Sustainable Olive Culture under Climate Change: The Potential of Biostimulants
Abstract
1. Introduction
2. Olive Tree and Culture Systems
| Country | Olive Oil Production | Olive Oil Consumption | Table Olives Production | Cultivars | ||
|---|---|---|---|---|---|---|
| 2020/2021 | 2021/2022 | 2020/2021 | 2021/2022 | |||
| Spain | 1389 | 1300 | 17 | 547 | 645 | Arbequina, Arbosana, Aloreña, Alfafara, Blanqueta, Cornicabra, Empeltre, Farga, Hojiblanca, Lechín, Manzanilla, Morisca, Negral, Nevadillo, Picual, Picudo, Redondilla, Royal, Sevillenca, Verdeal de Vélez-Málaga and Villalonga |
| Greece | 275 | 225 | 4 | 230 | 165 | Anphissis, Chalkidiki, Conservolia, Kalamon, Koroneiki, Kolybada, Liano-lia, Mastoidis, Megaritiki, Ntopia Atsicholou, Ntopia Pierias, Petrolia Serron, Smertolia and Chrysophylli |
| Italy | 274 | 315 | 15 | 80 | 59 | Ascolana, Bella di Cerignola, Biancolilla, Bosana, Canino, Carolea, Casaliva, Casasene, Cellina di Nardò, Cima di Melfi, Coratina, Frantoio, Giarraffa, Leccino, Maurino, Mele, Moraiolo, Nocellara del Belice, Nocellara etnea, Ogliarola, Olivastra Seggianese, Pendolino, Peranzana, Razzola, Taggiasca and Termite di Bitetto. |
| Portugal | 100 | 120 | 2 | 16 | 21 | Azeiteira, Blanqueta, Cobrançosa, Cordovil, Carrasquenha, Galega, Lentisca, Madural, Verdeal and Redondil |
| Tunisia | 140 | 240 | 1 | Na | Na | Baroni, Chétoui, Chemlali, Oueslati, Chemlali Tataouine, Esraadki, Gerboui, Meski, Neb Jmel, Rkhami, Roumi, Rajou and Zalmati |
| Turkey | 210 | 228 | 5 | 360 | 402 | Ayvalik, Balıkesir, Domat, Erkence, Çakir, Halhali, Memecik, Memeli, Uslu, Izmir Sofralik, Gemlik, Kilis, Kiraz and Otur |
| Morocco | 160 | 200 | 4 | 130 | 130 | Picholine Marocaine, Picholine Languedoc, Dahbia, Haouzia, Menara and Meslala |
| Algeria | 70 | 98 | 3 | 278 | 326 | Aaroun, Albani, Aedli, Azeradj, ‘Ballouti amzel, Blanquette, Bouchouk, Chemlal, Djbaili, Ferkani, Ferdel, Khadraya, Hamra, Limli, Mekki, Sigoise, Roulette and Zeboudj |
| Egypt | 30 | 20 | 1 | 500 | 500 | Wateken, Maraki, Wardan, Meloky, Sebhawy, Sinawy, Bez El Anza, Kosiem, Abou Monkar and Siwy |
3. Impact of Climate Changes on Olive Trees Performance and Quality
4. Sustainable Strategies to Improve Olive Culture under Climate Change Conditions: Biostimulants
4.1. Types of Biostimulants and Mode of Action
4.1.1. Seaweeds
4.1.2. Protein Hydrolysates
4.1.3. Humic Substances
4.1.4. Microorganisms
4.1.5. Nanoparticles or Nanomaterials
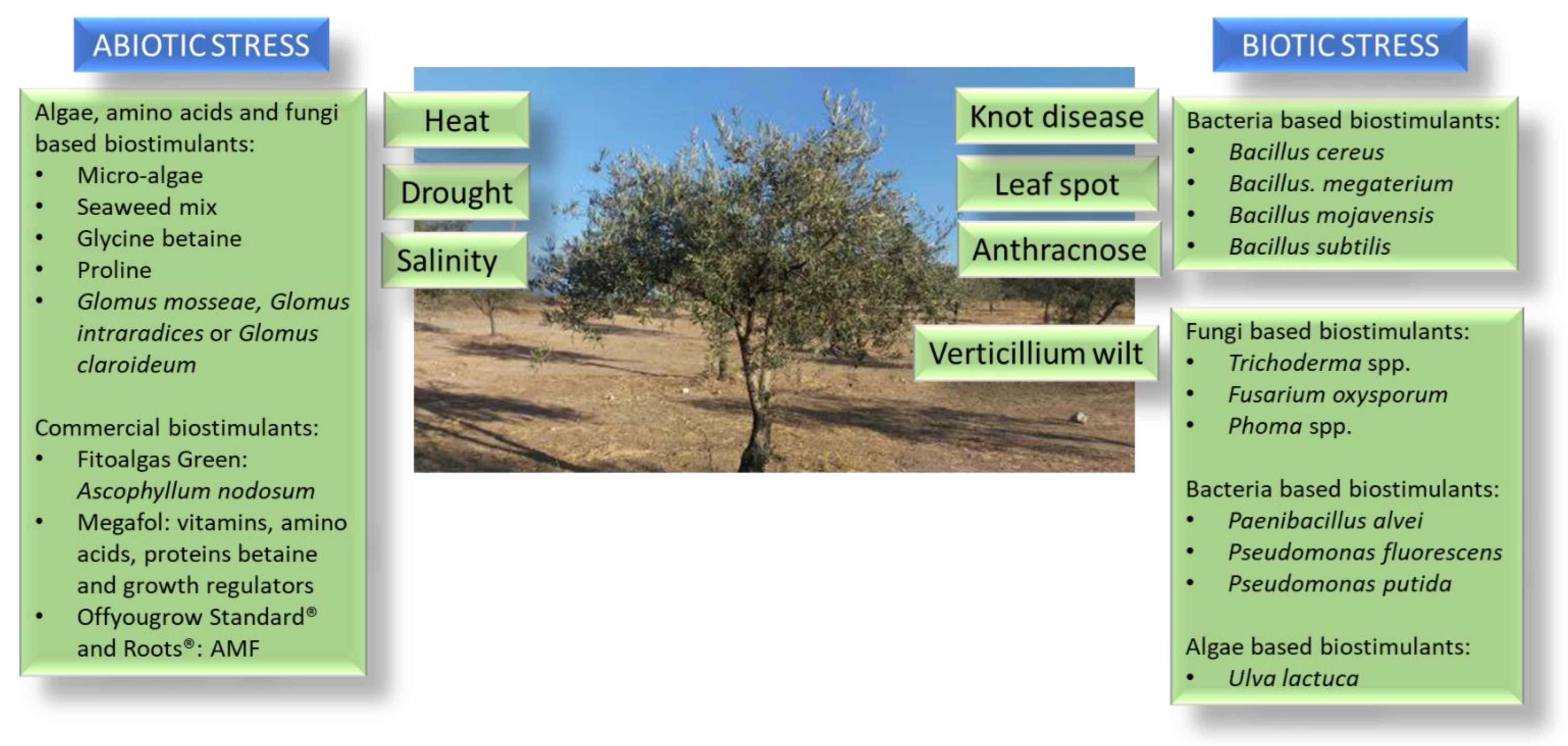
4.2. Biostimulants Use in Olive Culture and Their Impact on Stress Tolerance
4.2.1. Biotic Stress Tolerance
| Biostimulant | Pathogen | Effects | Reference |
|---|---|---|---|
| T. asperellum, T. harzianum, T. asperellum + T. gamsii | V. dahliae |
| [108] |
| F. oxysporum | V. dahliae |
| [107] |
| P. alvei | V. dahliae |
| [109] |
| P. fluorescens and P. putida | V. dahliae |
| [110,111,112,113,114] |
| Ulva lactuca | V. dahliae |
| [101] |
| Plant extracts | V. dahliae |
| [107] |
| B. cereus B. subtilis B. megaterium | S. oleaginea |
| [117,119] |
| B. mojavensis | P. savastanoi pv. Savastanoi |
| [116] |
| B. subtilis | C. acutatum |
| [120] |
4.2.2. Abiotic Stress Tolerance
4.3. Other Strategies and Techniques to Improve Olive Stress Performance
4.4. Biostimulant Effect on Olive Fruits, Oil Yield and Quality
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohanty, P.; Singh, P.K.; Chakraborty, D.; Mishra, S.; Pattnaik, R. Insight into the role of PGPR in sustainable agriculture and environment. Front. Sustain. Food Syst. 2021, 5, 667150. [Google Scholar] [CrossRef]
- Muluneh, M.G. Impact of climate change on biodiversity and food security: A global perspective—A review article. Agric. Food Secur. 2021, 10, 1–25. [Google Scholar] [CrossRef]
- IPCC. Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Shukla, P.R., Skea, J., Calvo Buendia, E., Masson-Delmotte, V., Pörtner, H.-O., Roberts, D.C., Zhai, P., Slade, R., Connors, S., van Diemen, R., et al., Eds.; 2019; Available online: https://www.ipcc.ch/site/assets/uploads/sites/4/2021/07/210714-IPCCJ7230-SRCCL-Complete-BOOK-HRES.pdf (accessed on 1 September 2022).
- Leogrande, R.; El Chami, D.; Fumarola, G.; Di Carolo, M.; Piegari, G.; Elefante, M.; Perrelli, D.; Dongiovanni, C. Biostimulants for Resilient Agriculture: A Preliminary Assessment in Italy. Sustainability 2022, 14, 6816. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- EBIC. Economic Overview of the Biostimulants Sector in Europe. Available online: https://biostimulants.eu/ (accessed on 2 August 2022).
