metabolism of bisphenol A by enhancing glutathione-dependent detoxification in Solanum lycopersicum L. J. Hazard. Mater. 2020, 388, 121727. [Google Scholar] [CrossRef]
- Zhang, J.J.; Yang, H. Metabolism and detoxification of pesticides in plants. Sci. Total Environ. 2021, 790, 148034. [Google Scholar] [CrossRef]
- Zhu, M.; Tang, J.; Shi, T.; Ma, X.; Wang, Y.; Wu, X.; Li, H.; Hua, R. Uptake, translocation and metabolism of imidacloprid loaded within fluorescent mesoporous silica nanoparticles in tomato (Solanum lycopersicum). Ecotoxicol. Environ. Saf. 2022, 232, 113243. [Google Scholar] [CrossRef]
- Deng, B.; Xia, C.; Tian, S.; Shi, H. Melatonin reduces pesticide residue, delays senescence, and improves antioxidant nutrient accumulation in postharvest jujube fruit. Postharvest Biol. Technol. 2021, 173, 111419. [Google Scholar] [CrossRef]
- Zhang, J.J.; Yang, H. Advance in methodology and strategies to unveil metabolic mechanisms of pesticide residues in food crops. J. Agric. Food Chem. 2021, 69, 2658–2667. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Kumar, P.S.; Vo, D.N. A review on the microbial degradation of chlorpyrifos and its metabolite TCP. Chemosphere 2021, 283, 131447. [Google Scholar] [CrossRef] [PubMed]
- Peuke, A.D.; Rennenberg, H. Phytoremediation. EMBO Rep. 2005, 6, 497–501. [Google Scholar] [CrossRef]
- Yu, G.B.; Chen, R.N.; Chen, Q.S.; Chen, F.Q.; Liu, H.L.; Ren, C.Y.; Zhang, Y.X.; Yang, F.J.; Wei, J.P. Jasmonic acid promotes glutathione assisted degradation of chlorothalonil during tomato growth. Ecotoxicol. Environ. Saf. 2022, 233, 113296. [Google Scholar] [CrossRef] [PubMed]
- De Freitas-Silva, L.; Araujo, H.H.; Meireles, C.S.; da Silva, L.C. Plant exposure to glyphosate-based herbicides and how this might alter plant physiological and structural processes. Botany 2022, 100, 473–480. [Google Scholar] [CrossRef]
- Wang, K.; Xing, Q.; Ahammed, G.J.; Zhou, J. Functions and prospects of melatonin in plant growth, yield and quality. J. Exp. Bot. 2022, 73, 5928–5946. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, P.; Zhao, G.; Li, L.; Shen, W. Phytomelatonin and gasotransmitters: A crucial combination for plant physiological functions. J. Exp. Bot. 2022, 73, 5851–5862. [Google Scholar] [CrossRef]
- Wei, J.; Li, D.-X.; Zhang, J.-R.; Shan, C.; Rengel, Z.; Song, Z.-B.; Chen, Q. Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure inArabidopsis thaliana. J. Pineal Res. 2018, 65, e12500. [Google Scholar] [CrossRef]
- Huangfu, L.; Chen, R.; Lu, Y.; Zhang, E.; Miao, J.; Zuo, Z.; Zhao, Y.; Zhu, M.; Zhang, Z.; Li, P.; et al. OsCOMT, encoding a caffeic acid O-methyltransferase in melatonin biosynthesis, increases rice grain yield through dual regulation of leaf senescence and vascular development. Plant Biotechnol. J. 2022, 20, 1122–1139. [Google Scholar] [CrossRef]
- Chen, Q.; Pu, X.; Li, X.; Li, R.; Yang, Q.; Wang, X.; Guan, M.; Rengel, Z. Secrets of phytomelatonin: Possible roles in darkness. J. Exp. Bot. 2022, 73, 5828–5839. [Google Scholar] [CrossRef]
- García-Sánchez, S.; Cano, A.; Hernández-Ruiz, J.; Arnao, M.B. Effects of temperature and light on the germination-promoting activity by melatonin in almond seeds without stratification. Agronomy 2022, 12, 2070. [Google Scholar] [CrossRef]
- Arnao, M.; Hernández-Ruiz, J. Melatonin and reactive oxygen and nitrogen species: A model for the plant redox network. Melatonin Res. 2019, 2, 152–168. [Google Scholar] [CrossRef]
