Chemical Priming with Brassinosteroids to Mitigate Responses of Avocado (Persea americana) Trees to Flooding Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Chemical Priming Treatments
2.3. Flooding Treatments
2.4. Leaf Gas Exchange
2.5. Chlorophyll Fluorescence
2.6. Statistical Analyses
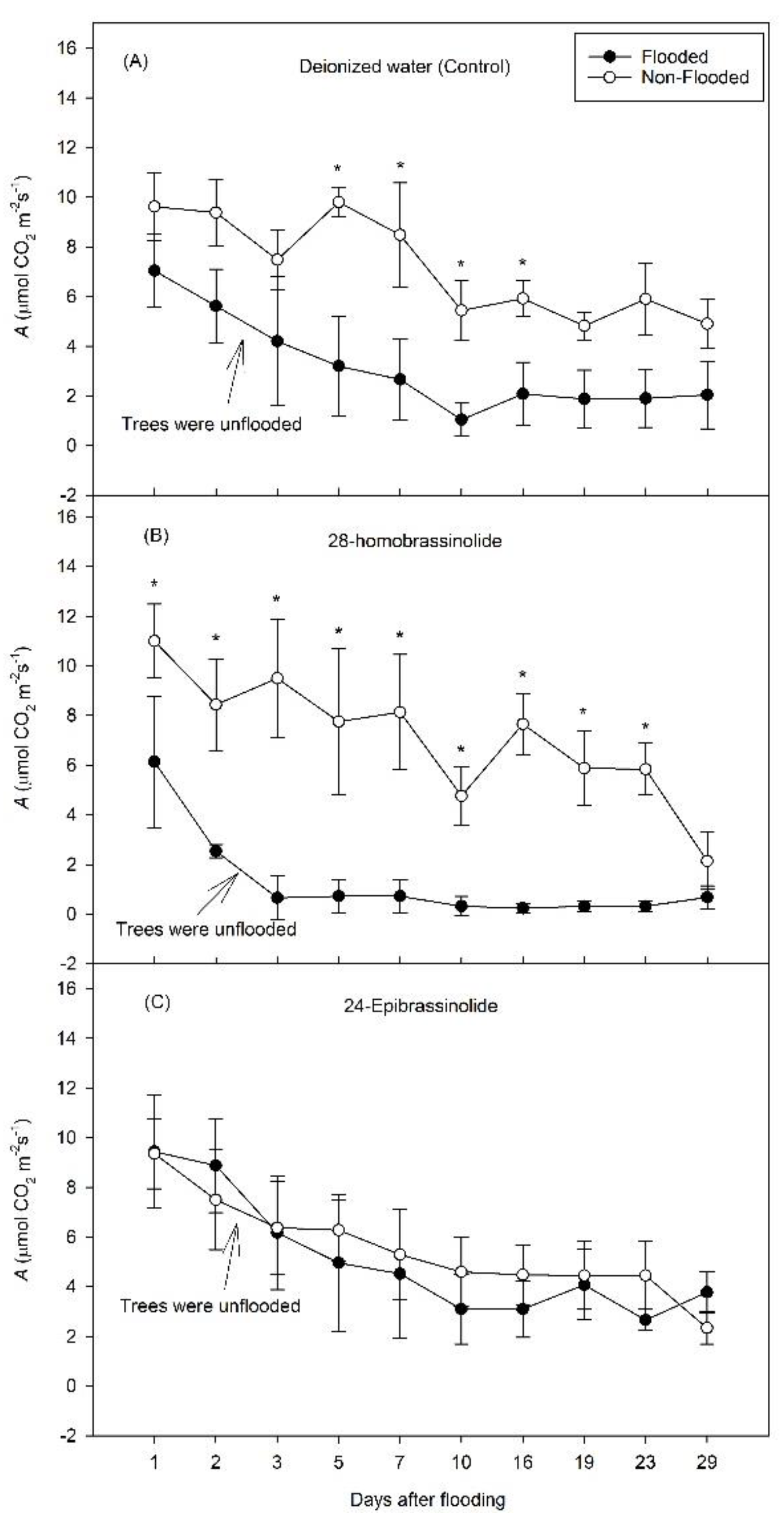
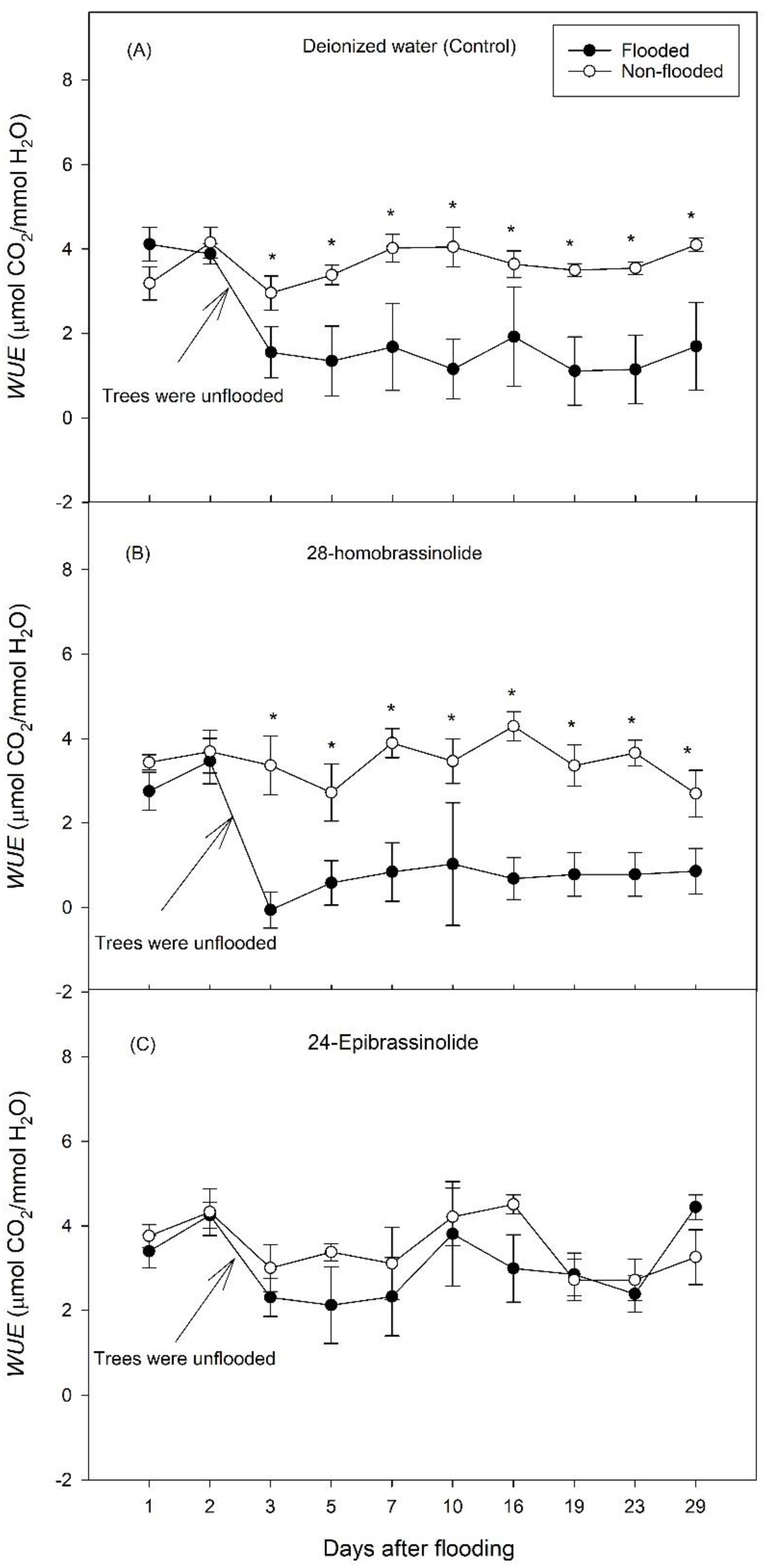
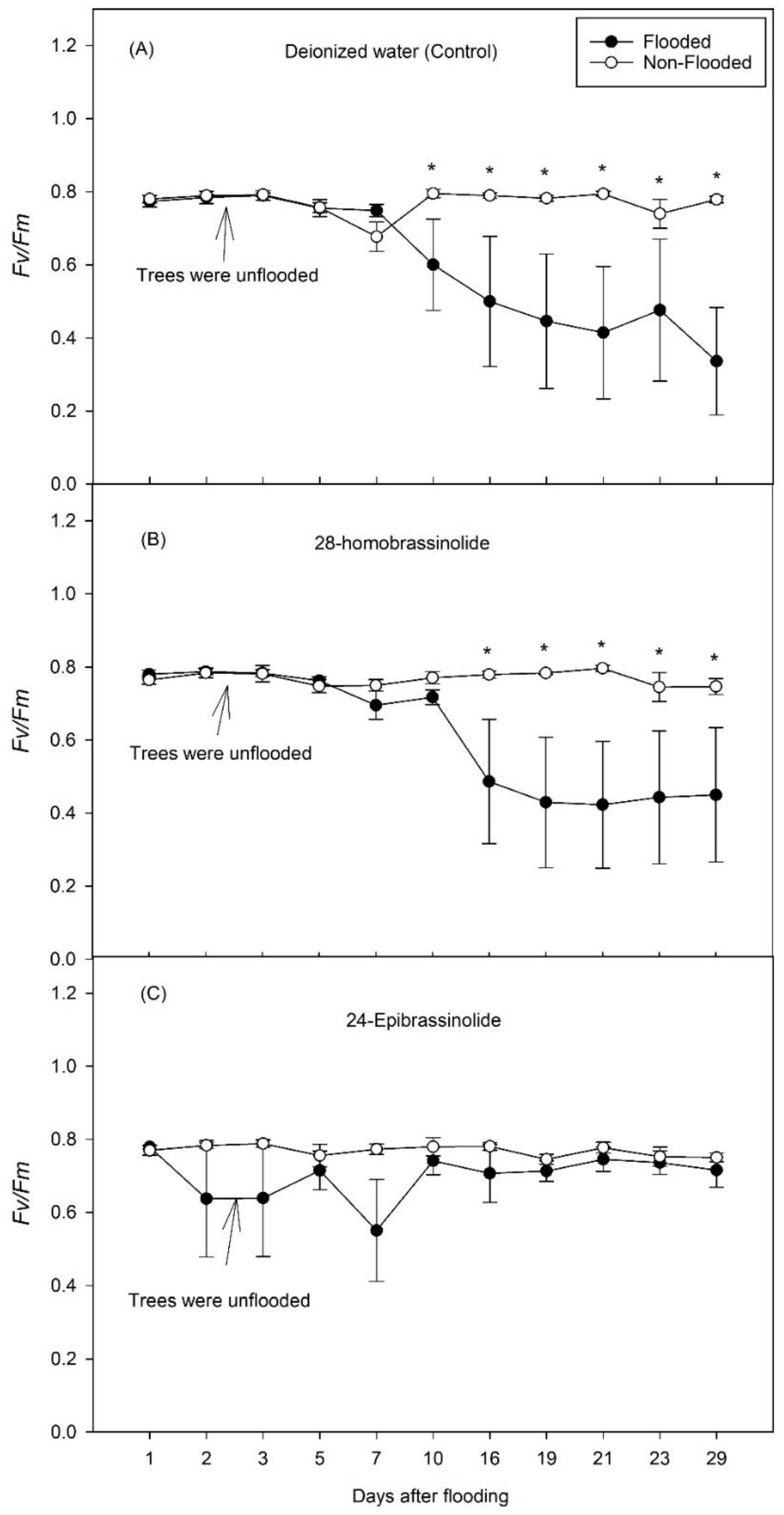
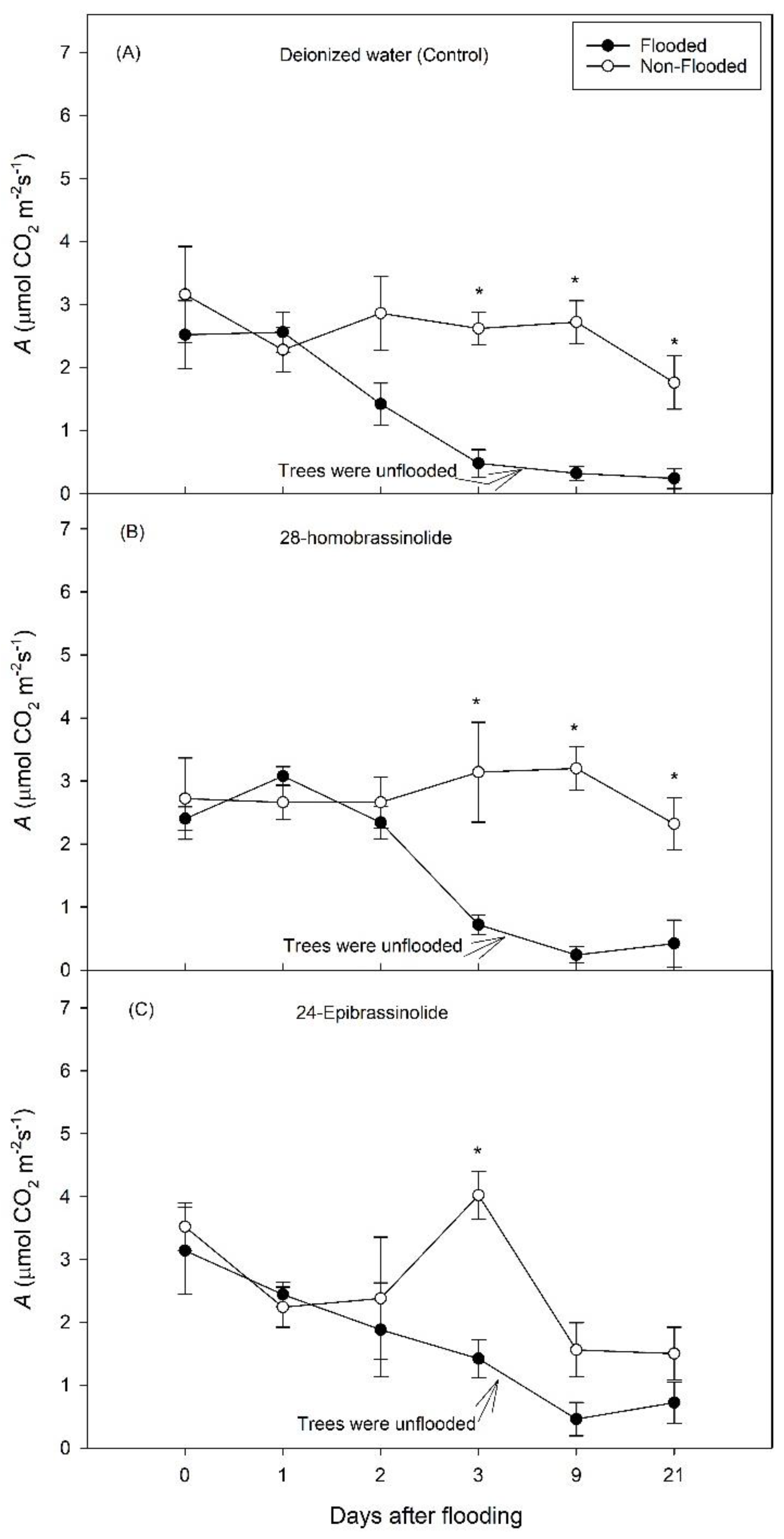
3. Results
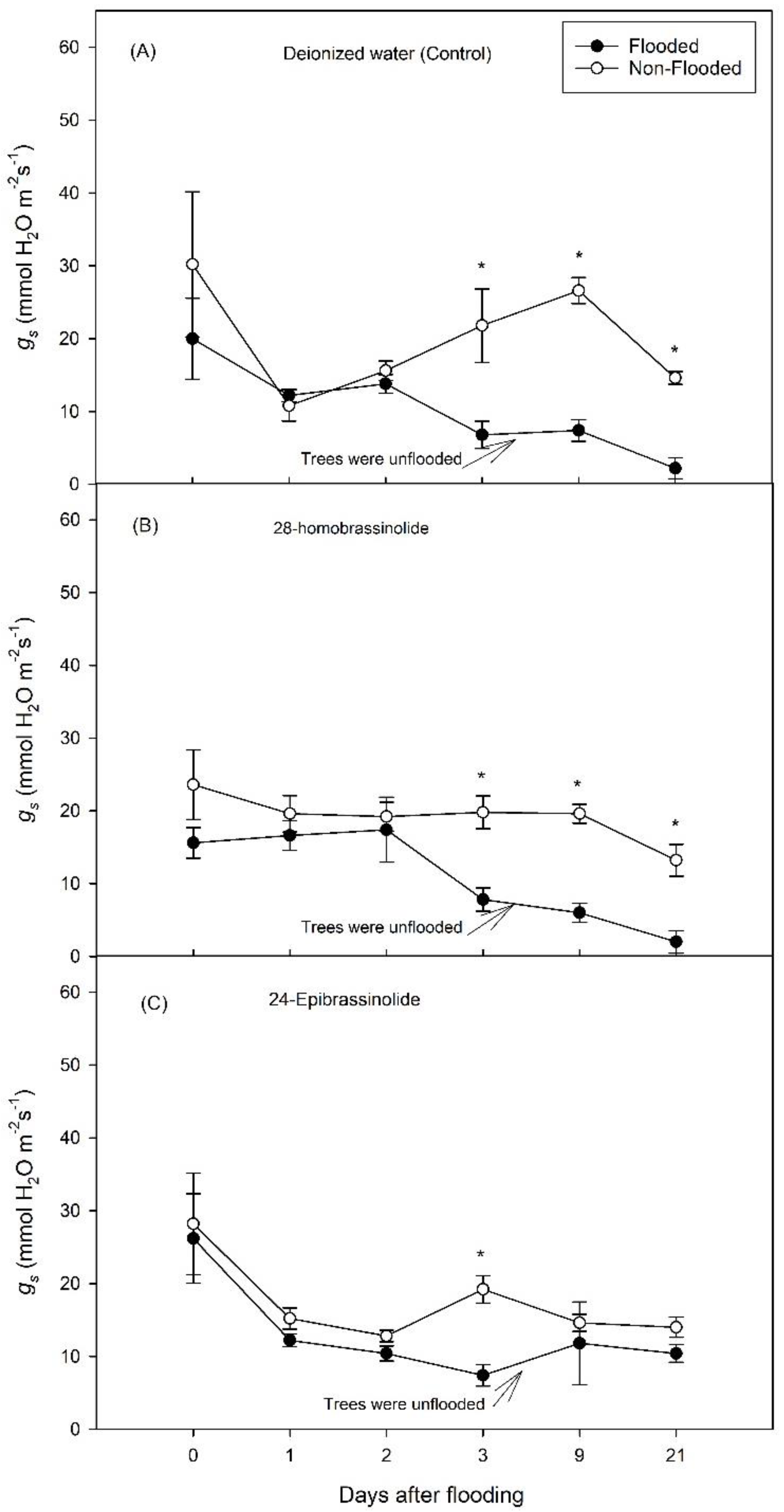
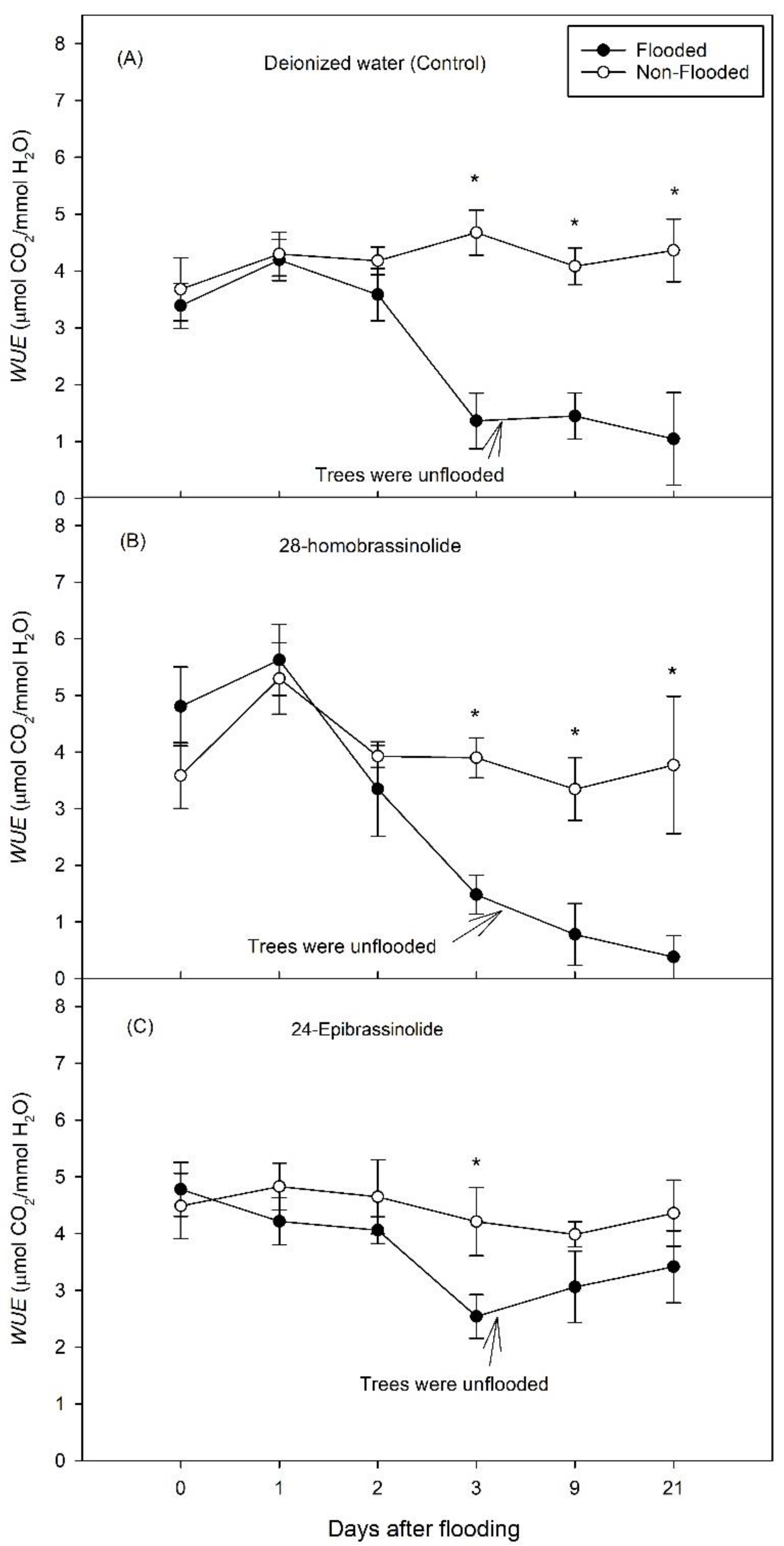
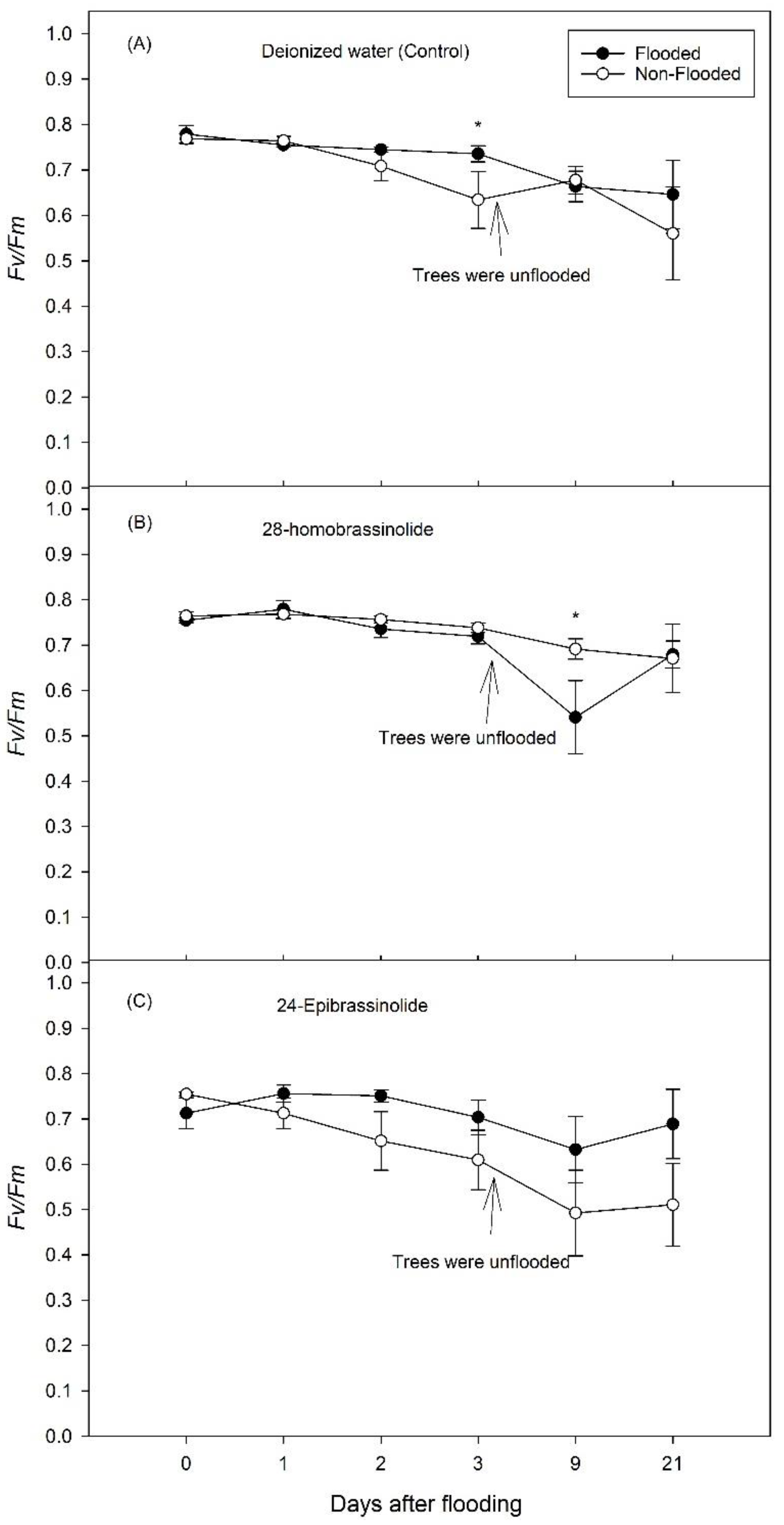
3.1. Experiment 1
3.2. Experiment 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ploetz, R.C.; Schaffer, B. Effects of flooding and Phytophthora root rot on net gas exchange and growth of avocado. Phytopathology 1989, 79, 204–208. [Google Scholar] [CrossRef]
- Sanclemente, M.A.; Schaffer, B.; Gil, P.M.; Davies, F.S.; Crane, J.H. Leaf removal before flooding influences recovery of avocado (Persea americana Mill.) trees from flooding stress. Sci. Hortic. 2013, 150, 154–163. [Google Scholar] [CrossRef]
- Sanclemente, M.A.; Schaffer, B.; Gil, P.M.; Vargas, A.I.; Davies, F.S. Pruning after flooding hastens recovery of flood-stressed avocado (Persea americana Mill.) trees. Sci. Hortic. 2014, 169, 27–35. [Google Scholar] [CrossRef]
