Genome-Wide Identification, Characterization and Expression Analysis of Mango (Mangifera indica L.) chalcone synthase (CHS) Genes in Response to Light
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification and Annotation of Mango CHS Genes
2.2. Sequence Alignment and Phylogenetic Tree Construction
2.3. Excavation of Motifs and Conserved Domains in MiCHSs
2.4. Gene Location and Duplication Analysis
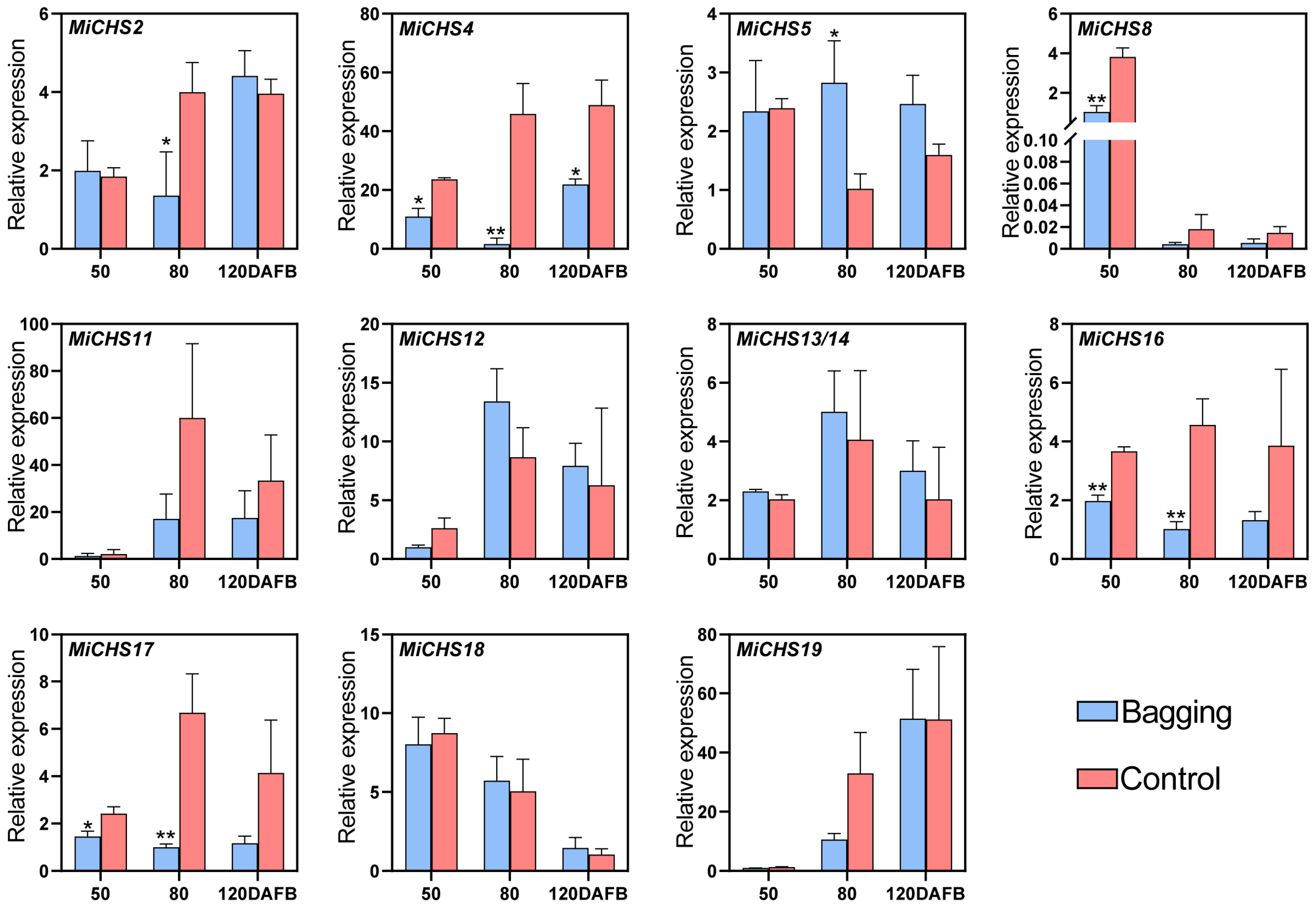
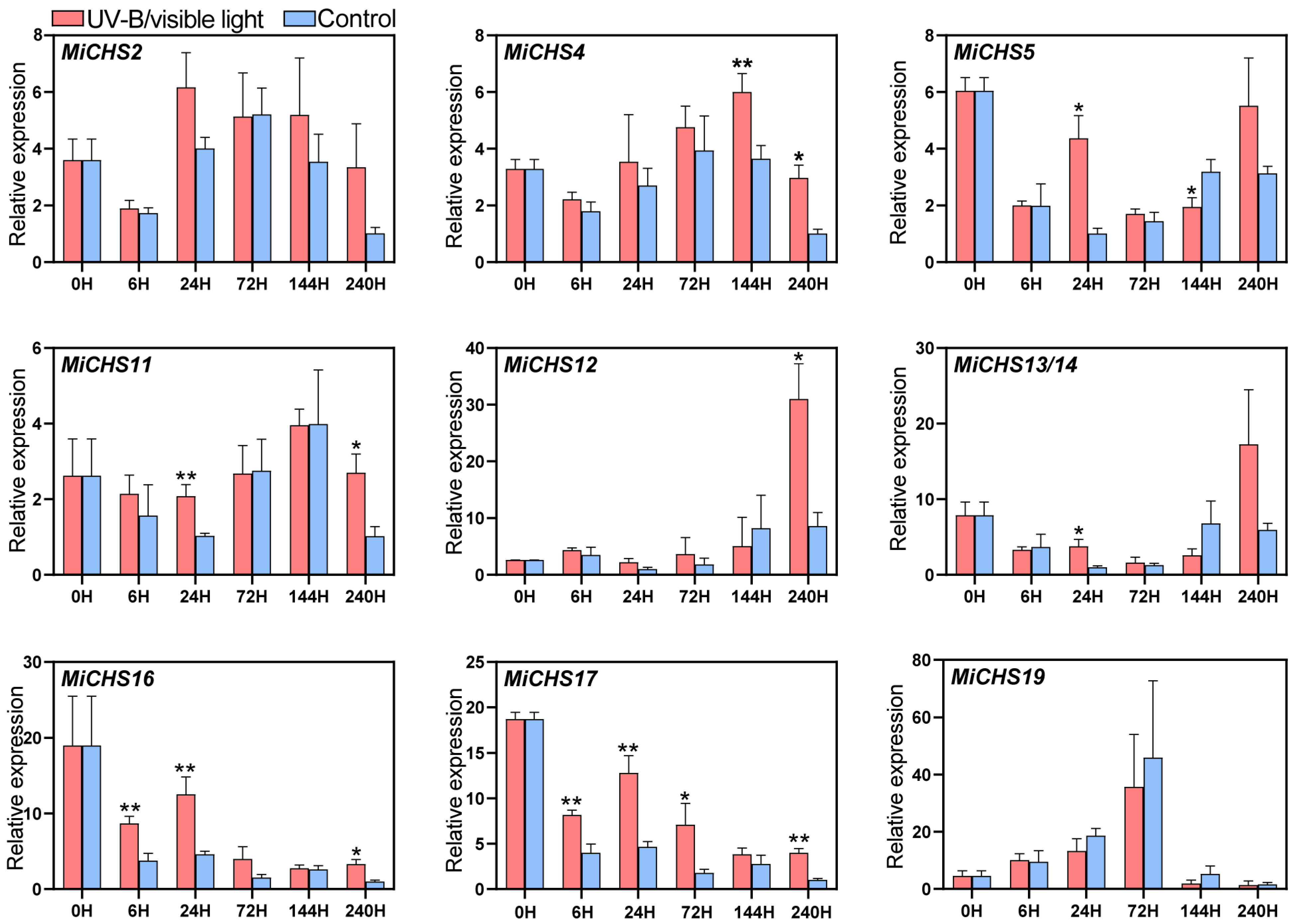
2.5. Materials and Treatments
2.6. Measurement of Flavonoid and Anthocyanin Contents in Mango Peel
2.7. Transcriptome Analysis of the MiCHS Expression
2.8. Analysis of Quantitative Real-Time PCR
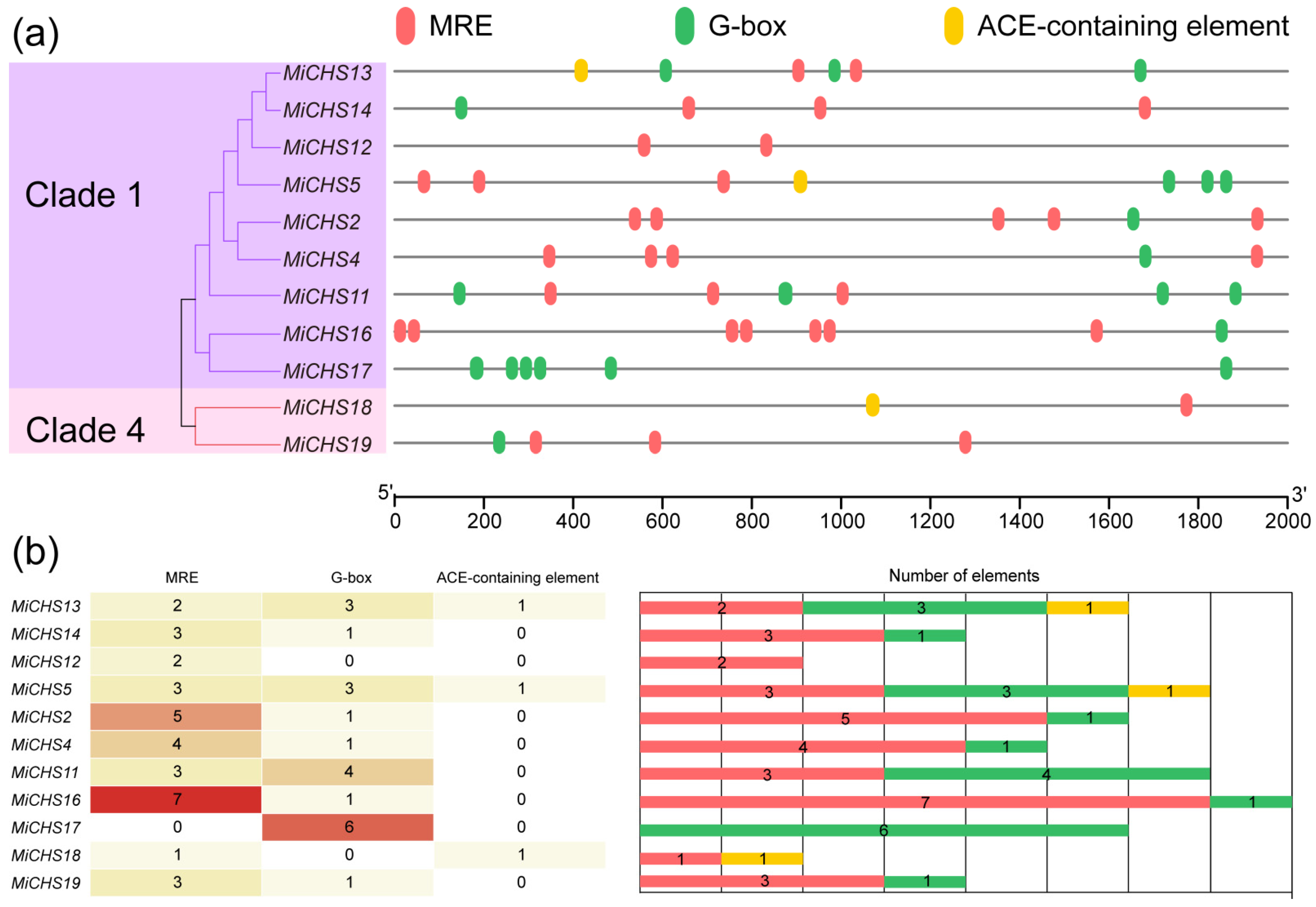
2.9. Promoter Cis-Acting Element Analysis
2.10. Statistical Analysis
3. Results
3.1. A Total of 21 Non-Redundant Putative Members of the MiCHS Gene Family Were Identified
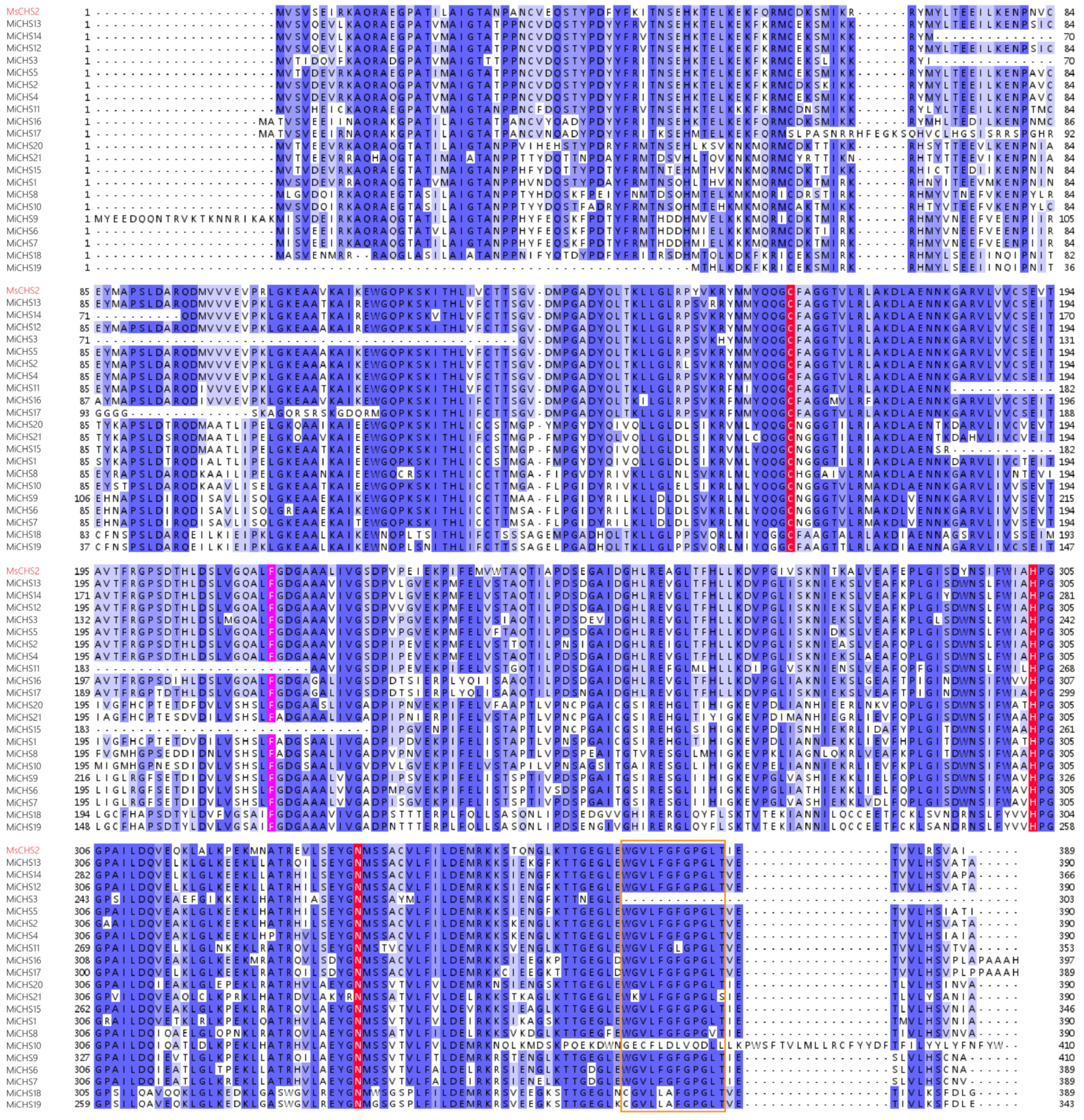
3.2. Multiple Sequence Alignment of Putative MiCHS Proteins
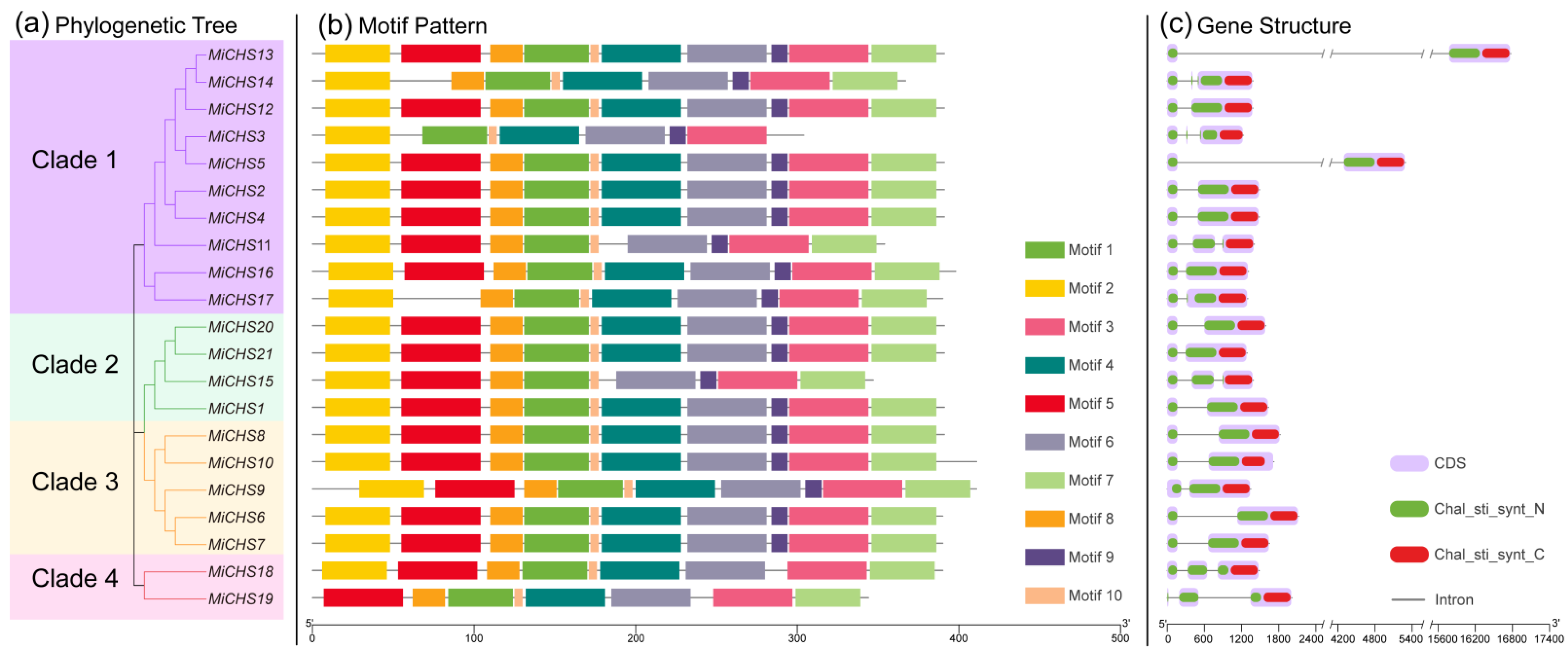
3.3. Construction of the Phylogenetic Tree, Motif Pattern, Domain and Gene Structure
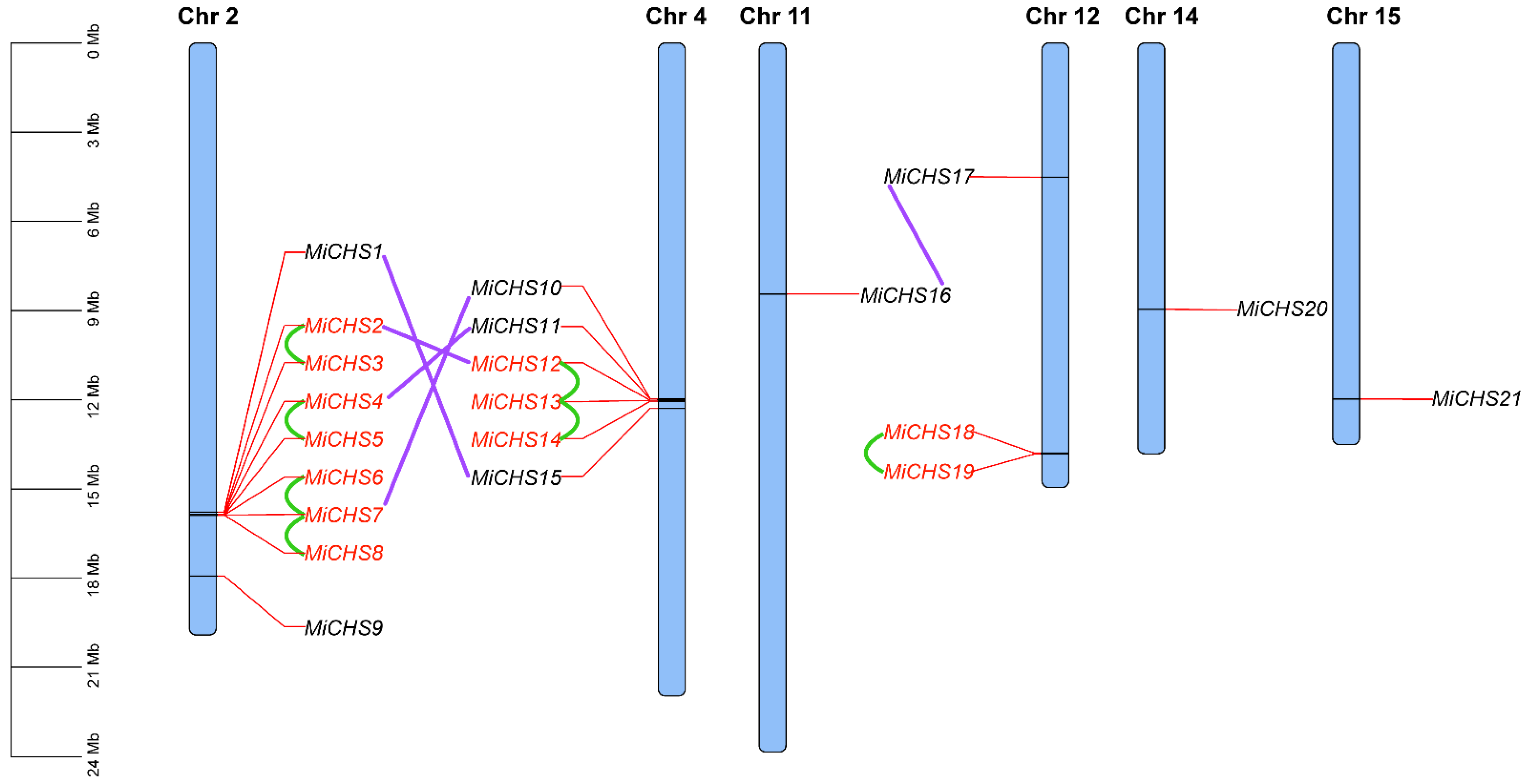
3.4. Synteny Analysis and Chromosomal Location of MiCHSs
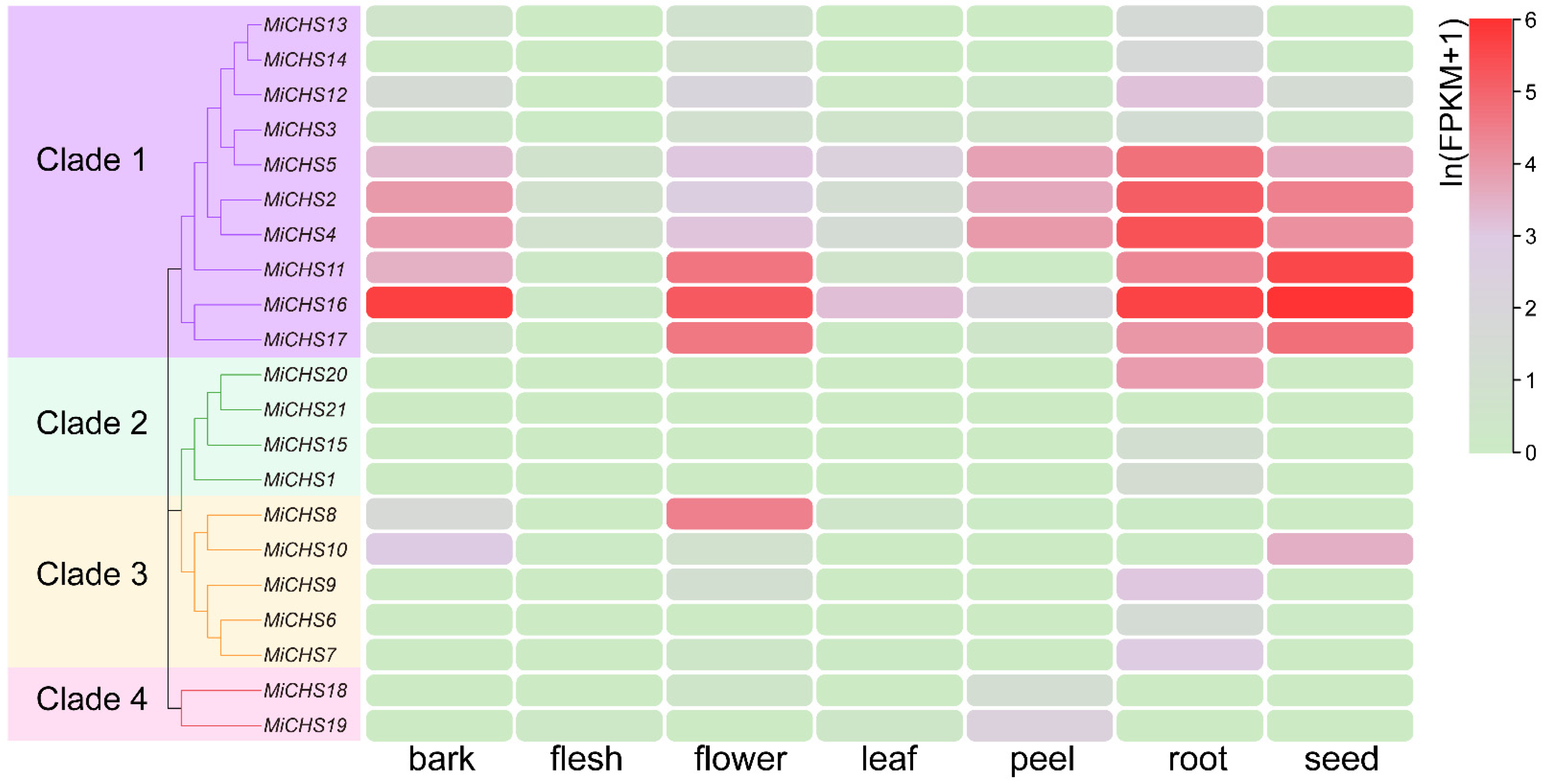
3.5. Tissue-Specific Expression Pattern Analysis of MiCHS Genes
3.6. Flavonoid and Anthocyanin Contents and MiCHS Expression in Differentially Colored Mango Cultivars
3.7. Expression of MiCHS Genes in Response to Light
3.8. Analysis of Light-Responsive Cis-Acting Elements in the Promoter Region of MiCHS Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, H.; Wang, X.; Mo, X.; Tang, C.; Zhong, S.; Deng, X.W. Arabidopsis DET1 degrades HFR1 but stabilizes PIF1 to precisely regulate seed germination. Proc. Natl. Acad. Sci. USA 2015, 112, 3817–3822. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhong, S.; Mo, X.; Liu, N.; Nezames, C.D.; Deng, X.W. HFR1 sequesters PIF1 to govern the transcriptional network underlying light-initiated seed germination in Arabidopsis. Plant Cell 2013, 25, 3770–3784. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Sun, N.; Yang, J.; Deng, Z.; Lan, J.; Qin, G.; He, H.; Deng, X.W.; Irish, V.F.; Chen, H.; et al. The transcription factors TCP4 and PIF3 antagonistically regulate organ-specific light induction of SAUR genes to modulate cotyledon opening during de-etiolation in Arabidopsis. Plant Cell 2019, 31, 1155–1170. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Wang, J.; Gao, Z.; Dong, J.; He, H.; Terzaghi, W.; Wei, N.; Deng, X.W.; Chen, H. Arabidopsis SAURs are critical for differential light regulation of the development of various organs. Proc. Natl. Acad. Sci. USA 2016, 113, 6071–6076. [Google Scholar] [CrossRef]
- Prisca, M.; Maarten, V.; Jan, V.D.; Bart, N.; Wouter, S.; Timo, H.; Barbara, D.C.; Bram, V.d.P. Blue and far-red light control flowering time of woodland strawberry (Fragaria vesca) distinctively via CONSTANS (CO) and FLOWERING LOCUS T1 (FT1) in the background of sunlight mimicking radiation. Environ. Exp. Bot. 2022, 198, 104866. [Google Scholar] [CrossRef]
- Sellaro, R.; Yanovsky, M.J.; Casal, J.J. Repression of shade-avoidance reactions by sunfleck induction of HY5 expression in Arabidopsis. Plant J. 2011, 68, 919–928. [Google Scholar] [CrossRef]
- Hersch, M.; Lorrain, S.; de Wit, M.; Trevisan, M.; Ljung, K.; Bergmann, S.; Fankhauser, C. Light intensity modulates the regulatory network of the shade avoidance response in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 6515–6520. [Google Scholar] [CrossRef]
- Heijde, M.; Ulm, R. UV-B photoreceptor-mediated signalling in plants. Trends Plant Sci. 2012, 17, 230–237. [Google Scholar] [CrossRef]
- Qian, M.; Rosenqvist, E.; Prinsen, E.; Pescheck, F.; Flygare, A.-M.; Kalbina, I.; Jansen, M.A.K.; Strid, Å. Downsizing in plants—UV light induces pronounced morphological changes in the absence of stress. Plant Physiol. 2021, 187, 378–395. [Google Scholar] [CrossRef]