- Zeng, W.; Mostafa, S.; Lu, Z.; Jin, B. Melatonin-mediated abiotic stress tolerance in plants. Front. Plant Sci. 2022, 13, 847175. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Hernandez-Ruiz, J. Phytomelatonin: An unexpected molecule with amazing performances in plants. J. Exp. Bot. 2022, 73, 5779–5800. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.Y.; Zhang, Y.; Xu, Y.P.; Qi, Z.Y.; Li, M.Q.; Ahammed, G.J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Reiter, R.J.; et al. HsfA1a upregulates melatonin biosynthesis to confer cadmium tolerance in tomato plants. J. Pineal Res. 2017, 62, e12387. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, J.; Sun, Y.; Zhang, L.; Zheng, S. Versatile roles of melatonin in growth and stress tolerance in plants. J. Plant Growth Regul. 2021, 41, 507–523. [Google Scholar] [CrossRef]
- Li, R.; Yang, R.; Zheng, W.; Wu, L.; Zhang, C.; Zhang, H. Melatonin promotes SGT1-involved signals to ameliorate drought stress adaption in rice. Int. J. Mol. Sci. 2022, 23, 599. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Wang, C.; Xiao, Q.; Chen, Z.; Han, Y. Melatonin confers plant cadmium tolerance: An update. Int. J. Mol. Sci. 2021, 22, 11704. [Google Scholar] [CrossRef] [PubMed]
- Moustafa-Farag, M.; Elkelish, A.; Dafea, M.; Khan, M.; Arnao, M.B.; Abdelhamid, M.T.; El-Ezz, A.A.; Almoneafy, A.; Mahmoud, A.; Awad, M.; et al. Role of melatonin in plant tolerance to soil stressors: Salinity, ph and heavy metals. Molecules 2020, 25, 5359. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Xu, W.; Liu, A.; Chen, S. Endogenous melatonin deficiency aggravates high temperature-induced oxidative stress in Solanum lycopersicum L. Environ. Exp. Bot. 2019, 161, 303–311. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Lan, Z.; Xu, K.; Chang, J.; Ahammed, G.J.; Ma, J.; Wei, C.; Zhang, X. Methyl jasmonate mediates melatonin-induced cold tolerance of grafted watermelon plants. Hortic. Res. 2021, 8, 57. [Google Scholar] [CrossRef]
- Liu, P.; Wu, X.; Gong, B.; Lü, G.; Li, J.; Gao, H. Review of the mechanisms by which transcription factors and exogenous substances regulate ROS metabolism under abiotic stress. Antioxidants 2022, 11, 2106. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, M.; Zhao, Y.; Han, P.; Dai, Y. Melatonin from different fruit sources, functional roles, and analytical methods. Trends Food Sci. Technol. 2014, 37, 21–31. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Alamri, S.; Nasir Khan, M.; Corpas, F.J.; Al-Amri, A.A.; Alsubaie, Q.D.; Ali, H.M.; Kalaji, H.M.; Ahmad, P. Melatonin and calcium function synergistically to promote the resilience through ROS metabolism under arsenic-induced stress. J. Hazard. Mater. 2020, 398, 122882. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.U.; Mahmood, A.; Awan, M.I.; Maqbool, R.; Aamer, M.; Alhaithloul, H.A.S.; Huang, G.; Skalicky, M.; Brestic, M.; Pandey, S.; et al. Melatonin-induced protection against plant abiotic stress: Mechanisms and prospects. Front. Plant Sci. 2022, 13, 902694. [Google Scholar] [CrossRef] [PubMed]
- Ayyaz, A.; Shahzadi, A.K.; Fatima, S.; Yasin, G.; Zafar, Z.U.; Athar, H.U.R.; Farooq, M.A. Uncovering the role of melatonin in plant stress tolerance. Theor. Exp. Plant Physiol. 2022, 34, 335–346. [Google Scholar] [CrossRef]
- Peng, X.; Wang, N.; Sun, S.; Geng, L.; Guo, N.; Liu, A.; Chen, S.; Ahammed, G.J. Reactive oxygen species signaling is involved in melatonin-induced reduction of chlorothalonil residue in tomato leaves. J. Hazard. Mater. 2023, 443, 130212. [Google Scholar] [CrossRef]
- Yan, Y.; Sun, S.; Zhao, N.; Yang, W.; Shi, Q.; Gong, B. COMT1 overexpression resulting in increased melatonin biosynthesis contributes to the alleviation of carbendazim phytotoxicity and residues in tomato plants. Environ. Pollut. 2019, 252, 51–61. [Google Scholar] [CrossRef]