- Schaffer, B.; Andersen, P.C.; Ploetz, R.C. Responses of fruit crops to flooding. Hortic. Rev. 1992, 13, 257–313. [Google Scholar]
- Schaffer, B. Flooding responses and water-use efficiency of subtropical and tropical fruit trees in an environmentally-sensitive wetland. Ann. Bot. 1998, 81, 475–481. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Attribution of Extreme Weather Events in the Context of Climate Change; The National Academies Press: Washington, DC, USA, 2016. [Google Scholar] [CrossRef]
- Her, Y.; Boote, K.; Migliaccio, K.W.; Fraisse, C.; Letson, D.; Mbuya, O.; Swamy, A.A.; Chi, H.; Ngatia, L.L.; Asseng, S. Climate change impacts and adaptation in Florida’s agriculture. In Florida’s Climate: Changes, Variations, and Impacts; Chassignet, E.P., Jones, J.W., Misra, V., Obeyesekera, J., Eds.; Florida Climate Institute, CreateSpace Independent Publishing: Gainesville, FL, USA, 2017; pp. 235–267. [Google Scholar]
- Bruce, T.J.; Matthes, M.C.; Napier, J.A.; Pickett, J.A. Stressful “memories” of plants: Evidence and possible mechanisms. Plant Sci. 2007, 173, 603–608. [Google Scholar] [CrossRef]
