The Contribution of Volatile Organic Compounds (VOCs) Emitted by Petals and Pollen to the Scent of Garden Roses
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. GC-MS Analysis
2.3. Calculation of the Percentage of the Relative Peak Area
2.4. Data Analysis
3. Results
3.1. Identification of VOCs in Garden Rose Petals
3.2. Identification of VOCs in Rose Pollen
3.3. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hirata, H.; Ohnishi, T.; Watanabe, N. Biosynthesis of floral scent 2-phenylethanol in rose flower. Biosci. Biotechnol. Biochem. 2016, 80, 1865–1873. [Google Scholar] [CrossRef]
- Negre-Zakharov, F.; Long, M.C.; Dudareva, N. Floral Scents and Fruit Aromas Inspired by Nature. In Plant-Derived Natural Products; Osbourn, A., Lanzonti, V., Eds.; Springer: New York, NY, USA, 2009; pp. 405–431. [Google Scholar]
- Loreto, F.; Schnitzler, J.P. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef]
- Bertoli, A.; Fambrini, M.; DOveri, S.; Leonardi, M.; Pugliesi, C.; Pistelli, L. Pollen aroma fingerprint of two sunflower (Helianthus annuus L.) genotypes characterized by different pollen colors. Chem. Biodivers. 2011, 8, 1766–1775. [Google Scholar] [CrossRef]
- Niederbacher, B.; Winkler, J.B.; Schnitzler, J.P. Volatile organic compounds as non-invasive markers for plant phenotyping. J. Exp. Bot. 2015, 66, 5403–5416. [Google Scholar] [CrossRef]
- Caser, M.; D’Angiolillo, F.; Chitarra, W.; Lovisolo, C.; Ruffoni, B.; Pistelli, L.; Pistelli, L.; Scariot, V. Water deficit regimes trigger changes in valuable physiological and phytochemical parameters in Helichrysum petiolare Hilliard & BL Burtt. Ind. Crop. Prod. 2016, 83, 680–692. [Google Scholar]
- Caser, M.; D’Angiolillo, F.; Chitarra, W.; Lovisolo, C.; Ruffoni, B.; Pistelli, L.; Pistelli, L.; Scariot, V. Ecophysiological and phytochemical response of Salvia sinaloensis Fern. To drought stress. Plant Growth Regul. 2018, 84, 383–394. [Google Scholar] [CrossRef]
- Caser, M.; Chitarra, W.; D’Angiolillo, F.; Perrone, I.; Demasi, S.; Lovisolo, C.; Pistelli, L.; Pistelli, L.; Scariot, V. Drought stress adaptation modulates plant secondary metabolite production in Salvia dolomitica Codd. Ind. Crop. Prod. 2019, 129, 85–96. [Google Scholar] [CrossRef]
- Najar, B.; Demasi, S.; Caser, M.; Gaino, W.; Cioni, P.L.; Pistelli, L.; Scariot, V. Cultivation substrate composition influences morphology, volatilome and essential oil of Lavandula angustifolia Mill. Agronomy 2019, 9, 411. [Google Scholar] [CrossRef]
- Demasi, S.; Caser, M.; Lonati, M.; Cioni, P.L.; Pistelli, L.; Najar, B.; Scariot, V. Latitude and altitude influence secondary metabolite production in peripheral alpine populations of the Mediterranean species Lavandula angustifolia Mill. Front. Plant Sci. 2018, 9, 983. [Google Scholar] [CrossRef]
- Tambe, E.; Gotmare, S.R. Study of varitation and identification of chemical composition in Rosa species oil collected from different countries. J. Appl. Chem. 2016, 9, 11–18. [Google Scholar]