- Rostami, S.; Azhdarpoor, A.; Baghapour, M.A.; Dehghani, M.; Samaei, M.R.; Jaskulak, M.; Jafarpour, S.; Samare-Najaf, M. The effects of exogenous application of melatonin on the degradation of polycyclic aromatic hydrocarbons in the rhizosphere of Festuca. Environ. Pollut. 2021, 274, 116559. [Google Scholar] [CrossRef]
- Liu, N.; Li, J.; Lv, J.; Yu, J.; Xie, J.; Wu, Y.; Tang, Z. Melatonin alleviates imidacloprid phytotoxicity to cucumber (Cucumis sativus L.) through modulating redox homeostasis in plants and promoting its metabolism by enhancing glutathione dependent detoxification. Ecotoxicol. Environ. Saf. 2021, 217, 112248. [Google Scholar] [CrossRef]
- Popek, E. Environmental Chemical Pollutants. In Sampling and Analysis of Environmental Chemical Pollutants; Elsevier: Amsterdam, The Netherlands, 2018; pp. 13–69. [Google Scholar]
- Mbachu, A.; Chukwura, E.I.; Amalachukwu, M. Role of microorganisms in the degradation of organic pollutants: A review. Energy Environ. Eng. 2020, 7, 1–11. [Google Scholar]
- Ahammed, G.J.; Ruan, Y.P.; Zhou, J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Brassinosteroid alleviates polychlorinated biphenyls-induced oxidative stress by enhancing antioxidant enzymes activity in tomato. Chemosphere 2013, 90, 2645–2653. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Li, X.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Enhanced photosynthetic capacity and antioxidant potential mediate brassinosteriod-induced phenanthrene stress tolerance in tomato. Environ. Pollut. 2015, 201, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Raza, I.; Deeba, F.; Jamil, M.; Naeem, R.; Azizullah, A.; Khattak, B.; Shah, A.; Ali, I.; Jin, Z.; et al. Pesticide-induced physiological, metabolic and ultramorphological alterations in leaves of young maize seedlings. Pol. J. Environ. Stud. 2020, 29, 2247–2258. [Google Scholar] [CrossRef]
- Váňová, L.; Kummerová, M.; Klemš, M.; Zezulka, Š. Fluoranthene influences endogenous abscisic acid level and primary photosynthetic processes in pea (Pisum sativum L.) plants in vitro. Plant Growth Regul. 2008, 57, 39–47. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Choudhary, S.P.; Chen, S.; Xia, X.; Shi, K.; Zhou, Y.; Yu, J. Role of brassinosteroids in alleviation of phenanthrene-cadmium co-contamination-induced photosynthetic inhibition and oxidative stress in tomato. J. Exp. Bot. 2013, 64, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Alkio, M.; Tabuchi, T.M.; Wang, X.; Colon-Carmona, A. Stress responses to polycyclic aromatic hydrocarbons in Arabidopsis include growth inhibition and hypersensitive response-like symptoms. J. Exp. Bot. 2005, 56, 2983–2994. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; He, B.B.; Qian, X.J.; Zhou, Y.H.; Shi, K.; Zhou, J.; Yu, J.Q.; Xia, X.J. 24-Epibrassinolide alleviates organic pollutants-retarded root elongation by promoting redox homeostasis and secondary metabolism in Cucumis sativus L. Environ. Pollut. 2017, 229, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Wang, Y.; Mao, Q.; Wu, M.; Yan, Y.; Ren, J.; Wang, X.; Liu, A.; Chen, S. Dopamine alleviates bisphenol A-induced phytotoxicity by enhancing antioxidant and detoxification potential in cucumber. Environ. Pollut. 2020, 259, 113957. [Google Scholar] [CrossRef]
- Jiao, L.; Ding, H.; Wang, L.; Zhou, Q.; Huang, X. Bisphenol A effects on the chlorophyll contents in soybean at different growth stages. Environ. Pollut. 2017, 223, 426–434. [Google Scholar] [CrossRef]
