Biofortified Rocket (Eruca sativa) with Selenium by Using the Nutrient Film Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Characterization of the Area
2.2. Treatments and Experimental Design
2.3. Installation and Conduction of the Experiment
2.4. Characteristics Evaluated
2.4.1. Biometric Analyses
2.4.2. Nutrient and Se Content
2.4.3. Carotenoids and Chlorophyll Content
2.4.4. Lipid Peroxidation
2.4.5. Hydrogen Peroxide (H2O2)
2.4.6. Stress Signaling Enzymes
2.4.7. Superoxide Dismutase (SOD, E.C. 1.15.1.1)
2.4.8. Catalase (CAT, E.C. 1.11.1.6)
2.4.9. Ascorbate Peroxidase (APX, E.C. 1.11.1.11)
2.5. Statistical Analysis
3. Results
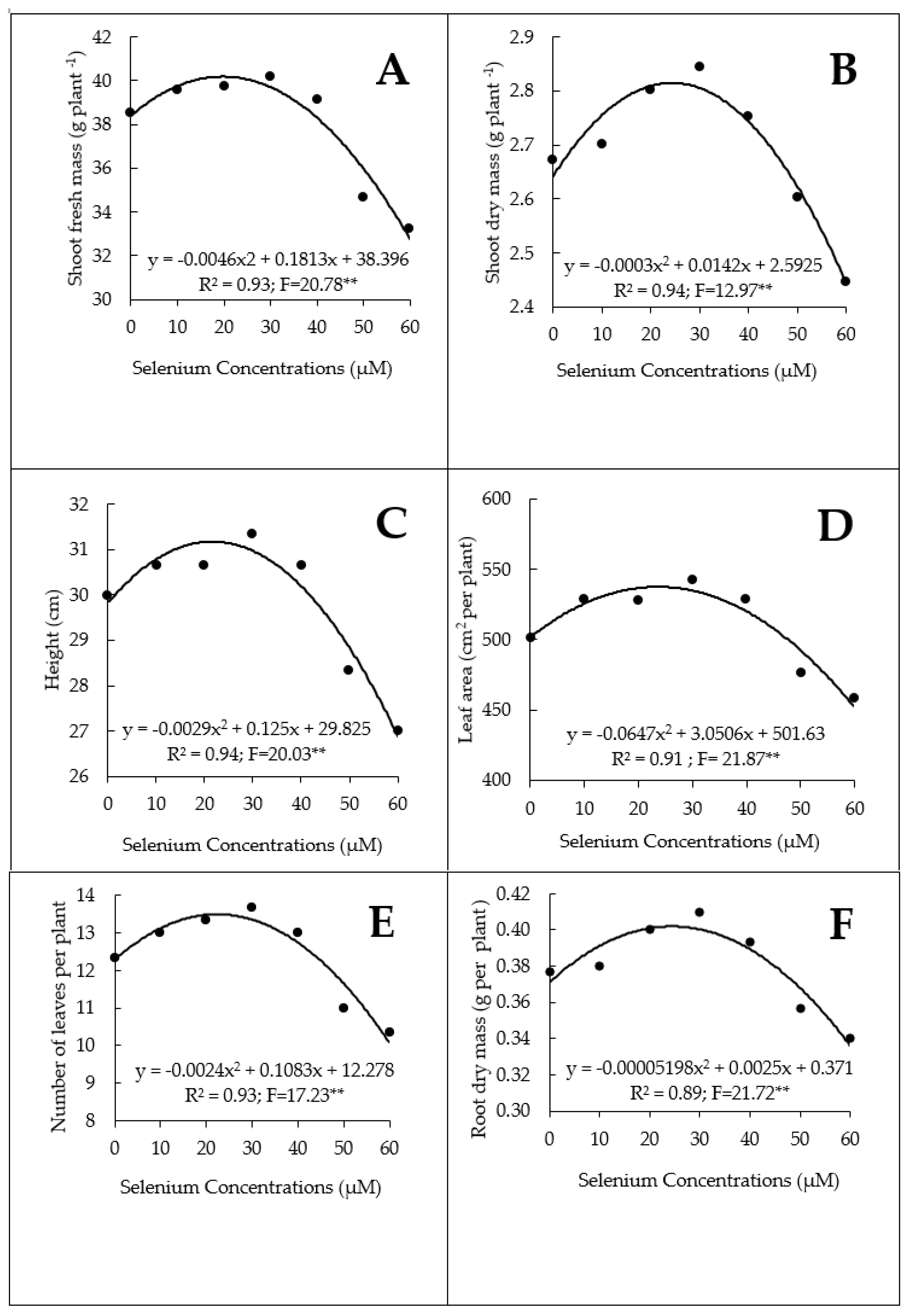
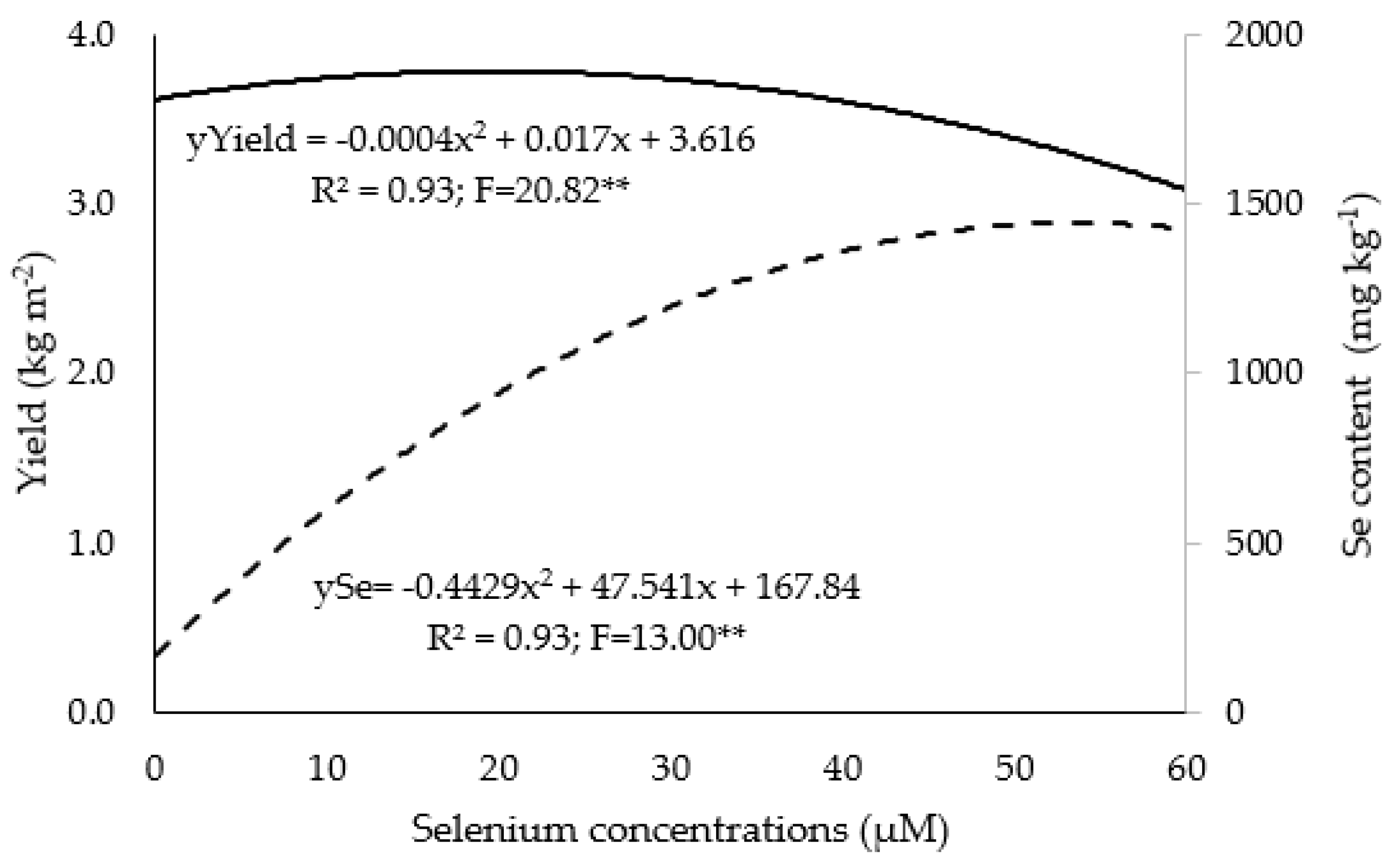
3.1. Growth, Yield, and Nutrient and Se Contents
3.2. Physiological and Enzymatic Characteristics
4. Discussion
4.1. Growth
4.2. Se biofortification in Hydroponic Systems
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schiavon, M.; Nardi, S.; Dalla Vecchia, F.; Ertani, A. Selenium biofortification in the 21st century: Status and challenges for healthy human nutrition. Plant Soil 2020, 453, 245–270. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Pan, P.; Feng, Y.; Kan, Z.; Li, Z.; Wei, F. Environmental water chemistry and possible correlation with Kaschin–Beck Disease (KBD) in northwestern Sichuan, China. Environ. Int. 2017, 99, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Floré, R. Selenium in human health and disease: An overview. In Molecular and Integrative Toxicology; Michalke, B., Ed.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Natasha, S.M.; Niazi, N.K.; Khalid, S.; Murtaza, B.; Bibi, I. A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ. Pollut. 2018, 234, 915–934. [Google Scholar] [CrossRef] [PubMed]
- Newman, R.; Waterland, N.; Moon, Y.; Tou, J.C. Selenium biofortification of agricultural crops and effects on plant nutrients and bioactive compounds important for human health and disease prevention—A review. Plant Food Hum. Nutr. 2019, 74, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, J.; Kronzucker, H.J.; Shi, W. Selenium biofortification and interaction with other elements in plants: A review. Front. Plant Sci. 2020, 11, 586421. