Chitosan Soaking Improves Seed Germination of Platycodon Grandiflorus and Enhances Its Growth, Photosynthesis, Resistance, Yield, and Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Seed Germination Experiment of P. grandiflorus
2.3. Growth Experiment of P. grandiflorus
2.4. Determination Methods
2.5. Statistical Analyses
3. Results
3.1. Effects of Chitosan Soaking on Seed Germination of P. grandiflorus
3.2. Effects of Chitosan on Overground Part Growth of P. grandiflorus Plants
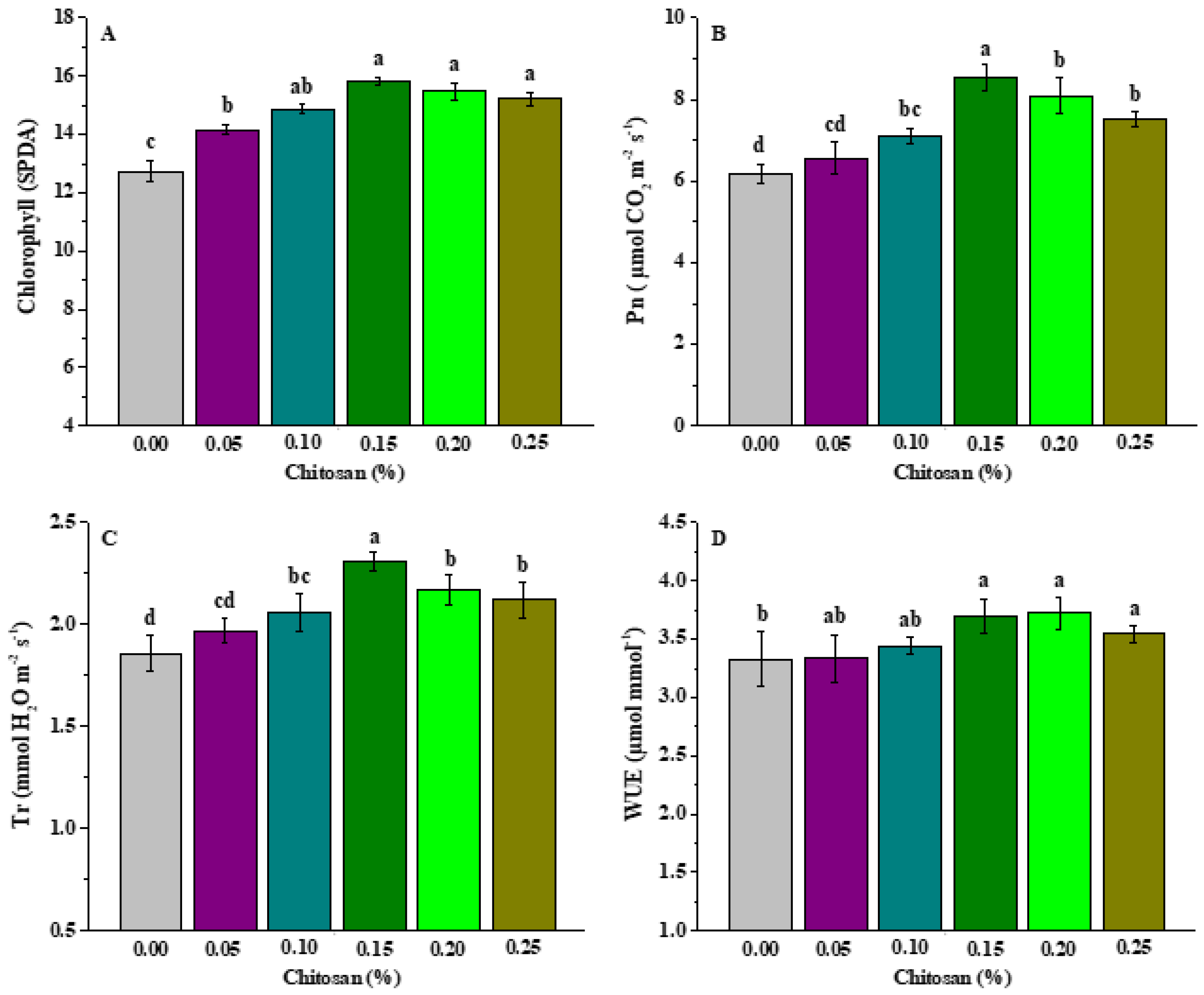
3.3. Effects of Chitosan Soaking on the Chlorophyll and Photosynthesis of P. grandiflorus Leaves
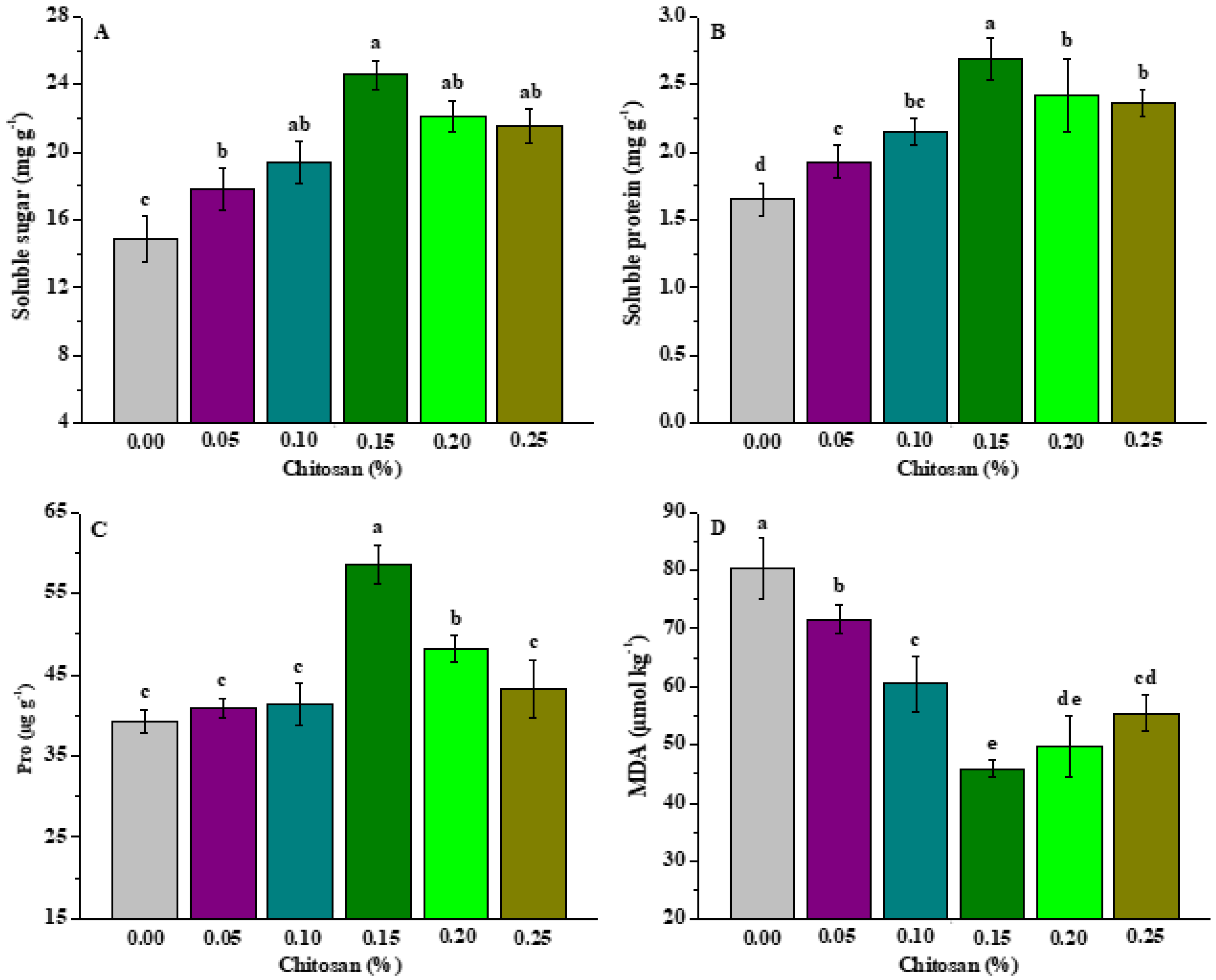
3.4. Effects of Chitosan Soaking on Resistance of P. grandiflorus Plants
3.5. Effects of Chitosan Soaking on Growth and Yield of P. grandiflorus Roots
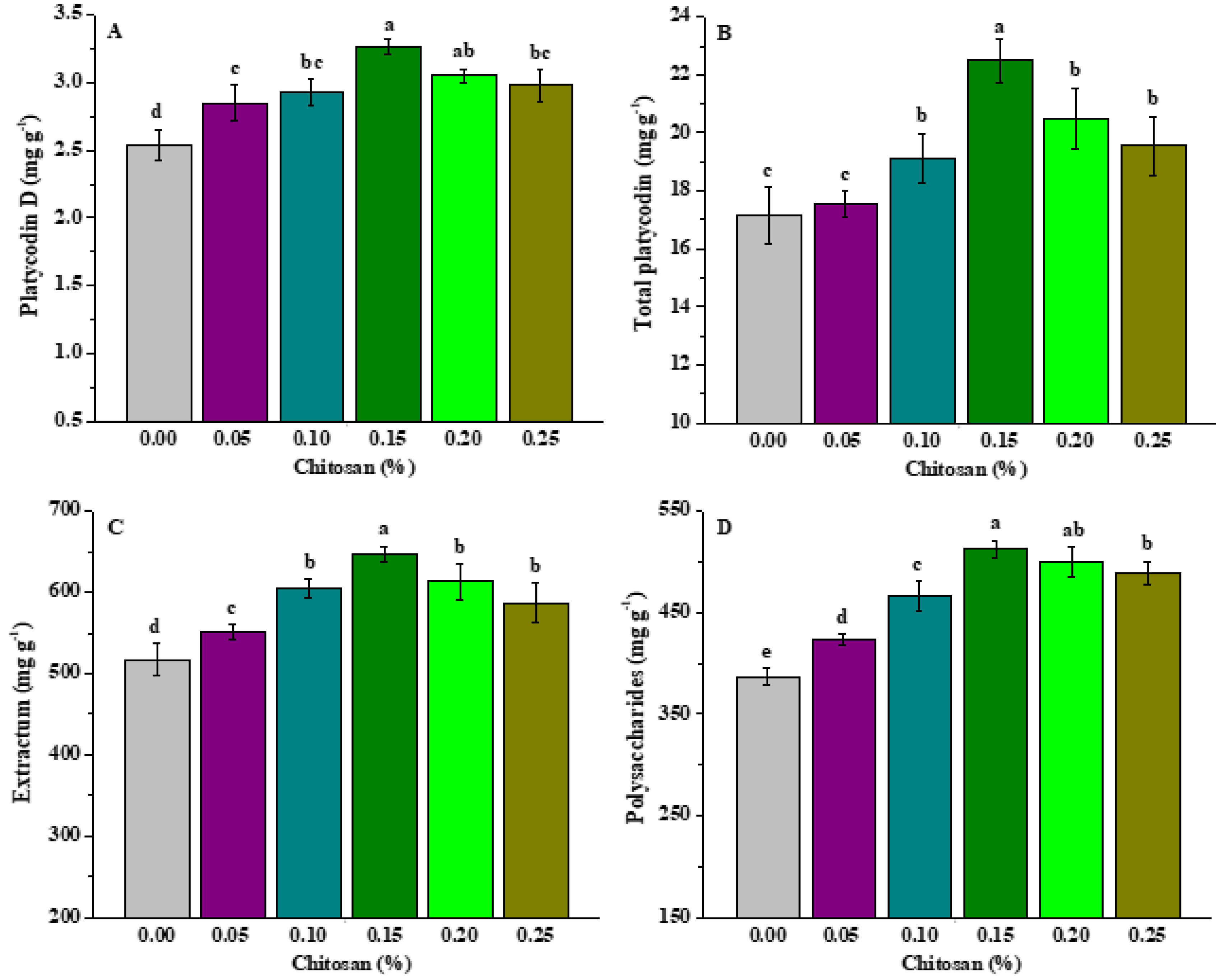
3.6. Effects of Chitosan Soaking on Medical Quality of P. grandiflorus
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Wang, Y.; Yang, D.; Zhang, C.; Zhang, N.; Li, M.; Liu, Y. Platycodon Grandiflorus—An Ethnopharmacological, Phytochemical and Pharmacological Review. J. Ethnopharmacol. 2015, 164, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, V.; Cho, Y.I.; Lee, H.J. Preliminary Study to Explore the Immune-Enhancement Mechanism of Platycodon grandiflorus Extract through Comparative Transcriptome Analysis. Appl. Sci. 2021, 11, 226. [Google Scholar] [CrossRef]
- Kim, G.; Rim, Y.; Cho, H.; Hyun, T.K. Identification and Functional Characterization of FLOWERING LOCUS T in Platycodon grandiflorus. Plants 2022, 11, 325. [Google Scholar] [CrossRef] [PubMed]