- Reid, T.E.; Kavamura, V.N.; Abadie, M.; Torres-Ballesteros, A.; Pawlett, M.; Clark, I.M.; Harris, J.; Mauchline, T.H. Inorganic chemical fertilizer application to wheat reduces the abundance of putative plant growth-promoting Rhizobacteria. Front. Microbiol. 2021, 12, 642587. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef]
- Obied, H.K.; Prenzler, P.D.; Ryan, D.; Servili, M.; Taticchi, A.; Esposto, S.; Robards, K. Biosynthesis and biotransformations of phenol-conjugated oleosidic secoiridoids from Olea europaea L. Nat. Prod. Rep. 2008, 25, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, J.M. The origin and expansion of olive cultivation. In World Olive Encyclopaedia; International Olive Oil Council: Madrid, Spain, 1996; pp. 19–58. ISBN 84-01-61881-9. [Google Scholar]
- Fraga, H.; Moriondo, M.; Leolini, L.; Santos, J.A. Mediterranean olive orchards under climate change: A review of future impacts and adaptation strategies. Agronomy 2021, 11, 56. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.T.; Moutinho-Pereira, J.; Correia, C.M. Drought stress effects and olive tree acclimation under a changing climate. Plants 2019, 8, 232. [Google Scholar] [CrossRef]
- Alonso, A.D.; Krajsic, V. Food heritage down under: Olive growers as Mediterranean ‘food ambassadors’. J. Herit. Tour. 2013, 8, 158–171. [Google Scholar] [CrossRef]
- Valente, S.; Machado, B.; Pinto, D.C.G.A.; Santos, C.; Silva, A.M.S.; Dias, M.C. Modulation of phenolic and lipophilic compounds of olive fruits in response to combined drought and heat. Food Chem. 2020, 329, 127191. [Google Scholar] [CrossRef] [PubMed]
- Reis, P. O Olival em Portugal Dinâmicas, Tecnologias e Relação com o Desenvolvimento Rural; Associação Portuguesa para o Desenvolvimento Local: Lisboa, Portugal, 2014; Volume 33. [Google Scholar]
- Calabrese, G.; Artaglini, N.; Ladisa, G. Study on Biodiversity in Century-Old Olive Groves; CIHEAM-Mediterranean Agronomic Institute: Bari, Italy, 2012; pp. 1–108. [Google Scholar]
- Guerrero-Casado, J.; Carpio, A.J.; Tortosa, F.S.; Villanueva, A.J. Environmental challenges of intensive woody crops: The case of super high-density olive groves. Sci. Total Environ. 2021, 798, 149212. [Google Scholar] [CrossRef] [PubMed]
- Therios, I. Olives; Cambridge University Press: Wallingford, UK, 2009. [Google Scholar]
- Belaj, A.; de la Rosa, R.; León, L.; Gabaldón-Leal, C.; Santos, C.; Porras, R.; de la Cruz-Blanco, M.; Lorite, I.J. Phenological diversity in a world olive germplasm bank: Potential use for breeding programs and climate change studies. Span. J. Agric. Res. 2020, 18, e0701. [Google Scholar] [CrossRef]
- Lo Bianco, R.; Proietti, P.; Regni, L.; Caruso, T. Planting systems for modern olive growing: Strengths and weaknesses. Agriculture 2021, 11, 494. [Google Scholar] [CrossRef]
- International Olive Council. Book of the IOC Network of Germplasm Banks; UCOLIVO—Universidad de Córdoba: Córdoba, Spain, 2019. [Google Scholar]
- Silva, S.; Santos, C.; Serôdio, J.; Silva, A.M.S.; Dias, M.C. Physiological performance of drought-stressed olive plants when exposed to a combined heat–UV-B shock and after stress relief. Funct. Plant Biol. 2018, 45, 1233–1240. [Google Scholar] [CrossRef]
- Regni, L.; Del Pino, A.M.; Mousavi, S.; Palmerini, C.A.; Baldoni, L.; Mariotti, R.; Mairech, H.; Gardi, T.; D’Amato, R.; Proietti, P. Behavior of four olive cultivars during salt stress. Front. Plant Sci. 2019, 10, 867. [Google Scholar] [CrossRef]
- Rapoport, H.F.; Fabbri, A.; Sebastiani, L. The Olive Tree Genome; Springer: Berlin/Heidelberg, Germany, 2016; pp. 13–26. [Google Scholar] [CrossRef]
- Tripepi, M.; Pöhlschroder, M.; Bitonti, M.B. Diversity of Dehydrins in Oleae europaea Plants Exposed to Stress. Open Plant Sci. J. 2011, 5, 9–13. [Google Scholar] [CrossRef]
- Chartzoulakis, K.S. Salinity and olive: Growth, salt tolerance, photosynthesis and yield. Agric. Water Manag. 2005, 78, 108–121. [Google Scholar] [CrossRef]
- Orlandi, F.; Rojo, J.; Picornell, A.; Oteros, J.; Pérez-Badia, R.; Fornaciari, M. Impact of climate change on olive crop production in Italy. Atmosphere 2020, 11, 595. [Google Scholar] [CrossRef]
- Martinelli, F.; Remorini, D.; Saia, S.; Massai, R.; Tonutti, P. Metabolic profiling of ripe olive fruit in response to moderate water stress. Sci. Hortic. 2013, 159, 52–58. [Google Scholar] [CrossRef]
- Ben-Gal, A. Salinity and olive: From physiological responses to orchard management. Isr. J. Plant Sci. 2011, 59, 15–28. [Google Scholar] [CrossRef]