- Ibrahim, M.; Agarwal, M.; Hardy, G.E.S.J.; Ren, Y. Optimized methods to analyze rose plant volatile organic compounds by HS-SPME-GC-FID/MSD. J. Biosci. Med. 2017, 5, 13–31. [Google Scholar] [CrossRef]
- Gudin, S. Rose: Genetics and breeding. Plant Breed. Rev. 2000, 17, 159–189. [Google Scholar]
- Khosh-Khui, M. Biotechnology of scented roses: A review. Int. J. Hortic. Sci. Technol. 2014, 1, 1–20. [Google Scholar]
- Martínez, M.C.; Santiago, J.L.; Boso, S.; Gago, P.; Álvarez-Acero, I.; De Vega, M.E.; Martínez-Bartolomé, M.; Álvarez-Nogal, R.; Molíst, P.; Caser, M.; et al. Narcea—An unknown, ancient cultivated rose variety from northern Spain. Hortic. Sci. 2020, 7, 44. [Google Scholar] [CrossRef]
- Lijun, Z.; Chao, Y.; Bixuan, C.; Huihua, W.; Le, L.; Huitang, P.; Qixiang, Z. Volatile compound analysis and aroma evaluation of tea-scented roses in China. Ind. Crop. Prod. 2020, 155, 112735. [Google Scholar]
- Ohloff, G. Importance of minor components in flavors and fragrance. Perfum. Flavorist 1978, 3, 11–22. [Google Scholar]
- Scalliet, G.; Piola, F.; Douady, C.J.; Réty, S.; Raymond, O.; Baudino, S.; Bordji, K.; Bendahmane, M.; Dumas, C.; Cock, J.M.; et al. Scent evolution in Chinese roses. Proc. Natl. Acad. Sci. USA 2008, 105, 5927–5932. [Google Scholar] [CrossRef]
- Tholl, D.; Gershenzon, J. The flowering of a new scent pathway in rose. Science 2015, 349, 28–29. [Google Scholar] [CrossRef]
- Magnard, J.L.; Roccia, A.; Caissard, J.; Vergne, P.; Sun, P.; Hecquet, R.; Dubois, A.; Oyant, L.H.; Jullien, F.; Nicolè, F.; et al. Biosynthesis of monoterpene scent compounds in roses. Science 2015, 349, 81–83. [Google Scholar] [CrossRef]
- Thammarat, P.; Kulsing, C.; Wongravee, K.; Leepipatpiboon, N.; Nhujak, T. Identification of Volatile Compounds and Selection of Discriminant Markers for Elephant Dung Coffee Using Static Headspace Gas Chromatography—Mass Spectrometry and Chemometrics. Molecules 2018, 23, 1910. [Google Scholar] [CrossRef]
- Cavalli, J.F.; Fernandez, X.; Lizzani-Cuvelier, L.; Loiseau, A.M. Comparison of Static Headspace, Headspace Solid Phase Microextraction, Headspace Sorptive Extraction, and Direct Thermal Desorption Techniques on Chemical Composition of French Olive Oils. J. Agric. Food Chem. 2003, 51, 7709–7716. [Google Scholar] [CrossRef]
- Zini, C.A.; Augusto, F.; Christensen, E.; Caramão, B.; Pawliszyn, J. SPME Applied to the Study of Volatile Organic Compounds Emitted by Three Species of Eucalyptus In Situ. J. Agric. Food Chem. 2002, 50, 7199–7205. [Google Scholar] [CrossRef]
- Helsper, J.; Davies, J.; Bouwmeester, H.; Krol, A.F.; van Kampen, M.H. Circadian rhythmicity in emission of volatile compounds by flowers of Rosa hybrida L. cv. Honesty. Planta 1998, 207, 88–95. [Google Scholar] [CrossRef]
- Feng, Y.; Cheng, X.; Lu, Y.; Wang, H.; Chen, D.; Luo, C.; Liu, H.; Gao, S.; Lei, T.; Huang, C.; et al. Gas chromatography-mass spectrometry analysis of floral fragrance-related compounds in scented rose (Rosa hybrida) varieties and a subsequent evaluation on the basis of the analytical hierarchy process. Plant Physiol. Biochem. 2022, 185, 368–377. [Google Scholar] [CrossRef]