- Vincent, C.I.; Rowland, D.; Schaffer, B.; Bassil, E.; Racette, K.; Zurweller, B. Primed acclimation: A physiological process offers a strategy for more resilient and irrigation-efficient crop production. Plant Sci. 2019, 295, 110240. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, I.T.; Halitschke, R.; Paschold, A.; Von Dahl, C.C.; Preston, C.A. Volatile signaling in plant-plant interactions: “talking trees” in the genomics era. Science 2006, 311, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Jakab, G.; Ton, J.; Flors, V.; Zimmerli, L.; Métraux, J.-P.; Mauch-Mani, B. Enhancing Arabidopsis salt and drought stress tolerance by chemical priming for its abscisic acid responses. Plant Physiol. 2005, 139, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Rowland, D.L.; Faircloth, W.H.; Payton, P.; Tissue, D.T.; Ferrell, J.A.; Sorensen, R.B.; Butts, C.L. Primed acclimation of cultivated peanut (Arachis hypogaea L.) through the use of deficit irrigation timed to crop developmental periods. Agric. Water Manag. 2012, 113, 85–95. [Google Scholar] [CrossRef]
- Teale, W.; Paponov, D.I.A.; Palme, K. Auxin in action: Signaling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Depuydt, S.; Hardtke, C.S. Hormone signaling crosstalk in plant growth regulation. Curr. Biol. 2011, 21, R365–R373. [Google Scholar] [CrossRef]