- Petridis, A.; Therios, I.; Samouris, G.; Koundouras, S.; Giannakoula, A. Effect of water deficit on leaf phenolic composition, gas exchange, oxidative damage and antioxidant activity of four Greek olive (Olea europaea L.) cultivars. Plant Physiol. Biochem. 2012, 60, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Koubouris, G.C.; Kavroulakis, N.; Metzidakis, I.T.; Vasilakakis, M.D.; Sofo, A. Ultraviolet-B radiation or heat cause changes in photosynthesis, antioxidant enzyme activities and pollen performance in olive tree. Photosynthetica 2015, 53, 279–287. [Google Scholar] [CrossRef]
- Araújo, M.; Santos, C.; Dias, M.C. Can young olive plants overcome heat shock? In Theory and Practice of Climate Adaptation; Alves, F., Leal Filho, W., Azeiteiro, U., Eds.; Springer: Berlin, Germany, 2018; pp. 193–203. [Google Scholar]
- Dias, M.C.; Pinto, D.C.G.A.; Correia, C.; Moutinho-Pereira, J.; Oliveira, H.; Freitas, H.; Silva, A.M.S.; Santos, C. UV-B radiation modulates physiology and lipophilic metabolite profile in Olea europaea. J. Plant Physiol. 2018, 222, 39–50. [Google Scholar] [CrossRef]
- Piccini, C.; Cai, G.; Dias, M.C.; Romi, M.; Longo, R.; Cantini, C. UV-B Radiation Affects Photosynthesis-Related Processes of Two Italian Olea europaea (L.) Varieties Differently. Plants 2020, 9, 1712. [Google Scholar] [CrossRef]
- Piccini, C.; Cai, G.; Dias, M.C.; Araújo, M.; Parri, S.; Romi, M.; Faleri, C.; Cantini, C. Olive varieties under UV-B stress show distinct responses in terms of antioxidant machinery and isoform/activity of rubisco. Int. J. Mol. Sci. 2021, 22, 11214. [Google Scholar] [CrossRef]
- Caruso, G.; Gucci, R.; Urbani, S.; Esposto, S.; Taticchi, A.; Di Maio, I.; Selvaggini, R.; Servili, M. Effect of different irrigation volumes during fruit development on quality of virgin olive oil of cv. Frantoio. Agric. Water Manag. 2014, 134, 94–103. [Google Scholar] [CrossRef]
- Nissim, Y.; Shloberg, M.; Biton, I.; Many, Y.; Doron-Faigenboim, A.; Zemach, H.; Hovav, R.; Kerem, Z.; Avidan, B.; Ben-Ari, G. High temperature environment reduces olive oil yield and quality. PLoS ONE 2020, 15, e0231956. [Google Scholar] [CrossRef]
- Nissim, Y.; Shlosberg, M.; Biton, I.; Many, Y.; Doron-Faigenboim, A.; Hovav, R.; Kerem, Z.; Avidan, B.; Ben-Ari, G. A high temperature environment regulates the olive oil biosynthesis network. Plants 2020, 9, 1135. [Google Scholar] [CrossRef]
- Fernández-Lobato, L.; García-Ruiz, R.; Jurado, F.; Vera, D. Life cycle assessment, C footprint and carbon balance of virgin olive oils production from traditional and intensive olive groves in southern Spain. J. Environ. Manage. 2021, 293, 112951. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jensen, C.R.; Liu, F. Nutritional responses to soil drying and rewetting cycles under partial root-zone drying irrigation. Agric. Water Manag. 2017, 179, 254–259. [Google Scholar] [CrossRef]
- Bedbabis, S.; Ben Rouina, B.; Boukhris, M.; Ferrara, G. Effects of irrigation with treated wastewater on root and fruit mineral elements of Chemlali olive cultivar. Sci. World J. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.; Fonseca, A.; Santos, J.A. Are land use options in viticulture and oliviculture in agreement with bioclimatic shifts in Portugal? Land 2021, 10, 869. [Google Scholar] [CrossRef]
- IPMA. Acompanhamento do Clima. Available online: https://www.ipma.pt/pt/oclima/monitorizacao/index.jsp?selTipo=m&selVar=tt&selAna=me&selAno=2017 (accessed on 7 August 2020).
- IPMA. Boletim Climático Portugal Continental Janeiro 2022. Available online: https://www.ipma.pt/resources.www/docs/im.publicacoes/edicoes.online/20220204/FGdTvyAzNYKcsCOxBZMy/cli_20220101_20220131_pcl_mm_co_pt.pdf (accessed on 1 September 2022).
- Almadi, L.; Paoletti, A.; Cinosi, N.; Daher, E.; Rosati, A.; Di Vaio, C.; Famiani, F. A biostimulant based on protein hydrolysates promotes the growth of young olive trees. Agriculture 2020, 10, 618. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop production. Plants 2021, 10, 531. [Google Scholar] [CrossRef]
- Franzoni, G.; Cocetta, G.; Prinsi, B.; Ferrante, A.; Espen, L. Biostimulants on crops: Their impact under abiotic stress conditions. Horticulturae 2022, 8, 189. [Google Scholar] [CrossRef]
- Bartucca, M.L.; Cerri, M.; Del Buono, D.; Forni, C. Use of biostimulants as a new approach for the improvement of phytoremediation performance—A Review. Plants 2022, 11, 1946. [Google Scholar] [CrossRef]