- Bae, N.; Ahn, T.; Chung, S.; Oh, M.S.; Ko, H.; Oh, H.; Park, G.; Yang, H.O. The Neuroprotective Effect of Modified Yeoldahanso-tang via Autophagy Enhancement in Models of Parkinson’s Disease. J. Ethnopharmacol. 2011, 134, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Nyakudya, E.; Jeong, J.H.; Lee, N.K.; Jeong, Y.S. Platycosides from the Roots of Platycodon Grandiflorum and Their Health Benefits. Prev. Nutr. Food Sci. 2014, 19, 59–68. [Google Scholar] [CrossRef]
- Tamura, K.; Teranishi, Y.; Ueda, S.; Suzuki, H.; Kawano, N.; Yoshimatsu, K.; Saito, K.; Kawahara, N.; Muranaka, T.; Seki, H. Cytochrome P450 Monooxygenase CYP716A141 is a Unique β -amyrin C-16 β Oxidase Involved in Triterpenoid Saponin Biosynthesis in Platycodon grandiflorus. Plant Cell Physiol. 2017, 58, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jeon, S.G.; Kim, K.A.; Kim, J.J.; Song, E.J.; Jeon, Y.; Kim, E.; Lee, K.B.; Kwak, J.H.; Moon, M. Platycodon grandiflorus Root Extract Improves Learning and Memory by Enhancing Synaptogenesis in Mice Hippocampus. Nutrients 2017, 9, 794. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Bo, A.; Yang, M.; Xu, J.; Jiang, L.; Zhou, B.; Li, M. The Pharmacological Effects and Health Benefits of Platycodon grandiflorus—A Medicine Food Homology Species. Foods 2020, 9, 142. [Google Scholar] [CrossRef]
- Kim, M.H. Contemplation on the Emergency Foods in Korea under the Japanese Occupation. J. East Asian Soc. Diet. Life 2015, 25, 721–738. [Google Scholar] [CrossRef]
- Zhao, M.; Xu, Z. Preliminary Study on the Characteristics of Endogenous Inhibitory Substances in Platycodon grandiflorum Seeds. J. Northeast Forestry Univ. 2000, 28, 51–54. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Wang, X.; Yu, H. Research Rrogress on Seed Vigor of Platycodon grandiflorus. Seed 2006, 25, 48–51. [Google Scholar] [CrossRef]
- Han, C.; Yang, P. Studies on the Molecular Mechanisms of Seed Germination. Proteomics 2015, 15, 1671–1679. [Google Scholar] [CrossRef]