- Qian, M.; Rosenqvist, E.; Flygare, A.-M.; Kalbina, I.; Teng, Y.; Jansen, M.A.K.; Strid, Å. UV-A light induces a robust and dwarfed phenotype in cucumber plants (Cucumis sativus L.) without affecting fruit yield. Sci. Hortic. 2020, 263, 109110. [Google Scholar] [CrossRef]
- Qian, M.; Kalbina, I.; Rosenqvist, E.; Jansen, M.A.K.; Teng, Y.; Strid, Å. UV regulates the expression of phenylpropanoid biosynthesis genes in cucumber (Cucumis sativus L.) in an organ and spectrum dependent manner. Photochem. Photobiol. Sci. 2019, 18, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Lario, L.D.; Ramirez-Parra, E.; Gutierrez, C.; Casati, P.; Spampinato, C.P. Regulation of plant MSH2 and MSH6 genes in the UV-B-induced DNA damage response. J. Exp. Bot. 2011, 62, 2925–2937. [Google Scholar] [CrossRef] [PubMed]
- Hideg, É.; Jansen, M.A.K.; Strid, Å. UV-B exposure, ROS, and stress: Inseparable companions or loosely linked associates? Trends Plant Sci. 2013, 18, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef]
- Zhang, X.; Abrahan, C.; Colquhoun, T.A.; Liu, C.-J. A proteolytic regulator controlling chalcone synthase stability and flavonoid biosynthesis in Arabidopsis. Plant Cell 2017, 29, 1157–1174. [Google Scholar] [CrossRef]
- Ferrer, J.L.; Jez, J.M.; Bowman, M.E.; Dixon, R.A.; Noel, J.P. Structure of chalcone synthase and the molecular basis of plant polyketide biosynthesis. Nat. Struct. Biol. 1999, 6, 775–784. [Google Scholar] [PubMed]
- Nivina, A.; Yuet, K.P.; Hsu, J.; Khosla, C. Evolution and diversity of assembly-line polyketide synthases. Chem. Rev. 2019, 119, 12524–12547. [Google Scholar] [CrossRef] [PubMed]