- Ali, I.; Liu, B.; Farooq, M.A.; Islam, F.; Azizullah, A.; Yu, C.; Su, W.; Gan, Y. Toxicological effects of bisphenol A on growth and antioxidant defense system in Oryza sativa as revealed by ultrastructure analysis. Ecotoxicol. Environ. Saf. 2016, 124, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Korte, F.; Kvesitadze, G.; Ugrekhelidze, D.; Gordeziani, M.; Khatisashvili, G.; Buadze, O.; Zaalishvili, G.; Coulston, F. Organic toxicants and plants. Ecotoxicol. Environ. Saf. 2000, 47, 1–26. [Google Scholar] [CrossRef]
- Pelaez-Vico, M.A.; Fichman, Y.; Zandalinas, S.I.; Van Breusegem, F.; Karpinski, S.M.; Mittler, R. ROS and redox regulation of cell-to-cell and systemic signaling in plants during stress. Free Radic. Biol. Med. 2022, 193, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Yang, S.J.; Huang, B.; Zhao, Y.Q.; Hu, D.; Chen, T.; Ding, C.B.; Chen, Y.E.; Yuan, S.; Yuan, M. Melatonin enhanced the tolerance of Arabidopsis thaliana to high light through improving anti-oxidative system and photosynthesis. Front. Plant Sci. 2021, 12, 752584. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.A.; Shahid, R.; Altaf, M.M.; Kumar, R.; Naz, S.; Kumar, A.; Alam, P.; Tiwari, R.K.; Lal, M.K.; Ahmad, P. Melatonin: First-line soldier in tomato under abiotic stress current and future perspective. Plant Physiol. Biochem. 2022, 185, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Nawaz, G.; Cao, Q.; Xu, T. Melatonin is a potential target for improving horticultural crop resistance to abiotic stress. Sci. Hortic. 2022, 291, 110560. [Google Scholar] [CrossRef]
- Hasan, M.K.; Ahammed, G.J.; Sun, S.; Li, M.; Yin, H.; Zhou, J. Melatonin inhibits cadmium translocation and enhances plant tolerance by regulating sulfur uptake and assimilation in Solanum lycopersicum L. J. Agric. Food Chem. 2019, 67, 10563–10576. [Google Scholar] [CrossRef]
- Hasan, M.K.; Liu, C.; Wang, F.; Ahammed, G.J.; Zhou, J.; Xu, M.X.; Yu, J.Q.; Xia, X.J. Glutathione-mediated regulation of nitric oxide, S-nitrosothiol and redox homeostasis confers cadmium tolerance by inducing transcription factors and stress response genes in tomato. Chemosphere 2016, 161, 536–545. [Google Scholar] [CrossRef]
- Yu, G.B.; Zhang, Y.; Ahammed, G.J.; Xia, X.J.; Mao, W.H.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Glutathione biosynthesis and regeneration play an important role in the metabolism of chlorothalonil in tomato. Chemosphere 2013, 90, 2563–2570. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Kumar, R.; Shahzad, B.; Thukral, A.K.; Bhardwaj, R.; Tejada Moral, M. Brassinosteroid-mediated pesticide detoxification in plants: A mini-review. Cogent Food Agric. 2018, 4, 1436212. [Google Scholar] [CrossRef]
- Tan, D.X.; Reiter, R.J. An evolutionary view of melatonin synthesis and metabolism related to its biological functions in plants. J. Exp. Bot. 2020, 71, 4677–4689. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Pernici, C.; Serio, G.; Gentile, C.; Bertea, C.M. Melatonin and phytomelatonin: Chemistry, biosynthesis, metabolism, distribution and bioactivity in plants and animals-An overview. Int. J. Mol. Sci. 2021, 22, 9996. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Guo, Y.; Yan, J.; Zhang, Z.; Yuan, L.; Wei, C.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X.; et al. The role of watermelon caffeic acid O-methyltransferase (ClCOMT1) in melatonin biosynthesis and abiotic stress tolerance. Hortic. Res. 2021, 8, 210. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.D.; Sun, X.S.; Liu, L.; Shi, H.D.; Chen, S.Y.; Zhao, D.K. Overexpression of the melatonin synthesis-related gene SlCOMT1 improves the resistance of tomato to salt Stress. Molecules 2019, 24, 1514. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Phytomelatonin versus synthetic melatonin in cancer treatments. Biomed. Res. Clin. Pract. 2018, 3, 1–6. [Google Scholar] [CrossRef]