- Santner, A.M.; Estelle, M. Recent advances and emerging trends in plant hormone signaling. Nature 2009, 459, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Peres, A.L.; Soares, G.J.S.; Tavares, R.G.; Righetto, G.; Zullo, M.A.; Mandava, N.B.; Menossi, M. Brassinosteroids, the sixth class of phytohormones: A molecular view from the discovery to hormonal interactions in plant development and stress adaptation. Int. J. Mol. Sci. 2019, 20, 331. [Google Scholar] [CrossRef] [PubMed]
- Vardhini, B.V.; Anjum, N.A. Brassinosteroids make plant life easier under abiotic stresses mainly by modulating major components of antioxidant defense system. Front. Environ. Sci. 2015, 2, 67. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Y.; Gong, W.; Chen, Y. Effect of brassinolide on the seedling growth and waterlogging resistance of soybean. Chin. Agric. Sci. Bull. 2006, 23, 37–38. [Google Scholar]
- Kang, Y.-Y.; Guo, S.-R.; Li, J.; Duan, J.-J. Effect of root applied 24-24-epibrassinolide on carbohydrate status and fermentative enzyme activities in cucumber (Cucumis sativus L.) seedlings under hypoxia. Plant Growth Reg. 2009, 57, 259–269. [Google Scholar] [CrossRef]
- Lu, X.; Guo, S. Effects of brassinolide on the polyamines, ATPase activity, and inorganic ion content in roots of cucumber seedlings under hypoxia stress. Chin. J. Ecol. 2013, 32, 611–614. [Google Scholar]