- Vishal, B.; Kumar, P.P. Regulation of Seed Germination and Abiotic Stresses by Gibberellins and Abscisic Acid. Front. Plant Sci. 2018, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Zhu, X.; Zhang, Z.; Li, Z.; Geng, X.; Cai, Q.; Liu, Z. Research Progress on Dormancy and Germination Mechanism of Forest Seeds. Sci. Silvae Sin. 2021, 57, 128–144. [Google Scholar] [CrossRef]
- Chen, J.; Lei, J.; Wang, L.; Zhao, W.; Zhang, L.; Mu, X.; Cheng, Q. Effects of Four Chemical Reagents on Seed Germination and Seedling Growth of Platycodon grandiflorus. J. Northwest Agric. 2010, 19, 100–105. [Google Scholar] [CrossRef]
- Zhang, C.; Long, Y.; He, G.; Wang, Q. Effects of Growth Regulator Soaking on the Seed Germination and Growth of Platycodon grandiflorus. J. Chin. Med. Mater. 2015, 38, 21–24. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Q.; Long, Y.; Li, M.; Wu, X.; He, G. Effects of Bihu Soaking on on the Seed Germination and Growth of Platycodon grandiflorus. Seed 2016, 35, 42–44. [Google Scholar] [CrossRef]
- Anitha, A.; Sowmya, S.; Kumar, P.T.S.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Chitosan Effects on Plant Systems-A Review. Int. J. Mol. Sci. 2016, 17, 996. [Google Scholar] [CrossRef] [PubMed]
- Dzung, N.A.; Khanh, V.T.P.; Dzung, T.T. Research on Impact of Chitosan Oligomers on Biophysical Characteristics, Growth, Development and Drought Resistance of Coffee. Carbohydr. Polym. 2011, 84, 751–755. [Google Scholar] [CrossRef]
- Chakraborty, M.; Hasanuzzaman, M.; Rahman, M.; Khan, M.; Bhowmik, P.; Mahmud, N.U.; Tanveer, M.; Islam, T. Mechanism of Plant Growth Promotion and Disease Suppression by Chitosan Biopolymer. Agriculture 2020, 10, 624. [Google Scholar] [CrossRef]