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.W.; Pilon-Smits, E.A.; Schiavon, M. Mechanisms of selenium hyperaccumulation in plants: A survey of molecular, biochemical and ecological cues. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2343–2353. [Google Scholar] [CrossRef] [PubMed]
- Fordyce, F.M. Selenium deficiency and toxicity in the environment. In Essentials of Medical Geology; Selinus, B., Alloway, J.A., Centeno, R.B., Finkelman, R., Fuge, U., Lindh, P., Smedley, Eds.; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Jones, D.L.; Winkel, L.H.E. Global Predictions of Selenium Distributions in Soils. In Global Advances in Selenium Research from Theory to Application; Banuelos, G.S., Lin, Z.Q., Moraes, M.F., Guilherme, L.R.G., Reis, A.R., Eds.; Taylor & Francis Group: London, UK, 2016; pp. 13–14. [Google Scholar]
- White, P.J.; Broadley, M.R. Biofortification of Crops with Seven Mineral Elements often Lacking Inhuman Diets—Iron, Zinc, Copper, Calcium, Magnesium, Selenium and Iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Ertani, A.; Pilon-Smits, E.A.H.; Fabrega-Prats, M.; Schiavon, M. Selenium Biofortification Differentially Affects Sulfur Metabolism and Accumulation of Phytochemicals in Two Rocket Species (Eruca sativa Mill. and Diplotaxis tenuifolia) Grown in Hydroponics. Plants 2019, 16, 68. [Google Scholar] [CrossRef]
- Bañuelos, G.S.; Freeman, J.; Arroyo, I. Accumulation and Speciation of Selenium in Biofortified Vegetables Grown under High Boron and Saline Field Conditions. Food Chem. 2020, 10, 100073. [Google Scholar] [CrossRef]
- Foroughbakhch, R.; Rocio, C.C.; Benavides-Mendoza, A.; Rosaura, S.C.L.; Maginot, N.H. Agronomic Biofortification with Selenium in Tomato Crops (Solanum lycopersicon L. Mill). Agriculture 2020, 10, 486. [Google Scholar] [CrossRef]
- Almeida Junior, H.; Carmona, V.V.; Dutra, A.F.; Cecilio Filho, A.B. Growth and Physiological Responses of Cabbage Cultivars Biofortified With Inorganic Selenium Fertilizers. Sci. Hortic. 2022, 302, 111154. [Google Scholar] [CrossRef]