- Lin, S.-Y.; Chen, P.-A.; Zhuang, B.-W. The stomatal conductance and Fv/Fm as the indicators of stress tolerance of avocado seedlings under short-term waterlogging. Agronomy 2022, 12, 1804. [Google Scholar] [CrossRef]
- Ikekawa, N.; Zhao, Y.-J. Application of 24-24-epibrassinolide in agriculture. In Brassinosteroids, Chemistry, Bioactivity, and Applications; Cutler, H.G., Yokota, T., Adam, G., Eds.; ACS Symposium Series 474; American Chemical Society: Washington, DC, USA, 1991; pp. 280–291. [Google Scholar]
- Crane, J.H.; Douhan, D.; Faber, B.A.; Arpaia, M.L.; Bender, G.S.; Balerdi, C.F.; Barrientos-Priego, A.F. Cultivars and rootstocks. In The Avocado: Botany Production and Uses; Schaffer, B., Wolstenholme, B.N., Whiley, A.W., Eds.; CABI Press: Waddington, UK, 2013; pp. 200–233. [Google Scholar]
- Schaffer, B.; Gil, P.M.; Mickelbart, M.V.; Whiley, A.W. Ecophysiology. In The Avocado: Botany, Production and Uses; Schaffer, B., Wolstenholme, B.N., Whiley, A.W., Eds.; CABI Press: Waddington, UK, 2013; pp. 168–199. [Google Scholar]
- Bergh, B.; Ellstrand, N. Taxonomy of the avocado. Calif. Avocado Soc. Yearb. 1986, 70, 135–145. [Google Scholar]