- Karami, A.; Khosh-Khui, M.; Salehi, H.; Saharkhiz, M.J.; Rowshan, V. Headspace analysis of floral scent from two distinct genotypes of Iranian damask rose (Rosa damascena Mill.). J. Essent. Oil Bear. Plant. 2013, 16, 489. [Google Scholar] [CrossRef]
- Samira, J.; Akbar, K. Seasonal Variation in Floral Scent of Persian Musk Rose (Rosa Moschata Hermm.). J. Med. Plant. Prod. 2015, 4, 243–247. [Google Scholar]
- Dobson, H.E.M.; Bergström, G.; Growth, I. Differences in fragrance chemistry between flower parts of Rosa rugosa. Isr. J. Bot. 1990, 39, 143–156. [Google Scholar]
- Dobson, H.E.M.; Danielson, E.M.; van Wesep, I.D. Pollen odor chemicals as modulators of bumble bee foraging on Rosa rugosa Thunb. (Rosaceae). Plant Species Biol. 1999, 14, 153–166. [Google Scholar] [CrossRef]
- Dobson, H.E.M.; Bergström, J.; Bergström, G.; Growth, I. Pollen and flower volatiles in two Rosa species. Phytochemistry 1987, 26, 3171–3173. [Google Scholar] [CrossRef]
- Cairns, T. Modern Roses 10; American Rose Society: Shreveport, LA, USA, 1993. [Google Scholar]
- HelpMeFind Roses. Available online: http://www.helpmefind.com/rose/index.php (accessed on 3 May 2022).
- Pubchem. Available online: www.pubchem.com (accessed on 3 May 2022).
- Semmelroch, P.; Grosch, W. Studies on character impact odorants of coffee brews. J. Agric. Food Chem. 1996, 44, 537–543. [Google Scholar] [CrossRef]
- Flavornet and Human Odor Space. Available online: http://flavornet.org/index.html (accessed on 3 May 2022).
- Servili, M.; Selvaggini, R.; Taticchi, A.; Montedoro, G.F. Headspace composition of virgin olive oil evaluated by solid phase microextraction: Relationship with the oil sensory characteristics. In Food Flavorus and Chemistry: Advances of the New Millennium; Spanier, A.M., Shahidi, F., Parliment, T.H., Mussinan, C., Ho, C.T., Contis, E.T., Eds.; The Royal Society of Chemistry Publisher: Cambridge, UK, 2001; pp. 236–247. [Google Scholar]
- Morales, M.T.; Rios, J.J.; Aparicio, R. Changes in the Volatile Composition of Virgin Olive Oil during Oxidation: Flavors and Off-Flavors. J. Agric. Food Chem. 1997, 45, 2666–2673. [Google Scholar] [CrossRef]
- Ramstad, T.; Nestrick, T.J. Purge vessel design in determinations of volatile organic compounds. Anal. Chim. Acta 1980, 121, 345–348. [Google Scholar] [CrossRef]
- Guth, H.; Grosh, W. A comparative study of the potent odorants of different virgin olive oils. Eur. J. Lipid Sci. Technol. 1991, 93, 335–339. [Google Scholar] [CrossRef]
- Ferreira, V.; Aznar, M.; López, R.; Cacho, J. Quantitative gas chromatography-Olfactometry carried out at different dilutions of an extract. Key differences in the odor profiles of four high-quality Spanish aged red wines. J. Agric. Food Chem. 2001, 49, 4818–4824. [Google Scholar] [CrossRef]