- Lopez-Moya, F.; Suarez-Fernandez, M.; Lopez-Llorca, L.V. Molecular Mechanisms of Chitosan Interactions with Fungi and Plants. Int. J. Mol. Sci. 2019, 20, 332. [Google Scholar] [CrossRef]
- Maluin, F.N.; Hussein, M.Z. Chitosan-Based Agronanochemicals as a Sustainable Alternative in Crop Protection. Molecules 2020, 25, 1611. [Google Scholar] [CrossRef] [PubMed]
- Salachna, P.; Zawadzinska, A. Effect of Chitosan on Plant Growth, Flowering and Corms Yield of Potted Freesia. J. Ecol. Eng. 2014, 15, 93–102. [Google Scholar] [CrossRef]
- Pirbalouti, A.G.; Malekpoor, F.; Salimi, A.; Golparvar, A. Exogenous Application of Chitosan on Biochemical and Physiological Characteristics, Phenolic Content and Antioxidant Activity of Two Species of Basil (Ocimum ciliatum and Ocimum basilicum) under reduced irrigation. Sci. Hortic. 2017, 217, 114–122. [Google Scholar] [CrossRef]
- Choudhary, R.C.; Kumaraswamy, R.V.; Kumari, S.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Cu-chitosan Nanoparticle Boost Defense Responses and Plant Growth in Maize (Zea mays L.). Sci. Rep. 2017, 7, 9754–9765. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, R.; Zhang, C.; Guo, Z.; Wu, X.; An, H. Co-Application of Allicin and Chitosan Increases Resistance of Rosa roxburghii against Powdery Mildew and Enhances Its Yield and Quality. Antibiotics 2021, 10, 1449. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.; Lei, Y.; Su, Y.; Long, Y. Chitosan as an Adjuvant to Improve Isopyrazam Azoxystrobin against Leaf Spot Disease of Kiwifruit and Enhance Its Photosynthesis, Quality, and Amino Acids. Agriculture 2022, 12, 373. [Google Scholar] [CrossRef]
- Pan, L.; Wei, H.; Zhang, H.; Wang, Y. Effects of Chitosan on Seed Germination and Seedling Growth of Trifolium repens under Salt Stress. Mol. Plant Breed. 2018, 16, 3740–3744. [Google Scholar] [CrossRef]