- Chanderbali, A.; Soltis, D.; Soltis, P.; Wolstenholme, B. Taxonomy and botany. In The Avocado: Botany Production and Uses; Schaffer, B., Wolstenholme, B.N., Whiley, A.W., Eds.; CABI Press: Waddington, UK, 2013; pp. 31–50. [Google Scholar]
- Yin, M.H. Physiological and Biochemical Responses of Avocado to Short-Term Flooding: Effects of Ecotype and Brassinosteroid Treatments. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2022. [Google Scholar]
- Schnell, R.J.; Brown, J.S.; Olano, C.T.; Power, E.J.; Krol, C.A. Evaluation of avocado germplasm using microsatellite markers. J. Am. Soc. Hortic. Sci. 2003, 128, 881–889. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, T.; Wu, B.; Tan, L.; Ma, F.; Zou, M.; Chen, H.; Pei, J.; Liu, Y.; Chen, Z.; et al. Genome-wide assessment of avocado germplasm determined from specific length amplified fragment sequencing and transcriptomes: Population structure, genetic diversity, identification, and application of race-specific markers. Genes 2019, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Vardhini, B.V. Modifications of morphological and anatomical characteristics of plants by application of brassinosteroids under various abiotic stress conditions—A review. Plant Gene 2017, 11, 70–89. [Google Scholar] [CrossRef]