- Lau, S.-E.; Fei, W.; Teo, A.; Teoh, E.Y.; Tan, B.C. Microbiome engineering and plant biostimulants for sustainable crop improvement and mitigation of biotic and abiotic stresses. Discov. Food 2022, 2, 9. [Google Scholar] [CrossRef]
- Lynch, J.; Cain, M.; Frame, D.; Pierrehumbert, R. Agriculture’s contribution to climate change and role in mitigation is distinct from predominantly fossil CO2-emitting sectors. Front. Sustain. Food Syst. 2021, 4, 518039. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Geelen, D. Developing biostimulants from agro-food and industrial by-products. Front. Plant Sci. 2018, 871, 1567. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Maldonado, A.; Ortega-Ortíz, H.; Morales-Díaz, A.B.; González-Morales, S.; Morelos-Moreno, Á.; Cabrera-De la Fuente, M.; Sandoval-Rangel, A.; Cadenas-Pliego, G.; Benavides-Mendoza, A. Nanoparticles and nanomaterials as plant biostimulants. Int. J. Mol. Sci. 2019, 20, 162. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.V.; Filgueiras, C.C.; Dória, J.; Peñaflor, M.F.G.V.; Willett, D.S. The effects of biostimulants on induced plant defense. Front. Agron. 2021, 3, 1–9. [Google Scholar] [CrossRef]
- Brouwers, E.; Draisma, M.; van Swam, K.; Veen, A.J.; Burger, L. Identification of the Seaweed Biostimulant Market (Phase 1); The North Sea Farm Foundation: AD Den Haag, The Netherlands, 2018; pp. 1–64. [Google Scholar]
- El Boukhari, M.E.M.; Barakate, M.; Bouhia, Y.; Lyamlouli, K. Trends in seaweed extract based biostimulants: Manufacturing process and beneficial effect on soil-plant systems. Plants 2020, 9, 359. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Baltazar, M.; Correia, S.; Guinan, K.J.; Sujeeth, N.; Bragança, R.; Gonçalves, B. Recent advances in the molecular effects of biostimulants in plants: An overview. Biomolecules 2021, 11, 1096. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Cristiano, G.; Pallozzi, E.; Conversa, G.; Tufarelli, V.; De Lucia, B. Effects of an animal-derived biostimulant on the growth and physiological parameters of potted snapdragon (Antirrhinummajus L.). Front. Plant Sci. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Stirk, W.A.; Rengasamy, K.R.R.; Kulkarni, M.G.; van Staden, J. Plant biostimulants from seaweed. In The Chemical Biology of Plant Biostimulants; Gleelen, D., Xu, L., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 31–55. [Google Scholar]
- Mukherjee, A.; Patel, J.S. Seaweed extract: Biostimulator of plant defense and plant productivity. Int. J. Environ. Sci. Technol. 2020, 17, 553–558. [Google Scholar] [CrossRef]
- Nanda, S.; Kumar, G.; Hussain, S. Utilization of seaweed-based biostimulants in improving plant and soil health: Current updates and future prospective. Int. J. Environ. Sci. Technol. 2021, 19, 12839–12852. [Google Scholar] [CrossRef]
- Ertani, A.; Francioso, O.; Tinti, A.; Schiavon, M.; Pizzeghello, D.; Nardi, S. Evaluation of seaweed extracts from laminaria and Ascophyllum nodosum spp. As biostimulants in Zea mays L. using a combination of chemical, biochemical and morphological approaches. Front. Plant Sci. 2018, 9, 428. [Google Scholar] [CrossRef] [PubMed]
- Cristofano, F.; El-Nakhel, C.; Rouphael, Y. Biostimulant substances for sustainable agriculture: Origin, operating mechanisms and effects on cucurbits, leafy greens, and nightshade vegetables species. Biomolecules 2021, 11, 1103. [Google Scholar] [CrossRef] [PubMed]
- Francesca, B.; Nicola, B.; Paolo, T.; Marcello, M. Classification of Biostimulants Origin Using Amino Acids Composition of Hydrolyzed Proteins. J. Hortic. Sci. Res. 2017, 1, 30–35. [Google Scholar] [CrossRef]
- Vaseva, I.I.; Simova-Stoilova, L.; Kostadinova, A.; Yuperlieva-Mateeva, B.; Karakicheva, T.; Vassileva, V. Heat-stress-mitigating effects of a protein-hydrolysate-based biostimulant are linked to changes in protease, DHN, and HSP gene expression in Maize. Agronomy 2022, 12, 1127. [Google Scholar] [CrossRef]
- Visconti, F.; de Paz, J.M.; Bonet, L.; Jordà, M.; Quiñones, A.; Intrigliolo, D.S. Effects of a commercial calcium protein hydrolysate on the salt tolerance of Diospyros kaki L. cv. “Rojo Brillante” grafted on Diospyros lotus L. Sci. Hortic. 2015, 185, 129–138. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 129–138. [Google Scholar] [CrossRef]
- Francesca, S.; Cirillo, V.; Raimondi, G.; Maggio, A.; Barone, A.; Rigano, M.M. A Novel protein hydrolysate-based biostimulant improves tomato performances under drought stress. Plants 2021, 10, 783. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 871, 1655. [Google Scholar] [CrossRef]