- Pandith, S.A.; Ramazan, S.; Khan, M.I.; Reshi, Z.A.; Shah, M.A. Chalcone synthases (CHSs): The symbolic type III polyketide synthases. Planta 2019, 251, 15. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Kim, S.Y.; Suh, D.-Y. Divergent evolution of the thiolase superfamily and chalcone synthase family. Mol. Phylogenet. Evol. 2008, 49, 691–701. [Google Scholar] [CrossRef]
- Han, Y.; Ding, T.; Su, B.; Jiang, H. Genome-wide identification, characterization and expression analysis of the chalcone synthase family in Maize. Int. J. Mol. Sci. 2016, 17, 161. [Google Scholar] [CrossRef]
- Austin, M.B.; Noel, J.P. The chalcone synthase superfamily of type III polyketide synthases. Nat. Prod. Rep. 2003, 20, 79–110. [Google Scholar] [CrossRef] [PubMed]
- Parage, C.; Tavares, R.; Réty, S.; Baltenweck-Guyot, R.; Poutaraud, A.; Renault, L.; Heintz, D.; Lugan, R.; Marais, G.A.B.; Aubourg, S.; et al. Structural, functional, and evolutionary analysis of the unusually large stilbene synthase gene family in grapevine. Plant Physiol. 2012, 160, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Jez, J.M.; Austin, M.B.; Ferrer, J.-L.; Bowman, M.E.; Schröder, J.; Noel, J.P. Structural control of polyketide formation in plant-specific polyketide synthases. Chem. Biol. 2000, 7, 919–930. [Google Scholar] [CrossRef]
- Lu, H.; Yang, M.; Liu, C.; Lu, P.; Cang, H.; Ma, L. Protein preparation, crystallization and preliminary X-ray analysis of Polygonum cuspidatum bifunctional chalcone synthase/benzalacetone synthase. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2013, 69, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Raeth, T.; Beuerle, T.; Beerhues, L. Biphenyl synthase, a novel type III polyketide synthase. Planta 2007, 225, 1495–1503. [Google Scholar] [CrossRef]
- Eckermann, S.; Schröder, G.; Schmidt, J.; Strack, D.; Edrada, R.A.; Helariutta, Y.; Elomaa, P.; Kotilainen, M.; Kilpeläinen, I.; Proksch, P.; et al. New pathway to polyketides in plants. Nature 1998, 396, 387–390. [Google Scholar] [CrossRef]