- Li, X.; Liu, R.; Hua, Z. Effects of Chitosan on Seed Germination and Seedling Drought Resistance of Sctellaria baicalensis. Acta Agric. Jiangxi 2020, 32, 75–81. [Google Scholar] [CrossRef]
- Zhang, C.; Li, H.; Wu, X.; Su, Y.; Long, Y. Co-Application of Tetramycin and Chitosan in Controlling Leaf Spot Disease of Kiwifruit and Enhancing Its Resistance, Photosynthesis, Quality and Amino Acids. Biomolecules 2022, 12, 500. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, C.; Long, Y.; Wu, X.; Su, Y.; Lei, Y.; Ai, Q. Bioactivity and Control Efficacy of the Novel Antibiotic Tetramycin against Various Kiwifruit Diseases. Antibiotics 2021, 10, 289. [Google Scholar] [CrossRef]
- Zhang, C.; Long, Y.; Wang, Q.; Li, J.; Wu, X.; Li, M. The Effect of Preharvest 28.6% Chitosan Composite Film Sprays for Controlling the Soft Rot on Kiwifruit. Hortic. Sci. 2019, 46, 180–194. [Google Scholar] [CrossRef]
- Liu, Q.; Cui, T.; Cheng, F. Effects of Soil Microorganism on Output and Quality of Platycodon grandiflorum. Guangdong Agric. Sci. 2009, 2, 36–38. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, H.; Hua, Z. Effects of Soil Compaction Stress on Growth, Quantity and Quality of Platycodon grandiflorum. J. Northwest AF Univ. 2013, 41, 176–181. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Kong, L.; Cao, H.; Lv, Y. Effects of Four Kinds of Active Organic Substances on Cucumber Seed Germination. Humic Acid. 2022, 4, 13–19. [Google Scholar] [CrossRef]
- Caffall, K.H.; Mohnen, D. The Structure, Function, and Biosynthesis of Plant Cell Wall Pectic Polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Vlot, A.C.; Sales, J.H.; Lenk, M.; Bauer, K.; Brambilla, A.; Sommer, A.; Nayem, S. Systemic Propagation of Immunity in Plants. New Phytol. 2020, 229, 1234–1250. [Google Scholar] [CrossRef]