- Popa, D.G.; Lupu, C.; Constantinescu-Aruxandei, D.; Oancea, F. Humic substances as microalgal biostimulants—Implications for microalgal biotechnology. Mar. Drugs 2022, 20, 327. [Google Scholar] [CrossRef]
- Nephali, L.; Piater, L.A.; Dubery, I.A.; Patterson, V.; Huyser, J.; Burgess, K.; Tugizimana, F. Biostimulants for plant growth and mitigation of abiotic stresses: A metabolomics perspective. Metabolites 2020, 10, 505. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, N.O.; Medici, L.O.; Olivares, F.L.; Dobbss, L.B.; Torres-Netto, A.; Silva, S.F.; Novotny, E.H.; Canellas, L.P. Metabolic profile and antioxidant responses during drought stress recovery in sugarcane treated with humic acids and endophytic diazotrophic bacteria. Ann. Appl. Biol. 2016, 168, 203–213. [Google Scholar] [CrossRef]
- Alsamadany, H. Physiological, biochemical and molecular evaluation of mungbean genotypes for agronomical yield under drought and salinity stresses in the presence of humic acid. Saudi J. Biol. Sci. 2022, 29, 103385. [Google Scholar] [CrossRef] [PubMed]
- Zanin, L.; Tomasi, N.; Cesco, S.; Varanini, Z.; Pinton, R. Humic substances contribute to plant iron nutrition acting as chelators and biostimulants. Front. Plant Sci. 2019, 10, 675. [Google Scholar] [CrossRef]
- Ferrara, G.; Loffredo, E.; Simeone, R.; Senesi, N. Evaluation of antimutagenic and desmutagenic effects of humic and fulvic acids on root tips of Vicia faba. Environ. Toxicol. 2000, 15, 513–517. [Google Scholar] [CrossRef]
- Ferrara, G.; Loffredo, E.; Senesi, N. Anticlastogenic, antitoxic and sorption effects of humic substances on the mutagen maleic hydrazide tested in leguminous plants. Eur. J. Soil Sci. 2004, 55, 449–458. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Gemin, L.G.; Mógor, Á.F.; De Oliveira Amatussi, J.; Mógor, G. Microalgae associated to humic acid as a novel biostimulant improving onion growth and yield. Sci. Hortic. 2019, 256, 108560. [Google Scholar] [CrossRef]
- Jung, H.; Kwon, S.; Kim, J.H.; Jeon, J.R. Which traits of humic substances are investigated to improve their agronomical value? Molecules 2021, 26, 760. [Google Scholar] [CrossRef]
- Del Buono, D. Can biostimulants be used to mitigate the effect of anthropogenic climate change on agriculture? It is time to respond. Sci. Total Environ. 2021, 751, 141763. [Google Scholar] [CrossRef]
- Rai, N.; Rai, S.P.; Sarma, B.K. Prospects for abiotic stress tolerance in crops utilizing phyto- and bio-stimulants. Front. Sustain. Food Syst. 2021, 5, 455. [Google Scholar] [CrossRef]
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Leontidou, K.; Genitsaris, S.; Papadopoulou, A.; Kamou, N.; Bosmali, I.; Matsi, T.; Madesis, P.; Vokou, D.; Karamanoli, K.; Mellidou, I. Plant growth promoting rhizobacteria isolated from halophytes and drought-tolerant plants: Genomic characterisation and exploration of phyto-beneficial traits. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Miceli, A.; Moncada, A.; Vetrano, F. Use of microbial biostimulants to increase the salinity tolerance of vegetable transplants. Agronomy 2021, 11, 1143. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Latef, A.A.H.A.; Miransari, M. Use of Microbes for the Alleviation of Soil Stresses. In Use of Microbes for the Alleviation of Soil Stresses: Alleviation of Soil Stress by Pgpr and Mycorrhizal Fungi; Miransari, M., Ed.; Springer: New York, NY, USA, 2014; pp. 23–38. [Google Scholar]
- Diagne, N.; Ngom, M.; Djighaly, P.I.; Fall, D.; Hocher, V.; Svistoonoff, S. Roles of arbuscular mycorrhizal fungi on plant growth and performance: Importance in biotic and abiotic stressed regulation. Diversity 2020, 12, 370. [Google Scholar] [CrossRef]
- Moradi Tarnabi, Z.; Iranbakhsh, A.; Mehregan, I.; Ahmadvand, R. Impact of arbuscular mycorrhizal fungi (AMF) on gene expression of some cell wall and membrane elements of wheat (Triticum aestivum L.) under water deficit using transcriptome analysis. Physiol. Mol. Biol. Plants 2020, 26, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Dias, M.C.; Silva, A.M.S. Titanium and zinc based nanomaterials in agriculture: A promising approach to deal with (a)biotic stresses? Toxics 2022, 10, 172. [Google Scholar] [CrossRef]
- Hassan, M.U.; Ghareeb, R.Y.; Nawaz, M.; Mahmood, A.; Shah, A.N.; Abdel-megeed, A.; Abdelsalam, N.R.; Hashem, M.; Alamri, S. Melatonin: A vital protectant for crops against heat stress: Mechanisms and prospects. Agronomy 2022, 12, 1116. [Google Scholar] [CrossRef]