- Morita, H.; Kondo, S.; Oguro, S.; Noguchi, H.; Sugio, S.; Abe, I.; Kohno, T. Structural insight into chain-length control and product specificity of pentaketide chromone synthase from Aloe arborescens. Chem. Biol. 2007, 14, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Saslowsky, D.E.; Dana, C.D.; Winkel-Shirley, B. An allelic series for the chalcone synthase locus in Arabidopsis. Gene 2000, 255, 127–138. [Google Scholar] [CrossRef]
- Han, Y.; Cao, Y.; Jiang, H.; Ding, T. Genome-wide dissection of the chalcone synthase gene family in Oryza sativa. Mol. Breed. 2017, 37, 119. [Google Scholar] [CrossRef]
- Anguraj Vadivel, A.K.; Krysiak, K.; Tian, G.; Dhaubhadel, S. Genome-wide identification and localization of chalcone synthase family in soybean (Glycine max [L.] Merr.). BMC Plant Biol. 2018, 18, 325. [Google Scholar] [CrossRef]
- Eom, S.H.; Hyun, T.K. Genome-wide identification and transcriptional expression analysis of chalcone synthase in flax (Linum usitatissimum L.). Gene Rep. 2016, 5, 51–56. [Google Scholar] [CrossRef]
- Koduri, P.K.H.; Gordon, G.S.; Barker, E.I.; Colpitts, C.C.; Ashton, N.W.; Suh, D.Y. Genome-wide analysis of the chalcone synthase superfamily genes of Physcomitrella patens. Plant Mol. Biol. 2010, 72, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Koes, R.E.; Spelt, C.E.; Mol, J.N.M. The chalcone synthase multigene family of Petunia hybrida (V30): Differential, light-regulated expression during flower development and UV light induction. Plant Mol. Biol. 1989, 12, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, S.; Liu, X.; Shang, J.; Zhang, A.; Zhu, Z.; Zha, D. Chalcone synthase (CHS) family members analysis from eggplant (Solanum melongena L.) in the flavonoid biosynthetic pathway and expression patterns in response to heat stress. PLoS ONE 2020, 15, e0226537. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, U.; Valentine, W.J.; Christie, J.M.; Hays, J.; Jenkins, G.I.; Weisshaar, B. Identification of UV/blue light-response elements in the Arabidopsis thaliana chalcone synthase promoter using a homologous protoplast transient expression system. Plant Mol. Biol. 1998, 36, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Blanding, C.R.; Simmons, S.J.; Casati, P.; Walbot, V.; Stapleton, A.E. Coordinated regulation of maize genes during increasing exposure to ultraviolet radiation: Identification of ultraviolet-responsive genes, functional processes and associated potential promoter motifs. Plant Biotechnol. J. 2007, 5, 677–695. [Google Scholar] [CrossRef]
- Schulze-Lefert, P.; Becker-André, M.; Schulz, W.; Hahlbrock, K.; Dangl, J.L. Functional architecture of the light-responsive chalcone synthase promoter from parsley. Plant Cell 1989, 1, 707–714. [Google Scholar]
- Schulze-Lefert, P.; Dangl, J.L.; Becker-André, M.; Hahlbrock, K.; Schulz, W. Inducible in vivo DNA footprints define sequences necessary for UV light activation of the parsley chalcone synthase gene. EMBO J. 1989, 8, 651–656. [Google Scholar] [CrossRef]
- Rocholl, M.; Talke-Messerer, C.; Kaiser, T.; Batschauer, A. Unit 1 of the mustard chalcone synthase promoter is sufficient to mediate light responses from different photoreceptors. Plant Sci. 1994, 97, 189–198. [Google Scholar] [CrossRef]
- Kaiser, T.; Emmler, K.; Kretsch, T.; Weisshaar, B.; Schäfer, E.; Batschauer, A. Promoter elements of the mustard CHS1 gene are sufficient for light regulation in transgenic plants. Plant Mol. Biol. 1995, 28, 219–229. [Google Scholar] [CrossRef]
- Faktor, O.; Loake, G.; Dixon, R.A.; Lamb, C.J. The G-box and H-box in a 39 bp region of a French bean chalcone synthase promoter constitute a tissue-specific regulatory element. Plant J. 1997, 11, 1105–1113. [Google Scholar] [CrossRef]
- Mehrotra, R.C.; Dilcher, D.L.; Awasthi, N. A palaeogene Mangifera-like leaf fossil from India. Phytomorphology 1998, 48, 91–100. [Google Scholar]