- Wang, Q.; Long, Y.; Ai, Q.; Su, Y.; Lei, Y. Oligosaccharins Used Together with Tebuconazole Enhances Resistance of Kiwifruit against Soft Rot Disease and Improves Its Yield and Quality. Horticulturae 2022, 8, 624. [Google Scholar] [CrossRef]
- Xoca-Orozco, L.Á.; Cuellar-Torres, E.A.; Gonz á lez-Morales, S.; Gutiérrez-Martínez, P.; López-García, U.; Herrera-Estrella, L.; Vega-Arreguín, J.; Chacón-López, A. Transcriptomic Analysis of Avocado Hass (Persea americana Mill) in the Interaction System Fruit-Chitosan-Colletotrichum. Front. Plant Sci. 2017, 8, 956. [Google Scholar] [CrossRef]
- Rahman, M.; Mukta, J.A.; Sabir, A.A.; Gupta, D.R.; Mohi-ud-din, M.; Hasanuzzaman, M.; Miah, M.G.; Rahman, M.; Islam, M.T. Chitosan Biopolymer Promotes Yield and Stimulates Accumulation of Antioxidants in Strawberry Fruit. PLoS ONE 2018, 13, e0203769. [Google Scholar] [CrossRef]
- Emami Bistgani, Z.; Siadat, S.A.; Bakhshandeh, A.; Ghasemi Pirbalouti, A.; Hashemi, M. Interactive Effects of Drought Stress and Chitosan Application on Physiological Characteristics and Essential Oil Yield of Thymus daenensis Celak. Crop J. 2017, 5, 407–415. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Q.; Li, J.; Su, Y.; Wu, X. Chitosan as an Adjuvant to Enhance the Control Efficacy of Low-Dosage Pyraclostrobin against Powdery Mildew of Rosa roxburghii and Improve Its Photosynthesis, Yield, and Quality. Biomolecules 2022, 12, 1304. [Google Scholar] [CrossRef]

| Indices | Content | Indices | Content |
|---|---|---|---|
| Organic matter | 15.36 g kg−1 | Available zinc | 0.83 mg kg−1 |
| Total nitrogen | 1.45 g kg−1 | Available iron | 7.04 mg kg−1 |
| Total phosphorus | 1.73 g kg−1 | Available boron | 0.16 mg kg−1 |