- Zafeiriou, I.; Gasparatos, D.; Ioannou, D.; Kalderis, D.; Massas, I. Selenium Biofortification of Lettuce Plants (Lactuca sativa L.) as Affected by Se Species, Se Rate, and a Biochar Co-Application in a Calcareous Soil. Agronomy 2022, 12, 131. [Google Scholar] [CrossRef]
- Zafeiriou, I.; Gasparatos, D.; Ioannou, D.; Massas, I. Selenium Uptake by Rocket Plants (Eruca sativa) Grown in a Calcareous Soil as Affected by Se Species, Se Rate and a Seaweed Extract-based Biostimulant Application. Crop Pasture Sci. 2022, 73. [Google Scholar] [CrossRef]
- Chatterjee, R.; Chowdhury, R.S.; Dukpa, P.; Thirumdasu, R.K. Iron Fortification in Leafy Vegetables: Present Status and Future Possibilities. Innovare J. Agric. Sci. 2016, 4, 1–3. [Google Scholar]
- Malagoli, M.; Schiavon, M.; Dall’Acqua, S.; Pilon-Smits, E.A.H. Effects of Selenium Biofortification on Crop Nutritional Quality. Front. Plant. Sci. 2015, 6, 280. [Google Scholar] [CrossRef]
- Sapkota, S.; Sapkota, S.; Liu, Z. Effects of Nutrient Composition and Lettuce Cultivar on Crop Production in Hydroponic Culture. Horticulturae 2019, 5, 72. [Google Scholar] [CrossRef]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Sarkar, S.; Ahmad, Z.; Vemuri, H.; Garai, S.; Mondal, M.; Bhatt, R.; et al. Selenium Biofortification: Roles, Mechanisms, Responses and Prospects. Molecules 2021, 26, 881. [Google Scholar] [CrossRef]
- Hatfield, D.L.; Tsuji, P.A.; Carlson, B.A.; Gladyshev, V.N. Selenium and Selenocysteine: Roles in Cancer, Health, and Development. Trends Biochem. Sci. 2014, 39, 112–120. [Google Scholar] [CrossRef]
- Zenda, T.; Liu, S.; Dong, A.; Duan, H. Revisiting Sulphur—The Once Neglected Nutrient: It’s Roles in Plant Growth, Metabolism, Stress Tolerance and Crop Production. Agriculture 2021, 11, 626. [Google Scholar] [CrossRef]
- Freitas, E.M.; Giovanelli, L.B.; Delazari, F.T.; Santos, M.L.; Pereira, S.B.; Silva, D.J.H. Arugula Production as a function of irrigation depths and potassium fertilization. Rev. Bras. Eng. Agríc. Ambient. 2017, 21, 197–202. [Google Scholar] [CrossRef]
- Traka, M.H. Health benefits of glucosinolates. Adv. Bot. Res. 2016, 80, 247–279. [Google Scholar]