- Sawangchote, P.; Grote, P.J.; Dilcher, D.L. Tertiary leaf fossils of Mangifera (Anacardiaceae) from Li Basin, Thailand as examples of the utility of leaf marginal venation characters. Am. J. Bot. 2009, 96, 2048–2061. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Luo, Y.; Huang, J.; Gao, S.; Zhu, G.; Dang, Z.; Gai, J.; Yang, M.; Zhu, M.; Zhang, H.; et al. The genome evolution and domestication of tropical fruit mango. Genome Biol. 2020, 21, 60. [Google Scholar] [CrossRef] [PubMed]
- Magadum, S.; Banerjee, U.; Murugan, P.; Gangapur, D.; Ravikesavan, R. Gene duplication as a major force in evolution. J. Genet. 2013, 92, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-h.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Pirie, A.; Mullins, M.G. Changes in anthocyanin and phenolic content of grapevine leaf and fruit tissue treated with sucrose, nitrate and abscisic acid. Plant Physiol. 1976, 58, 468–472. [Google Scholar] [CrossRef]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef]
- Panchy, N.; Lehti-Shiu, M.; Shiu, S.H. Evolution of gene duplication in plants. Plant Physiol. 2016, 171, 2294–2316. [Google Scholar] [CrossRef]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Burbulis, I.E.; Iacobucci, M.; Shirley, B.W. A null mutation in the first enzyme of flavonoid biosynthesis does not affect male fertility in Arabidopsis. Plant Cell 1996, 8, 1013–1025. [Google Scholar] [PubMed]
- Baumgarten, A.; Cannon, S.; Spangler, R.; May, G. Genome-level evolution of resistance genes in Arabidopsis thaliana. Genetics 2003, 165, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.; Peer, W.A.; Taiz, L. Regulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta 2000, 211, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Karanjalker, G.R.; Ravishankar, K.V.; Shivashankara, K.S.; Dinesh, M.R.; Roy, T.K.; Sudhakar Rao, D.V. A study on the expression of genes involved in carotenoids and anthocyanins during ripening in fruit peel of green, yellow, and red colored mango cultivars. Appl. Biochem. Biotechnol. 