- Munafo, J.P.; Didzbalis, J.; Schnell, R.J.; Steinhaus, M. Insights into the key aroma compounds in Mango (Mangifera indica L. ‘Haden’) fruits by stable isotope dilution quantitation and aroma simulation experiments. J. Agric. Food Chem. 2016, 64, 4312–4318. [Google Scholar] [CrossRef]
- Lin, J.; Rouseff, R.L. Characterization of aroma-impact compounds in cold-pressed grapefruit oil using time-intensity GC-olfactometry and GC-MS. Flavour Fragr. J. 2001, 16, 457–463. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corp: Carol Stream, IL, USA, 2007. [Google Scholar]
- Baudino, S.; Hugueney, P.; Caissard, J.-C. Chapter 12: Evolution of scent genes. In Biology of Plant Volatiles; Pichersky, E., Dudareva, N., Eds.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Rusanov, K.; Kovacheva, N.; Rusanova, M.; Atanassov, I. Low variability of flower volatiles of Rosa damascena Mill. plants from rose plantations along the Rose Valley, Bulgaria. Ind. Crop. Prod. 2012, 37, 6–10. [Google Scholar] [CrossRef]
- Shalit, M.; Guterman, I.; Volpin, H.; Bar, E.; Tamari, T.; Menda, N.; Adam, Z.; Zamir, D.; Vainstein, A.; Weiss, D.; et al. Volatile ester formation in roses. Identification of an acetyl-coenzyme A. Geraniol/citronellol acetyltransferase in developing rose petals. Plant Physiol. 2003, 131, 1868–1876. [Google Scholar] [CrossRef]
- Ibrahim, M.; Agarwal, M.; Yang, J.O.; Abdulhussein, M.; Du, X.; Hardy, G.; Ren, Y. Plant growth regulators improve the production of volatile organic compounds in two rose varieties. Plants 2019, 8, 35. [Google Scholar] [CrossRef]
- Ren, J.; Yang, L.; Wang, Y.; Yao, H. Chemical profile of floral scent at different flower developmental stages of rose de rescht (Rosa damascene Mill.) cultivated in Beijing. J. Essent. Oil Bear. Plants 2016, 19, 433–443. [Google Scholar] [CrossRef]
- Baydar, H. Oil-bearing rose (Rosa damascena Mill.) cultivation and rose oil industry in Turkey. Euro Cosmet. 2016, 14, 13–17. [Google Scholar]
- Rusanov, K.; Kovacheva, N.; Rusanova, M.; Atanassov, I. Traditional Rosa damascena flower harvesting practices evaluated through GC/MS metabolite profiling of flower volatiles. Food Chem. 2011, 129, 1851–1859. [Google Scholar] [CrossRef]
- Nedeltcheva-Antonova, D.; Stoicheva, P.; Antonov, L. Chemical profiling of Bulgarian rose absolute (Rosa damascena Mill.) using gas chromatography—Mass spectrometry and trimethylsilyl derivatives. Ind. Crop. Prod. 2017, 108, 36–43. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, R. Influence of harvesting stage and distillation time of Damask rose (Rosa damascena Mill.) flowers of essential oil content and composition in the Western Himalayas. J. Essent. Oil Bear. Plants 2018, 21, 92–102. [Google Scholar] [CrossRef]
- Vainstein, A.; Lewinsohn, E.; Pichersky, E.; Weiss, D. Floral Fragrance. New Inroads into an Old Commodity. Plant Physiol. 2001, 27, 1383–1389. [Google Scholar] [CrossRef]