- Wilson, D.W.; Nash, P.; Buttar, H.S.; Griffiths, K.; Singh, R.; De Meester, F.; Horiuchi, R.; Takahashi, T. The Role of Food Antioxidants, Benefits of Functional Foods, and Influence of Feeding Habits on the Health of the Older Person: An overview. Antioxidants 2017, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Ros, G.; van Rotterdam, A.; Bussink, D.; Bindraban, P. Selenium fertilization strategies for bio-fortification of food: An agro-ecosystem approach. Plant Soil 2016, 404, 99–112. [Google Scholar] [CrossRef]
- Sabatino, L.; Ntatsi, G.; Iapichino, G.; D’Anna, F.; De Pasquale, C. Effect of Selenium Enrichment and Type of Application on Yield, Functional Quality and Mineral Composition of Curly Endive Grown in a Hydroponic System. Agronomy 2019, 9, 207. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Nicola, S.; Savvas, D.; Voogt, W. Editorial: Soilless Cultivation Through an Intensive Crop Production Scheme. Management Strategies, Challenges and Future Directions. Front. Plant Sci. 2020, 11, 363. [Google Scholar] [CrossRef]
- Giordano, M.; El-Nakhel, C.; Pannico, A.; Kyriacou, M.C.; Stazi, S.R.; De Pascale, S.; Rouphael, Y. Iron Biofortification of Red and Green Pigmented Lettuce in Closed Soilless Cultivation Impacts Crop Performance and Modulates Mineral and Bioactive Composition. Agronomy 2019, 9, 290. [Google Scholar] [CrossRef]
- Sambo, P.; Nicoletto, C.; Giro, A.; Pii, Y.; Valentinuzzi, F.; Mimmo, T.; Lugli, P.; Orzes, G.; Mazzetto, F.; Astolfi, S.; et al. Hydroponic Solutions for Soilless Production Systems: Issues and Opportunities in a Smart Agriculture Perspective. Front. Plant Sci. 2019, 10, 923. [Google Scholar] [CrossRef]
- Frost, R.R.; Griffin, R.A. Effect of pH on Adsorption of Arsenic and Selenium from Landfill Leachate by Clay Minerals. Soil Sci. Soc. Am. J. 1977, 41, 53–57. [Google Scholar] [CrossRef]
- Eich-Greatorex, S.; Sogn, T.A.; Øgaard, A.F.; Aasen, I. Plant Availability of Inorganic and Organic Selenium Fertiliser as Influenced by Soil Organic Matter Content and pH. Nutr. Cycl. Agroecosyst. 2007, 79, 221–231. [Google Scholar] [CrossRef]