- Contente, M.L.; Farris, S.; Tamborini, L.; Molinari, F.; Paradisi, F. Flow-based enzymatic synthesis of melatonin and other high value tryptamine derivatives: A five-minute intensified process. Green Chem. 2019, 21, 3263–3266. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, X.; Tian, Y.; Li, W.; Wang, H.; Li, Q.; Li, Y.; Li, Z.; Wu, T. Synthesis of a new water-soluble melatonin derivative with low toxicity and a strong effect on sleep aid. ACS Omega 2020, 5, 6494–6499. [Google Scholar] [CrossRef]
- Perez-Llamas, F.; Hernandez-Ruiz, J.; Cuesta, A.; Zamora, S.; Arnao, M.B. Development of a phytomelatonin-rich extract from cultured plants with excellent biochemical and functional properties as an alternative to synthetic melatonin. Antioxidants 2020, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- Tijero, V.; Munoz, P.; Munne-Bosch, S. Melatonin as an inhibitor of sweet cherries ripening in orchard trees. Plant Physiol. Biochem. 2019, 140, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Cao, X.; Wang, X.; Zhang, W.; Li, W.; Wang, X.; Liu, S.; Lyu, D. RBOH-dependent hydrogen peroxide signaling mediatesmelatonin-induced anthocyanin biosynthesis in red pear fruit. Plant Sci. 2021, 313, 111093. [Google Scholar] [CrossRef]
- Moreno, J.E.; Campos, M.L. Waking up for defense! Melatonin as a regulator of stomatal immunity in plants. Plant Physiol. 2022, 188, 14–15. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, M.S.; Mukherjee, S.; Flores, F.B.; Arnao, M.B.; Luo, Z.; Corpas, F.J. Functions of melatonin during postharvest of horticultural crops. Plant Cell Physiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernandez-Ruiz, J. Melatonin as a plant biostimulant in crops and during post-harvest: A new approach is needed. J. Sci. Food Agric. 2021, 101, 5297–5304. [Google Scholar] [CrossRef]
- Xu, T.; Chen, Y.; Kang, H. Melatonin is a potential target for improving post-harvest preservation of fruits and vegetables. Front. Plant Sci. 2019, 10, 1388. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Bhat, J.A.; Siddiqui, M.H.; Rinklebe, J.; Ahmad, P. Integration of silicon and secondary metabolites in plants: A significant association in stress tolerance. J. Exp. Bot. 2020, 71, 6758–6774. [Google Scholar] [CrossRef]
- Gu, Q.; Chen, Z.; Yu, X.; Cui, W.; Pan, J.; Zhao, G.; Xu, S.; Wang, R.; Shen, W. Melatonin confers plant tolerance against cadmium stress via the decrease of cadmium accumulation and reestablishment of microRNA-mediated redox homeostasis. Plant Sci. 2017, 261, 28–37. [Google Scholar] [CrossRef]
- Debnath, B.; Islam, W.; Li, M.; Sun, Y.; Lu, X.; Mitra, S.; Hussain, M.; Liu, S.; Qiu, D. Melatonin mediates enhancement of stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 1040. [Google Scholar] [CrossRef]
- Hoque, M.N.; Tahjib-Ul-Arif, M.; Hannan, A.; Sultana, N.; Akhter, S.; Hasanuzzaman, M.; Akter, F.; Hossain, M.S.; Sayed, M.A.; Hasan, M.T.; et al. Melatonin modulates plant tolerance to heavy metal stress: Morphological responses to molecular mechanisms. Int. J. Mol. Sci. 2021, 22, 11445. [Google Scholar] [CrossRef]
- Nawaz, K.; Chaudhary, R.; Sarwar, A.; Ahmad, B.; Gul, A.; Hano, C.; Abbasi, B.H.; Anjum, S. Melatonin as master regulator in plant growth, development and stress alleviator for sustainable agricultural production: Current status and future perspectives. Sustainability 2020, 13, 294. [Google Scholar] [CrossRef]