- van der Niet, T.; Jurgens, A.; Johnson, S.D. Is the timing of scent emission correlated with insect visitor activity and pollination in long-spurred Satyrium species? Plant Biol. 2015, 17, 226–237. [Google Scholar] [CrossRef]
- Stensmyr, M.; Urru, I.; Collu, I.; Celander, M.; Hansson, B.S.; Angioy, A.-M. Rotting smell of dead-horse arum florets. Nature 2002, 420, 625–626. [Google Scholar] [CrossRef]
- Shuttleworth, A.; Johnson, S.D. Floral scents of chafer-pollinated asclepiads and a potential hybrid. S. Afr. J. Bot. 2010, 76, 770–778. [Google Scholar] [CrossRef][Green Version]
- Zito, P.; Dötterl, S.; Sajeva, M. Floral volatiles in a sapromyiophilous plant and their importance in attracting house fly pollinators. J. Chem. Ecol. 2015, 41, 340–349. [Google Scholar] [CrossRef]
- Farré-Armengol, G.; Filella, I.; Llusia, J.; Peñuelas, J. Floral volatile organic compounds: Between attraction and deterrence of visitors under global change. Perspectives in Plant Ecology. Evol. Syst. 2013, 15, 56–67. [Google Scholar]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef] [PubMed]
- Dobson, H.E.M.; Growth, I.; Bergström, G. Pollen advertisement: Chemical contrasts between flower and pollen odors. Am. J. Bot. 1996, 83, 877–885. [Google Scholar] [CrossRef]
- Flamini, G.; Cioni, P.L.; Morelli, I. Differences in the Fragrances of Pollen, Leaves, and Floral Parts of Garland (Chrysanthemum coronarium) and Composition of the Essential Oils from Flowerheads and Leaves. J. Agric. Food Chem. 2003, 51, 2267–2271. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.T.; Tollsten, L. Trends in floral scent chemistry in pollination syndromes: Floral scent composition in moth taxa. Bot. J. Linn. Soc. 1993, 113, 263–284. [Google Scholar] [CrossRef]
- Dobson, H.E.M.; Bergström, G. The ecology and evolution of pollen odors. Plant Syst. Evol. 2000, 222, 63–87. [Google Scholar] [CrossRef]
- Lunau, K. Innate recognition of flowers by bumble bees: Orientation of antennae to visual stamen signals. Can. J. Zool. 1992, 70, 2139–2144. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Cioni, P.L.; Giusti, G.; Pistelli, L.; Flamini, G. Patterns in volatile emission of different aerial parts of caper (Capparis spinosa L.). Chem. Biodivers. 2016, 13, 904–912. [Google Scholar] [CrossRef]
- Al-Jaber, H.I.; Obeidat, S.M.; Al-Qudah, M.A.; Abaza, I.F.; Lahham, J.N.; Zarga, M.H.A.; Afifi, F.U. Patterns in volatile emission of different organs of Inula Viscosa growing wild in Jordan. J. Essent. Oil Bear. Plant. 2017, 20, 24–35. [Google Scholar] [CrossRef]
- Smulders, M.J.M.; Arens, P.; Bourke, P.M.; Debener, T.; Linde, M.; De Riek, J.; Leus, L.; Ruttink, T.; Baudino, S.; Saint-Oyant, L.H.; et al. In the name of the rose: A roadmap for rose research in the genome era. Hortic. Res. 2019, 6, 65. [Google Scholar] [CrossRef]
- Giovannini, A.; Laura, M.; Nesi, B.; Savona, M.; Cardi, T. Genes and genome editing tools for breeding desirable phenotypes in ornamentals. Plant Cell Rep. 2021, 40, 461–478. [Google Scholar] [CrossRef]