- Winkel, L.; Vriens, B.; Jones, G.; Schneider, L.; Pilon-Smits, E.; Bañuelos, G. Selenium Cycling across Soil-Plant-Atmosphere Interfaces: A Critical Review. Nutrients 2015, 7, 4199. [Google Scholar] [CrossRef]
- Alves, L.R.; Rossatto, D.R.; Rossi, M.L.; Martinelli, A.P.; Gratão, P.L. Selenium improves photosynthesis and induces ultrastructural changes but does not alleviate cadmium-stress damages in tomato plants. Protoplasma 2020, 257, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, X.; Wassie, M.; Chen, L. Selenium supplementation alleviates cadmiuminduced damages in tall fescue through modulating antioxidant system, photosynthesis efficiency, and gene expression. Environ. Sci. Poll. Res. 2020, 1, 1–13. [Google Scholar] [CrossRef]
- Eiche, E.; Bardelli, F.; Nothstein, A.K.; Charlet, L.; Göttlicher, J.; Steininger, R.; Dhillon, K.S.; Sadana, U.S. Selenium distribution and speciation in plant parts of wheat (Triticum aestivum) and Indian mustard (Brassica juncea) from a seleniferous area of Punjab . Sci. Total Environ. 2015, 505, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Raza, A.; Hawrylak-Nowak, B.; Matraszek-Gawron, R.; Mahmud, J.A.; Nahar, K.; Fujita, M. Selenium in plants: Boon or bane? Environ. Exp. Bot. 2020, 178, 104170. [Google Scholar] [CrossRef]
- Supriatin, S.; Weng, L.; Comans, R.N. Selenium speciation and extractability in Dutch agricultural soils. Sci. Total Environ. 2015, 532, 368–382. [Google Scholar] [CrossRef]
- Sarwar, N.; Akhtar, M.; Kamran, M.A.; Imran, M.; Riaz, M.A.; Kamran, K.; Hussain, S. Selenium biofortification in food crops: Key mechanisms and future perspectives. J. Food Compos. Anal. 2020, 93, 103615. [Google Scholar] [CrossRef]
- Versini, A.; Tullo, P.; Aubry, E.; Bueno, M.; Thiry, Y.; Pannier, F.; Castrec-Rouelle, M. Influence of Se Concentrations and Species in Hydroponic Cultures on Se Uptake, Translocation and Assimilation in non-Accumulator Ryegrass. Plant Physiol. Biochem. 2016, 108, 372–380. [Google Scholar] [CrossRef]
- Furlani, P.R. Instrução Para o Cultivo de Hortaliça de Folha Pela Técnica de Hidroponia—NFT; Instituto Agronômico: Campinas, Brazil, 1998.