- Arora, N.; Bhardwaj, R.; Sharma, P.; Arora, H.K. Effects of 28-homobrassinolide on growth, lipid peroxidation and antioxidative enzyme activities in seedlings of Zea mays L. under salinity stress. Acta Physiol. Plant. 2008, 30, 833–839. [Google Scholar] [CrossRef]
- Kaur, H.; Sirhindi, G.; Bhardwaj, R.; Alyemeni, M.; Siddique, K.H.; Ahmad, P. 28-homobrassinolide regulates antioxidant enzyme activities and gene expression in response to salt-and temperature-induced oxidative stress in Brassica juncea. Sci. Rep. 2018, 8, 8735. [Google Scholar] [CrossRef] [PubMed]



| Experiment | Cultivar | Chemical Treatment | Flooding Treatment | Number of Replicates |
|---|---|---|---|---|
| 1 | Monroe | 24-Epibrassinolide | Flooded | 5 |
| Nonflooded | 5 | |||
| 28-Homobrassinolide | Flooded | 5 | ||
| Nonflooded | 5 | |||
| Deionized water (control) | Flooded | 5 | ||
| Non-flooded | 5 | |||
| 2 | Reed | 24-Epibrassinolide | Flooded | 10 |
| Nonflooded | 10 | |||
| 28-Homobrassinolide | Flooded | 10 | ||
| Nonflooded | 10 | |||
| Deionized water (control) | Flooded | 10 | ||
| Nonflooded | 10 |
| Chemical Priming Treatment | Flooding Treatment | |
|---|---|---|
| Nonflooded | Flooded | |
| Plant survival (%) | ||
| Control | 100 | 40 |
| 28-Homobrassinolide | 100 | 60 |
| 24-Epibrassinolide | 100 | 100 |
| Chemical Priming Treatment | Flooding Treatment | |
|---|---|---|