- Mukherjee, S. Insights into nitric oxide-melatonin crosstalk and N-nitrosomelatonin functioning in plants. J. Exp. Bot. 2019, 70, 6035–6047. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yan, J.; Su, Z.; Chang, J.; Yang, J.; Wei, C.; Zhang, Y.; Ma, J.; Zhang, X.; Li, H. Abscisic acid mediates grafting-induced cold tolerance of watermelon via interaction with melatonin and methyl jasmonate. Front. Plant Sci. 2021, 12, 785317. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; He, J.; Yang, X.; Li, X.; Luo, D.; Wei, C.; Ma, J.; Zhang, Y.; Yang, J.; Zhang, X. Glutathione-dependent induction of local and systemic defense against oxidative stress by exogenous melatonin in cucumber (Cucumis sativus L.). J. Pineal Res. 2016, 60, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Acosta, M.G.; Cano, A.; Hernández-Ruiz, J.; Arnao, M.B. Melatonin as a possible natural safener in crops. Plants 2022, 11, 890. [Google Scholar] [CrossRef]
- Zhou, Y.; Xia, X.; Yu, G.; Wang, J.; Wu, J.; Wang, M.; Yang, Y.; Shi, K.; Yu, Y.; Chen, Z.; et al. Brassinosteroids play a critical role in the regulation of pesticide metabolism in crop plants. Sci. Rep. 2015, 5, 9018. [Google Scholar] [CrossRef] [PubMed]

| Plant Species | Melatonin Concentrations * | Treatment Methods | Organic Pollutants | Melatonin Effects | References |
|---|---|---|---|---|---|
| Tomato (Solanum lycopersicum L.) | 20 μM | Foliar application | Bisphenol A (BPA, 10 mg L−1)-root treatment |
| [7] |
| Tomato (S. lycopersicum L.) | 100 μM | Foliar application | Chlorothalonil, 11.2 mM-foliar treatment |
| [37] |
| Tomato (S. lycopersicum L.) | 0.5 μM | Foliar application | Carbendazim (MBC, 1 mM)-foliar treatment |
| [38] |
| Lettuce (Lactuca sativa L.) | 0.5 μM | Foliar application | Carbendazim (MBC, 1 mM)-foliar treatment |
| [38] |
| Chinese cabbage (Brassica campestris L.) | 0.5 μM | Foliar application | Carbendazim (MBC, 1 mM)-foliar treatment |
| [38] |
| Spinach (Spinacia oleracea L.), | 0.5 μM | Foliar application | Carbendazim (MBC, 1 mM)-foliar treatment |
| [38] |
| Celery (Apium graveolens L.) | 0.5 μM | Foliar application | Carbendazim (MBC, 1 mM)-foliar treatment |
| [38] |
| Cucumber (Cucumis sativus L.) | 0.5 μM | Foliar application | Carbendazim (MBC, 1 mM)-foliar treatment |
| [38] |
| Cucumber (C. sativus L.) | 50 μM | Root pretreatment | Imidacloprid (IMD, 2.75 mM)-foliar treatment |
| [40] |
| Jujube (Ziziphus jujuba Mill. cv. Dongzao) | 0.1 mM | Mature jujube fruits (post-harvest spraying) | Fruits treated (immersed) with chlorothalonil (CHT, 10 mM), glyphosate (Gly, 2 mM), and malathion (Mal, 3 mM) solution for 2 h |
| [10] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahammed, G.J.; Li, X. Melatonin-Induced Detoxification of Organic Pollutants and Alleviation of Phytotoxicity in Selected Horticultural Crops. Horticulturae 2022, 8, 1142. https://doi.org/10.3390/horticulturae8121142
Ahammed GJ, Li X. Melatonin-Induced Detoxification of Organic Pollutants and Alleviation of Phytotoxicity in Selected Horticultural Crops. Horticulturae. 2022; 8(12):1142. https://doi.org/10.3390/horticulturae8121142
Chicago/Turabian StyleAhammed, Golam Jalal, and Xin Li. 2022. "Melatonin-Induced Detoxification of Organic Pollutants and Alleviation of Phytotoxicity in Selected Horticultural Crops" Horticulturae 8, no. 12: 1142. https://doi.org/10.3390/horticulturae8121142
APA StyleAhammed, G. J., & Li, X. (2022). Melatonin-Induced Detoxification of Organic Pollutants and Alleviation of Phytotoxicity in Selected Horticultural Crops. Horticulturae, 8(12), 1142. https://doi.org/10.3390/horticulturae8121142