- Miyazawa, M.; Pavan, M.A.; Muraoka, T.; Carmo, C.A.F.S.; Melo, W.J. Análises químicas de tecido vegetal. In Manual de Análises Químicas de Solos, Plantas e Fertilizantes; Silva, F.C., Ed.; EMBRAPA: Brasília, Brazil, 2009; pp. 191–233. [Google Scholar]
- United States Environmental Protection Agency. Method 3051A. 1998. Available online: https://www.epa.gov/sites/production/files/2015-12/documents/3051a.pdf (accessed on 20 January 2018).
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Azevedo, R.A.; Alas, R.M.; Smith, R.J.; Lea, P.J. Response of antioxidant enzymes to transfer from elevated carbon dioxide to air and ozone fumigation, in leaves and roots of wild-type and a catalase-deficient mutant of barley. Physiol. Plant. 1998, 104, 280–292. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Gratão, P.L.; Monteiro, C.C.; Carvalho, R.F.; Tezotto, T.; Piotto, F.A.; Peres, L.E.P.; Azevedo, R.A. Biochemical dissection of diageotropica and Never ripe tomato mutants to Cd-stressful conditions. Plant Physiol. Biochem. 2012, 56, 79–96. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Maldonado Júnior, W. Experimentação Agronômica & AgroEstat: Sistemas para Análises Estatísticas de Ensaios Agronômicos; Gráfica Multipress: Jaboticabal, Brazil, 2015. [Google Scholar]
- Trani, P.E.; Purquério, L.F.V.; Figueiredo, G.J.B.; Tivelli, S.W.; Blat, S.F. Calagem e Adubação da Alface, Almeirão, Agrião D’água, Chicória, Coentro, Espinafre e Rúcula; Instituto Agronômico de Campinas: Campinas, Brazil, 2014.
- Correia, B.; Hancock, R.; Amaral, J.; Gómez-Cadenas, A.; Valledor, L.; Pinto, G. Combined drought and heat activities protective responses in Eucalyptus globulus that are not activated when subjected to drought and heat stress alone. Front. Plant Sci. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Mitller, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Owusu-Sekyere, A.; Kontturi, J.; Hajiboland, R.; Rahmat, S.; Aliasgharzad, N.; Hartikainen, H.; Seppanen, M.M. Influence of selenium (Se) on carbohydrate metabolism, nodulation and growth in alfalfa (Medicago sativa L.). Plant Soil 2013, 373, 541–552. [Google Scholar] [CrossRef]
- Diao, M.; Ma, L.; Wang, J.; Cui, J.; Fu, A.; Liu, H. Selenium promotes the growth and photosynthesis of tomato seedlings under salt stress by enhancing chloroplast antioxidant defense system. J. Plant Growth Regul. 2014, 33, 671–682. [Google Scholar] [CrossRef]
- Mitller, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.J.; Blasco, B.; Cervilla, L.M.; Rosales, M.A.; Sanches-Rodriguez, E.; Romero, L.; Ruiz, J.M. Production and detoxification of H2O2 in lettuce plants exposed to selenium. Ann. Appl. Biol. 2008, 154, 107–116. [Google Scholar] [CrossRef]
- Ríos, J.J.; Blasco, B.; Leyva, R.; Sanchez-Rodrigues, E.; Rubio-Wilhelmi, M.M.; Romero, L.; Ruiz, J.M. Nutritional balance changes in lettuce plant grown under different doses and forms of selenium. J. Plant Nutr. 2013, 36, 1344–1354. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B.; Matraszek, R.; Pogorzelec, M. The dual effects of two inorganic selenium forms on the growth, selected physiological parameters and macronutrients accumulation in cucumber plants. Acta Physiol. Plant. 2015, 37, 37–41. [Google Scholar] [CrossRef]