- Yan, H.; Baudino, S.; Caissard, J.-C.; Florence, N.; Zhang, H.; Tang, K.; Li, S.; Lu, S. Functional characterization of the eugenol synthase gene (RcEGS1) in rose. Plant Physiol. Biochem. 2018, 129, 21–26. [Google Scholar] [CrossRef] [PubMed]

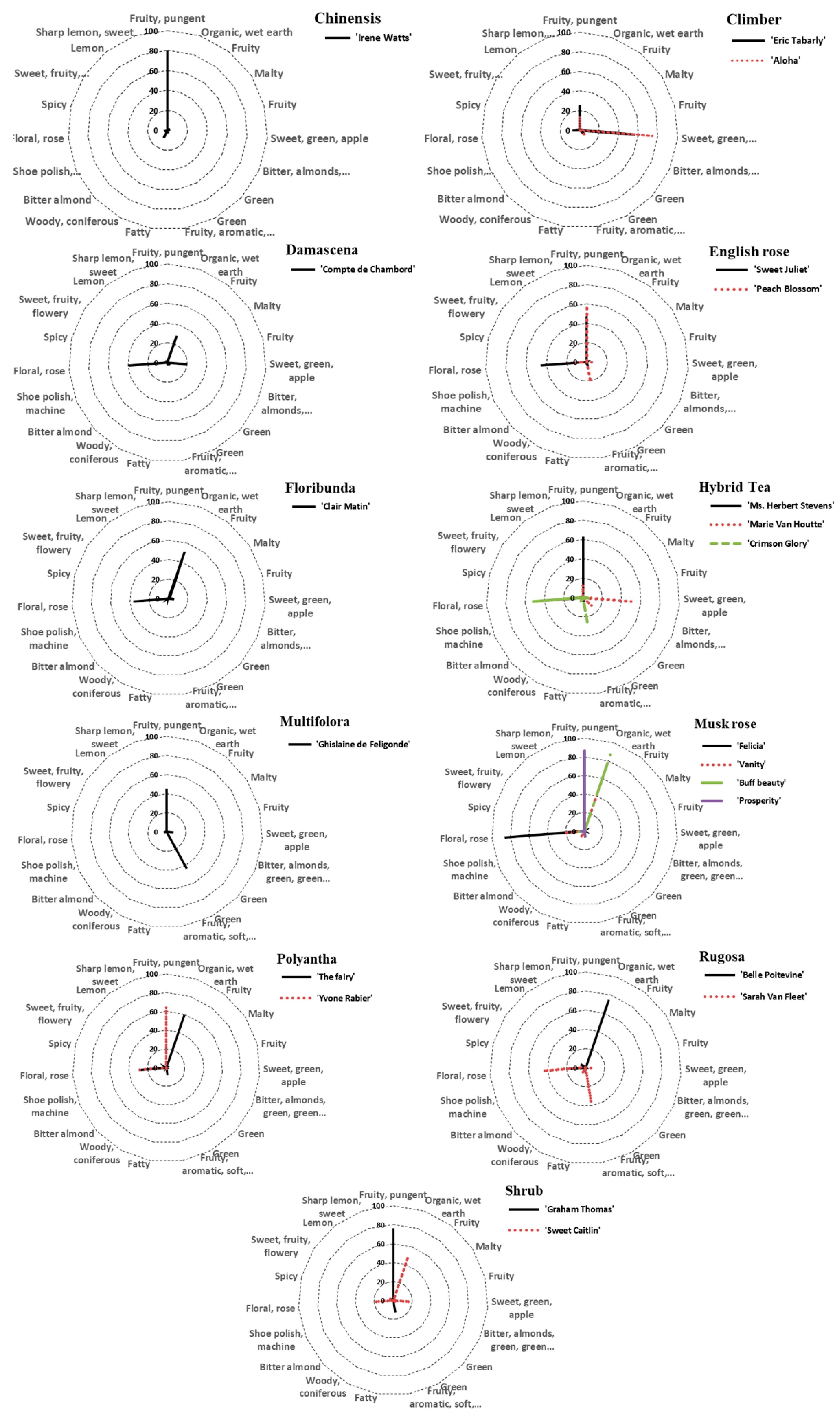
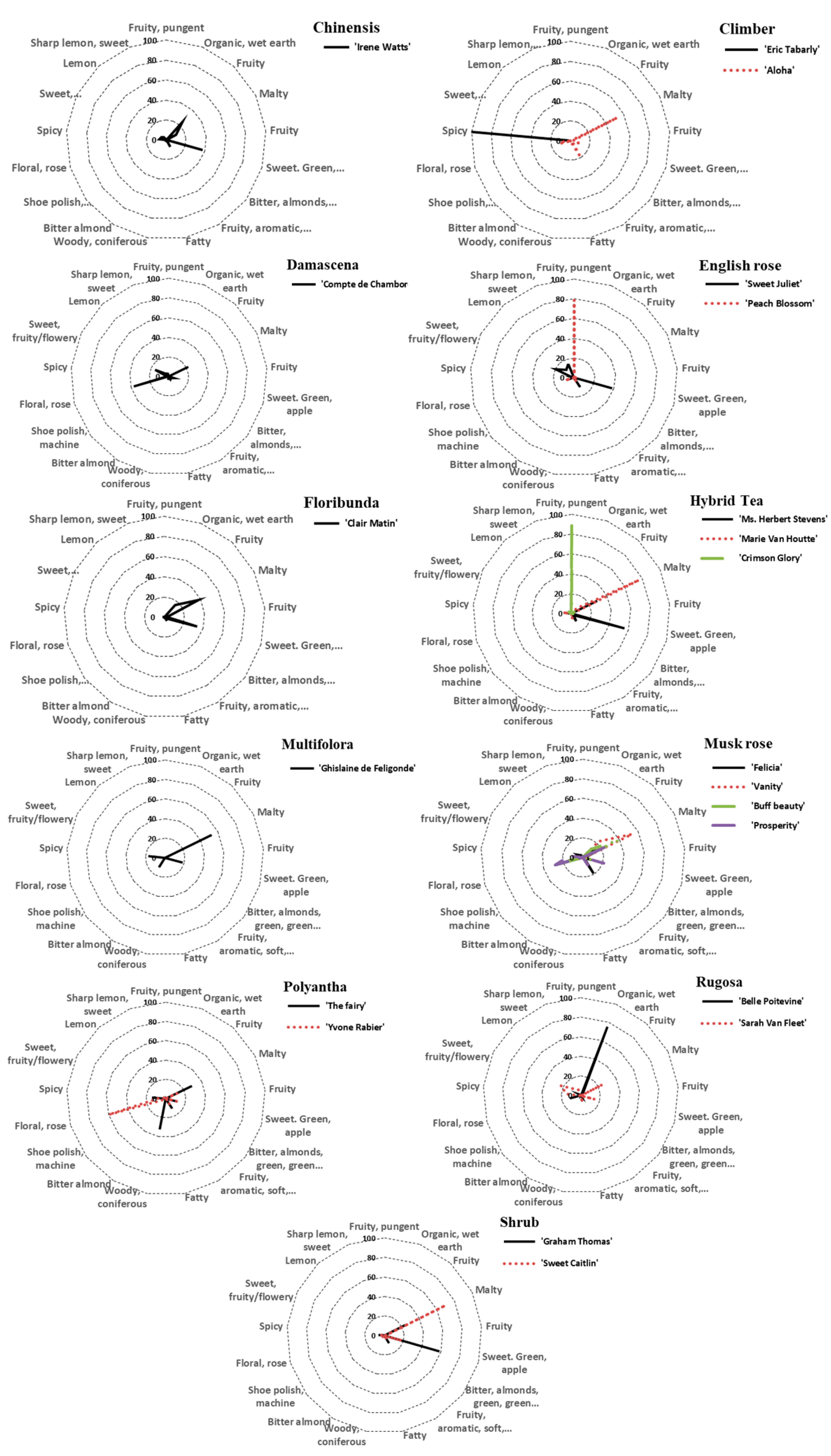
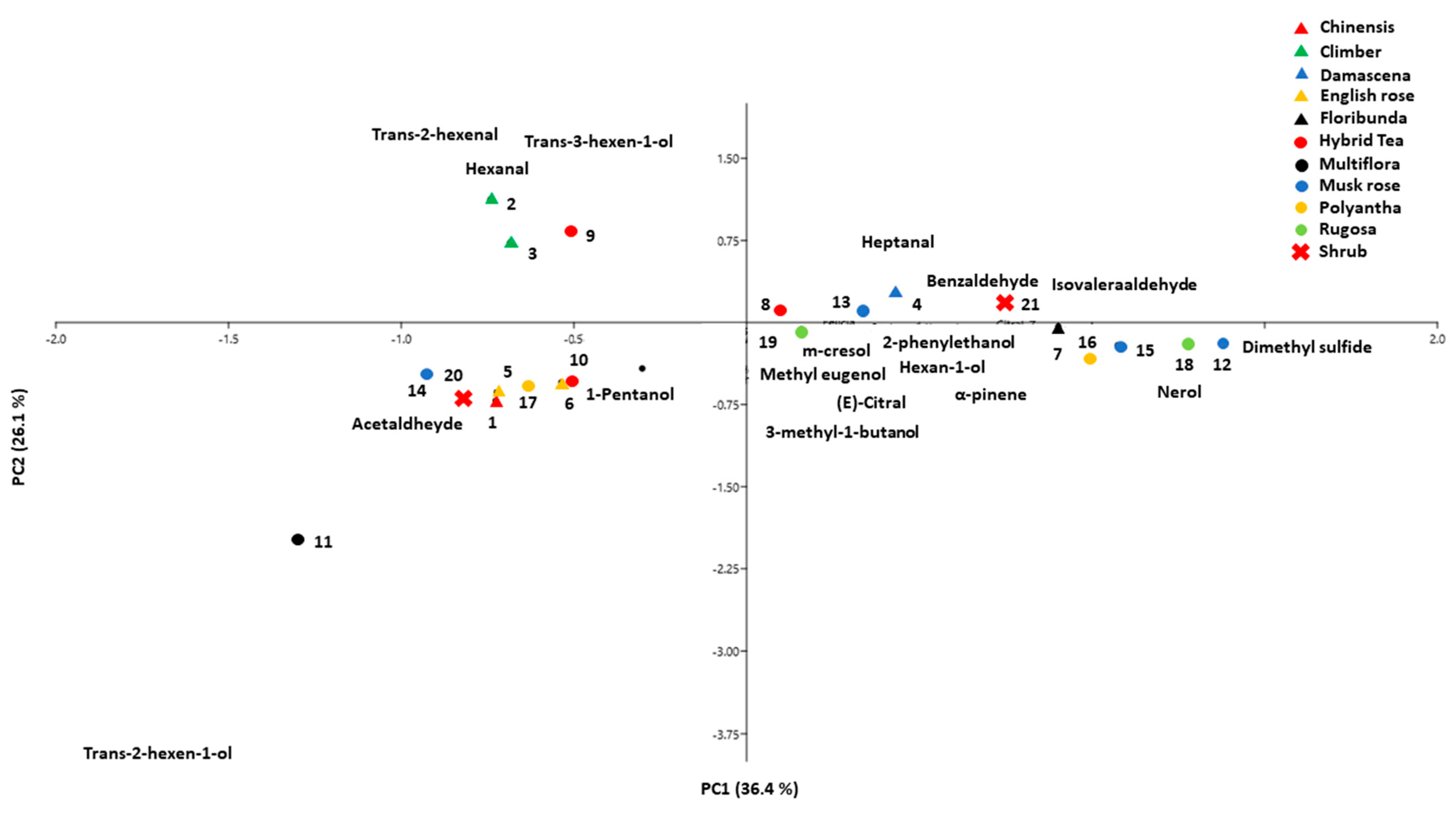
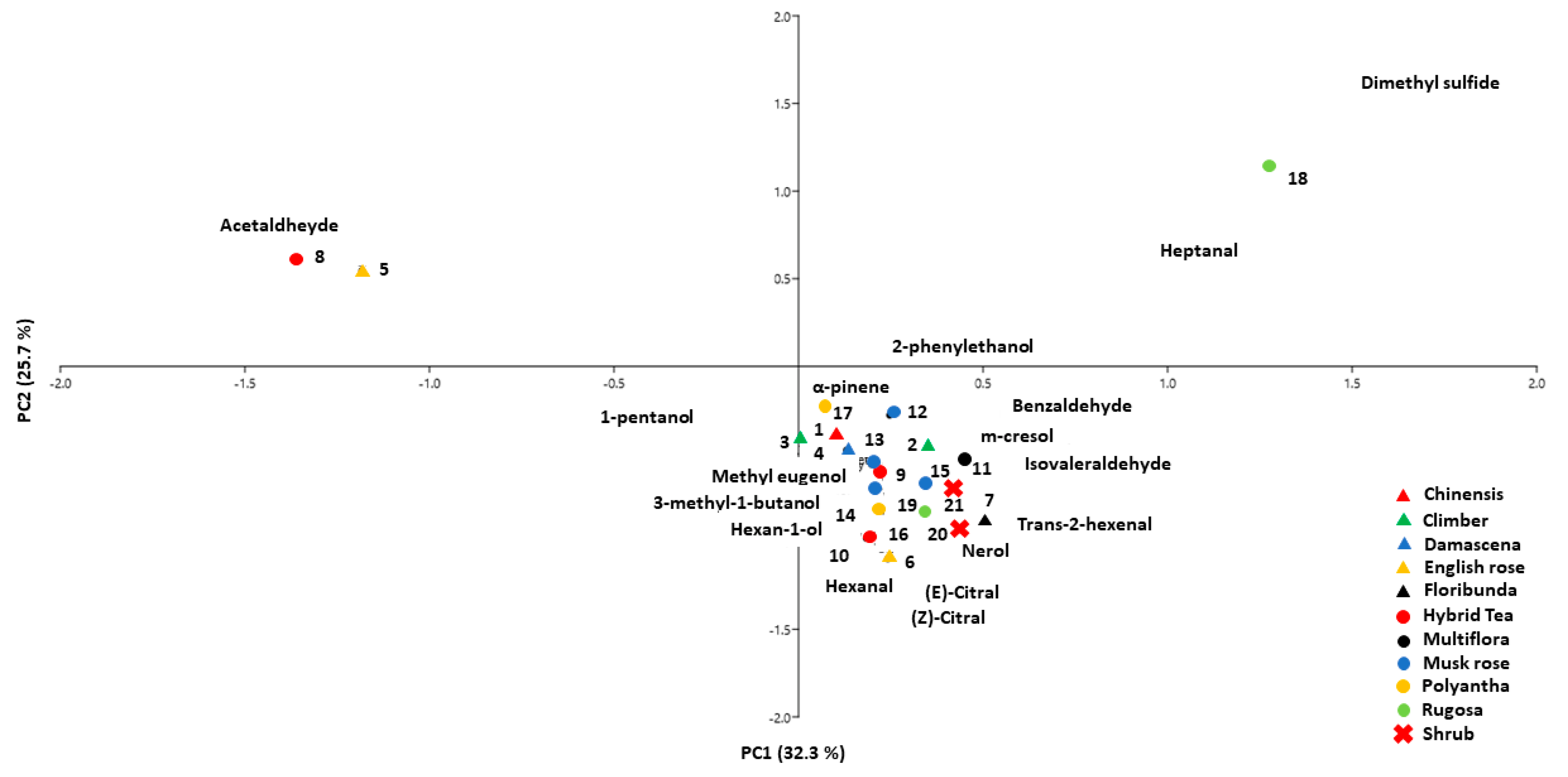
| ID | Cultivar | Class | Fragrance Intensity | Fragrance Type |
|---|---|---|---|---|
| 1 | ‘Irene Watts’ | Chinensis | ++ | Sweet |
| 2 | ‘Aloha’ | Climber | +++ | Apple |
| 3 | ‘Eric Tabarly’ | Climber | ++ | - |
| 4 | ‘Comte de Chambord’ | Damascena | +++ | Damask |
| 5 | ‘Peach Blossom’ | English rose | + | - |
| 6 | ‘Sweet Juliet’ | English rose | +++ | - |
| 7 | ‘Clair Matin’ | Floribunda | ++ | Sweetbriar |
| 8 | ‘Crimson Glory’ | Hybrid Tea | +++ | Clove, damask, rose |
| 9 | ‘Marie Van Houtte’ | Hybrid Tea | ++ | - |
| 10 | ‘Mrs. Herbert Stevens’ | Hybrid Tea | ++ | - |
| 11 | ‘Ghislaine de Feligonde’ | Multiflora | ++ | - |
| 12 | ‘Buff Beauty’ | Musk rose | +++ | Tea rose |
| 13 | ‘Felicia’ | Musk rose | +++ | Sweet |
| 14 | ‘Prosperity’ | Musk rose | ++ | - |
| 15 | ‘Vanity’ | Musk rose | ++ | Musk |
| 16 | ‘The Fairy’ | Polyantha | + | Apple |
| 17 | ‘Yvonne Rabier’ | Polyantha | ++ | - |
| 18 | ‘Belle Poitevine’ | Rugosa | ++ | Centifolia |
| 19 | ‘Sarah Van Fleet’ | Rugosa | +++ | Old rose |
| 20 | ‘Graham Thomas’ | Shrub | +++ | Tea rose |
| 21 | ‘Sweet Caitlin’ | Shrub | +++ | Anise, apricot, citrus, clove, myrrh, violets |
| Constituent | Rt | Ri | Odorant Description |
|---|---|---|---|
| Acetaldehyde | 1.53 | 358 [33] | Fruity, pungent [34] |
| Dimethyl sulfide | 1.63 | 505 [35] | Organic, wet earth [36] |
| Isovaleraldehyde | 2.45 | 632 [33] | Fruity [36] |
| 3-methyl-1-butanol | 3.35 | 736 [35] | Malty [34] |
| 1-pentanol | 3.90 | 772 [33] | Fruity [34] |
| Hexanal | 4.45 | 801 [35] | Sweet, green, apple [36] |
| Trans-2-hexenal | 5.49 | 854 [35] | Bitter, almonds, green, green apple-like, fatty, bitter almond like, cut grass [36] |
| Trans-3-hexen-1-ol | 5.61 | 873 [33] | Green [36] |
| Trans-2-hexen-1-ol | 5.95 | 879 [33] | Green [37] |
| Hexan-1-ol | 6.04 | 881 [33] | Fruity, aromatic, soft, cut grass [38] |
| Heptanal | 6.84 | 903 [35] | Fatty [39] |
| α-pinene | 7.73 | 939 [35] | Woody, coniferous [34] |
| Benzaldehyde | 8.73 | 960 [35] | Bitter almond [39] |
| m-cresol | 11.86 | 1084 [35] | Shoe polish, machine [40] |
| 2-phenylethanol | 14.89 | 1104 [33] | Floral, rose [41] |
| Nerol | 16.84 | 1233 [35] | Sweet, fruity, flower [42] |
| (E)-citral (Neral) | 18.77 | 1247 [35] | Lemon [34] |
| (Z)-citral (Geranial) | 19.21 | 1277 [35] | Sharp lemon, sweet [35] |
| Methyl eugenol | 20.11 | 1407 [35] | Spicy [34] |
| Genotype (ID) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Constituent | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 |