| Total potassium | 1.18 g kg−1 | Available manganese | 16.31 mg kg−1 |
| Available nitrogen | 57.33 mg kg−1 | Exchangeable magnesium | 315.78 mg kg−1 |
| Available phosphorus | 4.92 mg kg−1 | Exchangeable calcium | 16.85 cmol kg−1 |
| Available potassium | 28.27 mg kg−1 | pH value | 6.42 |
| Chitosan (%) | Germination Rate (%) | Germination Energy (%) | Germination Index | Cotyl Length (mm) | Radicle Length (mm) |
|---|---|---|---|---|---|
| 0.00 | 63.33 ± 2.52 d | 29.33 ± 1.53 e | 2.88 ± 0.11 d | 6.08 ± 0.16 e | 7.18 ± 0.14 e |
| 0.05 | 74.67 ± 3.51 c | 41.33 ± 1.53 d | 3.39 ± 0.16 c | 6.32 ± 0.12 d | 7.84 ± 0.26 d |
| 0.10 | 82.67 ± 3.21 b | 49.00 ± 2.00 c | 3.76 ± 0.15 b | 6.75 ± 0.13 c | 9.46 ± 0.27 c |
| 0.15 | 92.33 ± 3.51 a | 61.67 ± 3.51 a | 4.20 ± 0.16 a | 7.48 ± 0.14 a | 11.04 ± 0.24 a |
| 0.20 | 85.67 ± 2.52 b | 55.00 ± 3.00 b | 3.89 ± 0.11 b | 7.05 ± 0.06 b | 10.65 ± 0.28 ab |
| 0.25 | 84.00 ± 2.65 b | 53.67 ± 2.08 b | 3.82 ± 0.12 b | 6.83 ± 0.16 bc | 10.37 ± 0.27 b |
| Chitosan (%) | Leaf Length (mm) | Leaf Width (mm) | Leaf Area (mm2) |
|---|---|---|---|
| 0.00 | 36.38 ± 0.46 b | 23.90 ± 0.33 b | 869.46 ± 22.92 b |
| 0.05 | 36.34 ± 0.69 b | 23.86 ± 0.44 b | 867.27 ± 32.48 b |
| 0.10 | 36.95 ± 1.06 b | 24.26 ± 0.68 b | 897.01 ± 50.45 b |
| 0.15 | 40.46 ± 0.58 a | 26.57 ± 0.36 a | 1075.02 ± 29.90 a |
| 0.20 | 40.15 ± 0.56 a | 26.34 ± 0.37 a | 1057.82 ± 29.72 a |
| 0.25 | 40.13 ± 0.83 a | 26.33 ± 0.54 a | 1057.06 ± 43.30 a |
| Chitosan (%) | Plant Height (cm) | Stem Diameter (mm) | Overground Part Dry Weight (g Plant−1) |
|---|---|---|---|
| 0.00 | 27.13 ± 1.32 c | 1.80 ± 0.15 c | 1.48 ± 0.14 e |
| 0.05 | 30.32 ± 0.07 b | 1.79 ± 0.13 c | 1.53 ± 0.08 e |
| 0.10 | 29.68 ± 1.09 b | 1.86 ± 0.18 bc | 1.69 ± 0.07 d |
| 0.15 | 35.13 ± 1.01 a | 2.16 ± 0.12 a | 2.86 ± 0.08 a |