- White, P.J. Selenium accumulation by plants (Review). Ann. Bot. 2016, 117, 213–235. [Google Scholar] [CrossRef]
- Boldrin, P.F.; Faquin, V.; Ramos, S.J.; Guilherme, L.R.G.; Bastos, C.E.A.; Carvalho, G.S.; Costa, E.T.S. Selenato e selenito sobre produção e biofortificação agronômica com selênio em arroz. Pesq. Agropec. Bras. 2012, 47, 831–837. [Google Scholar] [CrossRef][Green Version]
- Santos, N.; Teixeira, N.; Valim, J.; Almeida, E.; Oliveira, M.; Campos, W. Sulfur fertilization increases defense metabolites and nitrogen but decreases plant resistance against a host-specific insect. Bull. Entomol. Res. 2017, 108, 479–486. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Aminoácidos, Peptídeos e proteínas. In Princípios de Bioquímica de Lehninger; Dalmaz, C., Termignoni, C., Nelson, D.L., Cox, M.M., Eds.; Artmed: Porto Alegre, Brazil, 2022; pp. 75–114. [Google Scholar]
- Soares, M.M.; Bardiviesso, D.M.; Barbosa, W.F.S.; Barcelos, M.N. Adubação de cobertura com enxofre na cultura da rúcula. Rev. Agric. Neotrop. 2017, 4, 49–52. [Google Scholar] [CrossRef]
- Jákli, B.; Tavakol, E.; Tränkner, M.; Senbayram, M.; Dittert, K. Quantitative limitations to photosynthesis in K deficient sunflower and their implications on water-use efficiency. J. Plant. Physiol. 2017, 209, 20–30. [Google Scholar] [CrossRef]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef]
- Reilly, C. Selenium in Food and Health; Blackie Academic and Professional: London, UK, 1996. [Google Scholar]
- Kipp, A.P.; Strohm, D.; Brigelius-Flohé, R.; Schomburg, L.; Bechthold, A.; Leschik-Bonnet, E.; Heseker, H. Revised reference values for selenium intake. J. Trace Elem. Med. Biol. 2015, 32, 195–199. [Google Scholar] [CrossRef]

| N | P | K | Ca | Mg | S | |
| g kg−1 | ||||||
| Observ. | 42.9–46.0 | 4.5–5.0 | 77.0–111.0 | 24.6–26.7 | 6.2–6.8 | 7.4–8.5 |
| Adeq. | 40.0–50.0 | 3.0–8.0 | 30.0–60.0 | 20.0–40.0 | 4.0–7.0 | 4.0–9.0 |
| B | Cu | Fe | Mn | Zn | ||
| mg kg−1 | ||||||
| Observ. | 74.4–88.7 | 3.7–5.0 | 400.0–466.7 | 166.7–216.7 | 300 | |
| Adeq. | 25.0–60.0 | 5.0–20.0 | 100.0–300.0 | 50.0–160.0 | 45.0–80.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, C.S.; Nascimento, C.S.; Lopes, G.; Carrasco, G.; Gratão, P.L.; Cecílio Filho, A.B. Biofortified Rocket (Eruca sativa) with Selenium by Using the Nutrient Film Technique. Horticulturae 2022, 8, 1088. https://doi.org/10.3390/horticulturae8111088
Nascimento CS, Nascimento CS, Lopes G, Carrasco G, Gratão PL, Cecílio Filho AB. Biofortified Rocket (Eruca sativa) with Selenium by Using the Nutrient Film Technique. Horticulturae. 2022; 8(11):1088. https://doi.org/10.3390/horticulturae8111088
Chicago/Turabian StyleNascimento, Carolina Seno, Camila Seno Nascimento, Guilherme Lopes, Gilda Carrasco, Priscila Lupino Gratão, and Arthur Bernardes Cecílio Filho. 2022. "Biofortified Rocket (Eruca sativa) with Selenium by Using the Nutrient Film Technique" Horticulturae 8, no. 11: 1088. https://doi.org/10.3390/horticulturae8111088
APA StyleNascimento, C. S., Nascimento, C. S., Lopes, G., Carrasco, G., Gratão, P. L., & Cecílio Filho, A. B. (2022). Biofortified Rocket (Eruca sativa) with Selenium by Using the Nutrient Film Technique. Horticulturae, 8(11), 1088. https://doi.org/10.3390/horticulturae8111088