| Nonflooded | Flooded | |
| Plant survival (%) | ||
| Control | 100 | 20 |
| 28-Homobrassinolide | 100 | 40 |
| 24-Epibrassinolide | 100 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, M.H.; Gutierrez-Rodriguez, E.A.; Vargas, A.I.; Schaffer, B. Chemical Priming with Brassinosteroids to Mitigate Responses of Avocado (Persea americana) Trees to Flooding Stress. Horticulturae 2022, 8, 1115. https://doi.org/10.3390/horticulturae8121115
Yin MH, Gutierrez-Rodriguez EA, Vargas AI, Schaffer B. Chemical Priming with Brassinosteroids to Mitigate Responses of Avocado (Persea americana) Trees to Flooding Stress. Horticulturae. 2022; 8(12):1115. https://doi.org/10.3390/horticulturae8121115
Chicago/Turabian StyleYin, Melinda H., Edwin A. Gutierrez-Rodriguez, Ana I. Vargas, and Bruce Schaffer. 2022. "Chemical Priming with Brassinosteroids to Mitigate Responses of Avocado (Persea americana) Trees to Flooding Stress" Horticulturae 8, no. 12: 1115. https://doi.org/10.3390/horticulturae8121115
APA StyleYin, M. H., Gutierrez-Rodriguez, E. A., Vargas, A. I., & Schaffer, B. (2022). Chemical Priming with Brassinosteroids to Mitigate Responses of Avocado (Persea americana) Trees to Flooding Stress. Horticulturae, 8(12), 1115. https://doi.org/10.3390/horticulturae8121115