| Air | 85.8 | 53.3 | 60.5 | 68.3 | 80.2 | 80.7 | 76.4 | 93.7 | 55.2 | 83.7 | 77.5 | 78.3 | 91.2 | 80.6 | 67.1 | 78.1 | 77.9 | 65.5 | 95.6 | 84.3 | 74.5 |
| Acetaldheyde | 9.9 | 6.1 | 9.8 | - | 9.2 | 8.1 | - | - | 6.0 | 9.0 | 8.7 | - | - | 15.9 | - | - | 12.9 | - | - | 9.1 | - |
| Dimethyl sulfide | - | - | - | 8.4 | - | - | 11.0 | - | - | - | - | 17.7 | - | - | 16.7 | 11.8 | - | 23.9 | - | - | 10.3 |
| Isovaleraldehyde | - | - | - | 0.4 | - | - | 0.5 | - | 0.2 | - | - | 0.1 | - | - | - | - | - | - | - | - | - |
| 3-methyl-1-butanol | 0.1 | - | - | 0.1 | 0.1 | 0.1 | - | 0.1 | 0.1 | 0.1 | - | 0.4 | 0.1 | 0.1 | 0.2 | 0.2 | 0.3 | 0.1 | - | 0.1 | 0.1 |
| 1-pentanol | 0.3 | - | 0.2 | - | 0.3 | 0.2 | 0.2 | 0.1 | 0.2 | - | 0.1 | 0.2 | 0.1 | - | - | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | - |
| Hexanal | - | 31.6 | 21.8 | 5.9 | 0.8 | 0.1 | 1.3 | 0.4 | 21.8 | 0.1 | 1.4 | - | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | - | 0.2 | 0.3 | 3.9 |
| Trans-2-hexenal | - | 0.1 | 0.1 | - | - | - | - | - | 0.1 | - | - | - | - | - | - | - | - | - | - | - | - |
| Trans-3-hexen-1-ol | - | 3.6 | 2.4 | 1.1 | - | - | - | - | 5.5 | - | - | - | - | - | - | - | - | - | - | - | 0.6 |
| Trans-2-hexen-1-ol | - | - | - | - | - | - | - | - | - | - | 8.5 | - | - | - | - | - | - | - | - | - | - |
| Hexan-1-ol | 0.4 | 0.3 | 0.6 | 0.9 | 3.4 | 0.8 | 0.1 | 1.3 | 0.7 | 0.7 | - | 0.3 | 0.5 | 0.8 | 1.6 | 1.6 | 0.7 | 0.5 | 1.1 | 1.5 | 0.1 |
| Heptanal | - | - | - | - | - | - | - | - | 0.1 | - | - | - | - | - | - | - | - | - | - | - | - |
| α-pinene | 1.1 | - | 0.1 | - | 0.1 | - | 0.1 | 0.3 | - | - | - | 0.1 | - | - | 2.3 | - | - | - | 0.2 | - | 0.1 |
| Benzaldehyde | - | - | 0.1 | 0.1 | - | - | 0.1 | 0.1 | 0.1 | - | - | - | - | - | - | - | - | - | - | - | 0.5 |
| m-cresol | - | - | - | 0.1 | - | - | 0.1 | - | 0.1 | - | - | - | - | - | 0.1 | - | 0.1 | 0.1 | - | 0.1 | 0.1 |
| 2-phenylethanol | 0.5 | 0.3 | 2.9 | 11.5 | 1.8 | 7.9 | 7.8 | 2.4 | 8.7 | 4.0 | 0.6 | 1.2 | 6.4 | 1.5 | 6.2 | 5.8 | 5.6 | 5.2 | 1.4 | - | 4.2 |
| Nerol | - | - | - | - | - | 0.1 | 0.1 | - | - | 0.2 | - | - | 0.1 | - | 0.7 | 0.2 | - | 1.9 | - | 0.4 | 0.9 |
| (E)-citral (neral) | - | - | - | - | - | - | - | - | - | 0.1 | - | - | - | - | 0.1 | - | - | 0.1 | - | 0.1 | 0.1 |
| (Z)-citral (geranial) | - | - | - | 0.4 | - | - | 0.1 | - | - | - | - | 0.1 | 0.1 | - | - | - | - | 0.1 | - | 0.1 | 0.1 |
| Methyl eugenol | 0.1 | - | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | - | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | - | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Total | 98.3 | 95.4 | 98.6 | 97.3 | 96.0 | 98.1 | 97.8 | 98.3 | 98.5 | 98.0 | 96.8 | 98.4 | 98.5 | 99.0 | 95.1 | 97.8 | 97.9 | 97.5 | 98.7 | 96.2 | 95.6 |
| Genotype (ID) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Constituent | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 |
| Air | 97.6 | 93.6 | 99.1 | 92.3 | 83.7 | 83.4 | 93.0 | 84.0 | 92.6 | 95.8 | 97.6 | 94.0 | 94.6 | 97.4 | 92.4 | 97.5 | 95.8 | 80.6 | 97.9 | 93.8 | 94.2 |