- Hidangmayum, A.; Dwivedi, P. Chitosan based nanoformulation for sustainable agriculture with special reference to abiotic stress: A Review. J. Polym. Environ. 2022, 30, 1264–1283. [Google Scholar] [CrossRef]
- Regni, L.; Del Buono, D.; Micheli, M.; Facchin, S.L.; Tolisano, C.; Proietti, P. Effects of biogenic ZnO nanoparticles on growth, physiological, biochemical traits and antioxidants on olive tree in vitro. Horticulturae 2022, 8, 161. [Google Scholar] [CrossRef]
- Ntasiou, P.; Kerou, A.K.; Karamanidou, T.; Vlachou, A.; Tziros, G.T.; Tsouknidas, A.; Karaoglanidis, G.S. Synthesis and characterization of novel copper nanoparticles for the control of leaf spot and anthracnose diseases of olive. Nanomaterials 2021, 11, 1667. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, B.; Jiang, D.; Chen, G. Silicon improves photosynthetic performance by optimizing thylakoid membrane protein components in rice under drought stress. Environ. Exp. Bot. 2019, 158, 117–124. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, X.; Zhang, J.; He, Y.; Zhu, X.; Zhou, X.; Gong, H.; Yin, J.; Liu, Y. Silicon confers cucumber resistance to salinity stress through regulation of proline and cytokinins. Plant Physiol. Biochem. 2020, 156, 209–220. [Google Scholar] [CrossRef]
- Morales-Díaz, A.B.; Ortega-Ortíz, H.; Juárez-Maldonado, A.; Cadenas-Pliego, G.; González-Morales, S.; Benavides-Mendoza, A. Application of nanoelements in plant nutrition and its impact in ecosystems. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 013001. [Google Scholar] [CrossRef]
- Muzzalupo, I.; Badolati, G.; Chiappetta, A.; Picci, N.; Muzzalupo, R. In vitro antifungal activity of Olive (Olea europaea) leaf extracts loaded in chitosan nanoparticles. Front. Bioeng. Biotechnol. 2020, 8, 151. [Google Scholar] [CrossRef]
- Salah, I.B.; Aghrouss, S.; Douira, A.; Aissam, S.; El Alaoui-Talibi, Z.; Filali-Maltouf, A.; El Modafar, C. Seaweed polysaccharides as bio-elicitors of natural defenses in olive trees against verticillium wilt of olive. J. Plant Interact. 2018, 13, 248–255. [Google Scholar] [CrossRef]
- Montes-Osuna, N.; Mercado-Blanco, J. Verticillium wilt of olive and its control: What did we learn during the last decade? Plants 2020, 9, 735. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Díaz, R.M.; Castillo, P.; Jiménez-Gasco, M.d.M.; Landa, B.B.; Navas-Cortés, J.A. Fusarium wilt of chickpeas: Biology, ecology and management. Crop Prot. 2015, 73, 16–27. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-ściseł, J. Trichoderma: The current status of its application in agriculture for the biocontrol of fungal phytopathogens and stimulation of plant growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef]
- Carrero-Carrón, I.; Trapero-Casas, J.L.; Olivares-García, C.; Monte, E.; Hermosa, R.; Jiménez-Díaz, R.M. Trichoderma asperellum is effective for biocontrol of Verticillium wilt in olive caused by the defoliating pathotype of Verticillium dahliae. Crop Prot. 2016, 88, 45–52. [Google Scholar] [CrossRef]
- Carrero-Carrón, I.; Rubio, M.B.; Niño-Sánchez, J.; Navas-Cortés, J.A.; Jiménez-Díaz, R.M.; Monte, E.; Hermosa, R. Interactions between Trichoderma harzianum and defoliating Verticillium dahliae in resistant and susceptible wild olive clones. Plant Pathol. 2018, 67, 1758–1767. [Google Scholar] [CrossRef]
- Varo, A.; Raya-Ortega, M.C.; Trapero, A. Selection and evaluation of micro-organisms for biocontrol of Verticillium dahliae in olive. J. Appl. Microbiol. 2016, 121, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Mulero-Aparicio, A.; Varo, A.; Agustí-Brisach, C.; López-Escudero, F.J.; Trapero, A. Biological control of Verticillium wilt of olive in the field. Crop Prot. 2020, 128, 104993. [Google Scholar] [CrossRef]
- Markakis, E.A.; Tjamos, S.E.; Antoniou, P.P.; Paplomatas, E.J.; Tjamos, E.C. Biological control of Verticillium wilt of olive by Paenibacillus alvei, strain K165. BioControl 2016, 61, 293–303. [Google Scholar] [CrossRef]
- Mercado-Blanco, J.; Rodríguez-Jurado, D.; Hervás, A.; Jiménez-Diaz, R.M. Suppression of Verticillium wilt in olive planting stocks by root-associated fluorescent Pseudomonas spp. Biol. Control 2004, 30, 474–486. [Google Scholar] [CrossRef]