| 0.20 | 31.68 ± 1.30 b | 2.10 ± 0.14 ab | 2.24 ± 0.10 b |
| 0.25 | 31.32 ± 1.19 b | 2.05 ± 0.03 ab | 2.07 ± 0.04 c |
| Chitosan (%) | Root Diameter (mm) | Fresh Weight of Root (g Plant−1) | Dry Weight of Root (g Plant−1) |
|---|---|---|---|
| 0.00 | 5.28 ± 0.20 d | 0.73 ± 0.03 d | 0.20 ± 0.01 d |
| 0.05 | 5.35 ± 0.10 d | 0.79 ± 0.10 d | 0.22 ± 0.01 cd |
| 0.10 | 5.88 ± 0.23 c | 0.87 ± 0.03 cd | 0.23 ± 0.03 cd |
| 0.15 | 7.13 ± 0.12 a | 1.43 ± 0.07 a | 0.38 ± 0.03 a |
| 0.20 | 6.71 ± 0.17 b | 1.25 ± 0.09 b | 0.32 ± 0.03 b |
| 0.25 | 5.84 ± 0.08 c | 0.98 ± 0.13 c | 0.26 ± 0.02 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zheng, Z.; Han, X.; Zhang, C.; Li, H.; Wu, M. Chitosan Soaking Improves Seed Germination of Platycodon Grandiflorus and Enhances Its Growth, Photosynthesis, Resistance, Yield, and Quality. Horticulturae 2022, 8, 943. https://doi.org/10.3390/horticulturae8100943
Liu H, Zheng Z, Han X, Zhang C, Li H, Wu M. Chitosan Soaking Improves Seed Germination of Platycodon Grandiflorus and Enhances Its Growth, Photosynthesis, Resistance, Yield, and Quality. Horticulturae. 2022; 8(10):943. https://doi.org/10.3390/horticulturae8100943
Chicago/Turabian StyleLiu, Hai, Zhihong Zheng, Xue Han, Cheng Zhang, Haitao Li, and Mingkai Wu. 2022. "Chitosan Soaking Improves Seed Germination of Platycodon Grandiflorus and Enhances Its Growth, Photosynthesis, Resistance, Yield, and Quality" Horticulturae 8, no. 10: 943. https://doi.org/10.3390/horticulturae8100943
APA StyleLiu, H., Zheng, Z., Han, X., Zhang, C., Li, H., & Wu, M. (2022). Chitosan Soaking Improves Seed Germination of Platycodon Grandiflorus and Enhances Its Growth, Photosynthesis, Resistance, Yield, and Quality. Horticulturae, 8(10), 943. https://doi.org/10.3390/horticulturae8100943