| Acetaldheyde | - | - | - | - | 11.4 | - | - | 8.4 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Dimethyl sulfide | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 12.2 | - | - | - |
| Isovaleraldehyde | 0.3 | - | - | - | - | - | 0.5 | - | 0.1 | - | - | 0.3 | 0.2 | - | 0.7 | - | - | 0.4 | - | - | - |
| Methyl-1-butanol | 0.1 | 0.9 | - | 1.0 | 0.5 | 0.1 | 1.2 | 0.3 | 0.7 | 0.2 | 0.3 | 1.0 | 0.5 | 0.2 | 1.6 | 0.2 | 0.2 | 0.3 | 0.1 | 0.3 | 1.5 |
| 1-pentanol | - | 0.1 | - | - | 0.2 | - | 0.1 | - | - | - | - | - | - | - | 0.1 | - | - | - | - | - | - |
| Hexanal | 0.5 | 0.2 | - | 0.3 | 0.3 | 4.3 | 1.1 | 0.1 | - | 0.2 | 0.1 | 0.3 | 0.2 | 0.2 | 0.1 | 0.1 | 0.2 | 0.3 | 0.1 | 0.7 | 0.4 |
| Trans-2-hexenal | - | 0.1 | - | 0.1 | - | - | 0.1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Hexan-1-ol | 0.1 | 0.3 | - | 0.2 | 0.6 | 1.2 | 0.1 | - | - | 0.1 | - | - | 0.5 | 0.1 | 0.3 | 0.1 | 0.1 | 0.2 | - | 0.1 | 0.1 |
| Heptanal | - | - | - | - | - | - | - | - | 0.1 | - | - | - | - | - | - | - | - | 0.1 | - | - | - |
| α-pinene | - | - | - | 0.2 | - | 0.1 | - | - | - | - | - | - | - | - | - | 0.2 | - | - | - | - | - |
| Benzaldehyde | - | - | - | - | - | - | 0.1 | - | - | - | 0.1 | 0.1 | - | - | - | - | - | 0.1 | - | - | - |
| m-cresol | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.1 |
| 2-phenylethanol | - | 0.2 | - | 1.7 | 1.3 | 0.2 | - | - | - | 1.1 | - | 0.7 | 0.7 | 0.2 | 0.2 | - | 1.1 | 1.9 | - | - | - |
| Nerol | 0.1 | - | - | 0.7 | - | 2.2 | - | 0.3 | - | - | - | - | 0.2 | - | - | - | - | 0.4 | 0.1 | - | - |
| (E)-citral (neral) | - | - | - | 0.2 | - | 1.2 | - | 0.1 | - | - | - | - | 0.1 | - | - | - | - | 0.1 | 0.1 | - | - |
| (Z)-citral (geranial) | - | - | - | 0.2 | - | 1.7 | - | 0.1 | - | - | - | - | 0.1 | - | - | - | - | 0.1 | - | - | - |
| Methyl eugenol | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Total | 98.8 | 95.5 | 99.2 | 96.8 | 98.2 | 94.2 | 96.2 | 93.2 | 93.6 | 97.7 | 98.2 | 96.4 | 97.3 | 98.3 | 95.3 | 98.2 | 97.7 | 96.7 | 98.5 | 95.0 | 96.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caser, M.; Scariot, V. The Contribution of Volatile Organic Compounds (VOCs) Emitted by Petals and Pollen to the Scent of Garden Roses. Horticulturae 2022, 8, 1049. https://doi.org/10.3390/horticulturae8111049
Caser M, Scariot V. The Contribution of Volatile Organic Compounds (VOCs) Emitted by Petals and Pollen to the Scent of Garden Roses. Horticulturae. 2022; 8(11):1049. https://doi.org/10.3390/horticulturae8111049
Chicago/Turabian StyleCaser, Matteo, and Valentina Scariot. 2022. "The Contribution of Volatile Organic Compounds (VOCs) Emitted by Petals and Pollen to the Scent of Garden Roses" Horticulturae 8, no. 11: 1049. https://doi.org/10.3390/horticulturae8111049
APA StyleCaser, M., & Scariot, V. (2022). The Contribution of Volatile Organic Compounds (VOCs) Emitted by Petals and Pollen to the Scent of Garden Roses. Horticulturae, 8(11), 1049. https://doi.org/10.3390/horticulturae8111049