- Prieto, P.; Navarro-Raya, C.; Valverde-Corredor, A.; Amyotte, S.G.; Dobinson, K.F.; Mercado-Blanco, J. Colonization process of olive tissues by Verticillium dahliae and its in planta interaction with the biocontrol root endophyte Pseudomonas fluorescens PICF7. Microb. Biotechnol. 2009, 2, 499–511. [Google Scholar] [CrossRef]
- Deketelaere, S.; Tyvaert, L.; França, S.C.; Hofte, M. Desirable traits of a good biocontrol agent against Verticillium wilt. Front. Microbiol. 2017, 8, 1186. [Google Scholar] [CrossRef]
- Schilirò, E.; Ferrara, M.; Nigro, F.; Mercado-Blanco, J. Genetic responses induced in olive roots upon colonization by the biocontrol endophytic bacterium Pseudomonas fluorescens PICF7. PLoS ONE 2012, 7, e48646. [Google Scholar] [CrossRef]
- Cabanás, C.G.L.; Schilirò, E.; Valverde-Corredor, A.; Mercado-Blanco, J. The biocontrol endophytic bacterium Pseudomonas fluorescens PICF7 induces systemic defense responses in aerial tissues upon colonization of olive roots. Front. Microbiol. 2014, 5, 427. [Google Scholar] [CrossRef]
- Sharma, H.S.S.; Fleming, C.; Selby, C.; Rao, J.R.; Martin, T. Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 2014, 26, 465–490. [Google Scholar] [CrossRef]
- Ghanney, N.; Locantore, P. Potential biocontrol effect of the phylloplane bacterium Bacillus mojavensis ABC-7 on the olive knot disease. J. Plant Pathol. Microbiol. 2016, 7, 3–5. [Google Scholar] [CrossRef]
- Salman, M. Biological control of Spilocaea oleagina, the causal agent of olive leaf spot disease, using antagonistic bacteria. J. Plant Pathol. 2017, 99, 741–744. [Google Scholar] [CrossRef]
- Cabanás, C.G.-L.; Ruano-Rosa, D.; Legarda, G.; Pizarro-Tobías, P.; Valverde-Corredor, A.; Triviño, J.C.; Roca, A.; Mercado-Blanco, J. Bacillales members from the olive rhizosphere are effective biological control agents against the defoliating pathotype of Verticillium dahliae. Agriculture 2018, 8, 90. [Google Scholar] [CrossRef]
- Al-Khatib, M. Biological control of olive leaf spot (peacock spot disease) caused by Cycloconium oleaginum (Spilocea oleaginea). J. Microbiol. 2010, 2, 64–67. [Google Scholar]
- Nigro, F.; Antelmi, I.; Labarile, R.; Sion, V.; Pentimone, I. Biological control of olive anthracnose. Acta Hortic. 2018, 1199, 439–444. [Google Scholar] [CrossRef]
- Graziani, G.; Cirillo, A.; Giannini, P.; Conti, S.; El-Nakhel, C.; Rouphael, Y.; Ritieni, A.; Di Vaio, C. Biostimulants improve plant growth and bioactive compounds of young olive trees under abiotic stress conditions. Agriculture 2022, 12, 227. [Google Scholar] [CrossRef]
- Dias, M.C.; Figueiras, R.; Sousa, M.; Araújo, M.; Santos, C. Aplicação de bioestimulante na cultura de oliveira em rega deficitária. In Proceedings of IX Simpósio Nacional de Olivicultura, Oeiras, Portugal, 25 October 2021; Ramos, A.C., Pereira, J.A., Rodrigues, N., Eds.; Associação Portuguesa de Horticultura (APH): Lisboa, Portugal, 2021; p. 92. [Google Scholar]
- Del Buono, D.; Regni, L.; Del Pino, A.M.; Bartucca, M.L.; Palmerini, C.A.; Proietti, P. Effects of megafol on the olive cultivar ‘Arbequina’ grown under severe saline stress in terms of physiological traits, oxidative stress, antioxidant defenses, and cytosolic Ca2+. Front. Plant Sci. 2021, 11, 603576. [Google Scholar] [CrossRef]
- Montes-Osuna, N.; Cabanás, C.G.-L.; Valverde-Corredor, A.; Legarda, G.; Prieto, P.; Mercado-Blanco, J. Evaluation of indigenous olive biocontrol rhizobacteria as protectants against drought and salt stress. Microorganisms 2021, 9, 1209. [Google Scholar] [CrossRef]
- Ahmed, C.B.; Rouina, B.B.; Sensoy, S.; Boukhriss, M.; Abdullah, F.B. Exogenous Proline Effects on Photosynthetic Performance and Antioxidant Defense System of Young Olive Tree. J. Agr. Food Chem. 2010, 58, 4216–4222. [Google Scholar] [CrossRef]
- Porras-Soriano, A.; Soriano-Martín, M.L.; Porras-Piedra, A.; Azcón, R. Arbuscular mycorrhizal fungi increased growth, nutrient uptake and tolerance to salinity in olive trees under nursery conditions. J. Plant Physiol. 2009, 166, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.I.; Arrobas, M.; Brito, C.; Gonçalves, A.; Silva, E.; Martins, S.; Raimundo, S.; Rodrigues, M.A.; Correia, C.M. Mycorrhizal Fungi were More Effective than Zeolites in Increasing the Growth of Non-Irrigated Young Olive Trees. Sustainability 2020, 12, 10630. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Fahadi Hoveizeh, N.; Gholami, R.; Abdelrahman, M.; Tran, L.-S.P. Exogenous melatonin mitigates salinity-induced damage in olive seedlings by modulating ion homeostasis, antioxidant defense, and phytohormone balance. Physiol. Plant. 2021, 173, 1682–1694. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.T.; Silva, E.; Gonçalves, A.; Matos, C.; Rodrigues, M.A.; Moutinho-Pereira, J.; Barros, A.; Correia, C. Kaolin and salicylic acid foliar application modulate yield, quality and phytochemical composition of olive pulp and oil from rainfed trees. Sci. Hortic. 2018, 237, 176–183. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.T.; Meijón, M.; Ferreira, H.; Pinto, G.; Moutinho-Pereira, J.; Correia, C. Salicylic acid modulates olive tree physiological and growth responses to drought and rewatering events in a dose dependent manner. J. Plant Physiol. 2018, 230, 21–32. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.T.; Luzio, A.; Silva, E.; Gonçalves, A.; Meijón, M.; Escandón, M.; Arrobas, M.; Rodrigues, M.Â.; Moutinho-Pereira, J.; et al. Kaolin and salicylic acid alleviate summer stress in rainfed olive orchards by modulation of distinct physiological and biochemical responses. Sci. Hortic. 2019, 246, 201–211. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.T.; Ferreira, H.; Coutinho, J.; Moutinho-Pereira, J.; Correia, C.M. Salicylic acid increases drought adaptability of young olive trees by changes on redox status and ionome. Plant Physiol. Biochem. 2019, 141, 315–324. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.T.; Ferreira, H.; Moutinho-Pereira, J.; Correia, C.M. Foliar pre-treatment with abscisic acid enhances olive tree drought adaptability. Plants 2020, 9, 341. [Google Scholar] [CrossRef]
- Silvestri, C.; Celletti, S.; Cristofori, V.; Astolfi, S.; Ruggiero, B.; Rugini, E. Olive (Olea europaea L.) plants transgenic for tobacco osmotin gene are less sensitive to in vitro-induced drought stress. Acta Physiol. Plant. 2017, 39, 229. [Google Scholar] [CrossRef]
- Bashir, M.A.; Silvestri, C.; Coppa, E.; Brunori, E.; Cristofori, V.; Rugini, E.; Ahmad, T.; Hafiz, I.A.; Abbasi, N.A.; Shah, M.K.N.; et al. Response of olive shoots to salinity stress suggests the involvement of sulfur metabolism. Plants 2021, 10, 350. [Google Scholar] [CrossRef]
- Monteiro, E.; Gonçalves, B.; Cortez, I.; Castro, I. The role of biostimulants as alleviators of biotic and abiotic stresses in grapevine: A Review. Plants 2022, 11, 396. [Google Scholar] [CrossRef]
- Mazeh, M.; Almadi, L.; Paoletti, A.; Cinosi, N.; Daher, E.; Tucci, M.; Lodolini, E.M.; Rosati, A.; Famiani, F. Use of an organic fertilizer also having a biostimulant action to promote the growth of young olive trees. Agriculture 2021, 11, 593. [Google Scholar] [CrossRef]
- Chouliaras, V.; Tasioula, M.; Chatzissavvidis, C.; Therios, I.; Tsabolatidou, E. The effects of a seaweed extract in addition to nitrogen and boron fertilization on productivity, fruit maturation, leaf nutritional status and oil quality of the olive (Olea europaea L.) cultivar Koroneiki. J. Sci. Food Agric. 2009, 89, 984–988. [Google Scholar] [CrossRef]
- Zouari, I.; Mechri, B.; Tekaya, M.; Dabbaghi, O.; Cheraief, I.; Mguidiche, A.; Annabi, K.; Laabidi, F.; Attia, F.; Hammami, M.; et al. Olive oil quality influenced by biostimulant foliar fertilizers. Brazilian J. Biol. Sci. 2020, 7, 3–18. [Google Scholar] [CrossRef]
- Hernández-Hernandez, G.; Salazar, D.M.; Martínez-Tomé, J.; López-Cortés, I. The use of biostimulants in high-density olive growing: Quality and production. Asian J. Adv. Agric. Res. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Tejada, M.; Caballero, P.; Parrado, J. Effects of foliar fertilization of biostimulants obtained from sewage sludge on olive yield. Cogent Food Agric. 2022, 8, 2124702. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, M.C.; Araújo, M.; Silva, S.; Santos, C. Sustainable Olive Culture under Climate Change: The Potential of Biostimulants. Horticulturae 2022, 8, 1048. https://doi.org/10.3390/horticulturae8111048
Dias MC, Araújo M, Silva S, Santos C. Sustainable Olive Culture under Climate Change: The Potential of Biostimulants. Horticulturae. 2022; 8(11):1048. https://doi.org/10.3390/horticulturae8111048
Chicago/Turabian StyleDias, Maria Celeste, Márcia Araújo, Sónia Silva, and Conceição Santos. 2022. "Sustainable Olive Culture under Climate Change: The Potential of Biostimulants" Horticulturae 8, no. 11: 1048. https://doi.org/10.3390/horticulturae8111048
APA StyleDias, M. C., Araújo, M., Silva, S., & Santos, C. (2022). Sustainable Olive Culture under Climate Change: The Potential of Biostimulants. Horticulturae, 8(11), 1048. https://doi.org/10.3390/horticulturae8111048