2018, 184, 140–154. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Sun, Y.; Allan, A.C.; Teng, Y.; Zhang, D. The red sport of ‘Zaosu’ pear and its red-striped pigmentation pattern are associated with demethylation of the PyMYB10 promoter. Phytochemistry 2014, 107, 16–23. [Google Scholar] [CrossRef]
- Qian, M.; Zhang, D.; Yue, X.Y.; Wang, S.K.; Li, X.G.; Teng, Y.W. Analysis of different pigmentation patterns in ‘Mantianhong’ (Pyrus pyrifolia Nakai) and ‘Cascade’ (Pyrus communis L.) under bagging treatment and postharvest UV-B/visible irradiation conditions. Sci. Hortic. 2013, 151, 75–82. [Google Scholar] [CrossRef]

| Gene Name | Gene ID | Chr ID | Length (aa) | pI | Mw (Da) |
|---|---|---|---|---|---|
| MiCHS1 | mango002786.t1 | chr 2 | 391 | 6.5 | 42,943.65 |
| MiCHS2 | mango002798.t1 | chr 2 | 391 | 6.53 | 42,647.35 |
| MiCHS3 | mango002799.t1 | chr 2 | 304 | 6.09 | 33,475.56 |
| MiCHS4 | mango002800.t1 | chr 2 | 391 | 6.18 | 42,700.39 |
| MiCHS5 | mango002801.t1 | chr 2 | 391 | 6.18 | 42,754.52 |
| MiCHS6 | mango002802.t1 | chr 2 | 390 | 5.97 | 42,756.57 |
| MiCHS7 | mango002803.t1 | chr 2 | 390 | 5.97 | 42,845.66 |
| MiCHS8 | mango002804.t1 | chr 2 | 391 | 7.15 | 42,882.67 |
| MiCHS9 | mango003116.t1 | chr 2 | 411 | 6.44 | 45,379.59 |
| MiCHS10 | mango007035.t1 | chr 4 | 411 | 6.38 | 46,069.54 |
| MiCHS11 | mango007036.t1 | chr 4 | 354 | 6.41 | 39,076.37 |
| MiCHS12 | mango007038.t1 | chr 4 | 391 | 6.12 | 42,672.46 |
| MiCHS13 | mango007039.t1 | chr 4 | 391 | 6.22 | 42,735.56 |
| MiCHS14 | mango007040.t1 | chr 4 | 367 | 6.72 | 39,992.42 |
| MiCHS15 | mango007048.t1 | chr 4 | 347 | 6.38 | 38,199.12 |
| MiCHS16 | mango018655.t1 | chr 11 | 398 | 6.03 | 43,295.06 |
| MiCHS17 | mango020263.t1 | chr 12 | 390 | 8.74 | 42,118.31 |
| MiCHS18 | mango021008.t1 | chr 12 | 390 | 6.67 | 42,755.25 |
| MiCHS19 | mango021009.t1 | chr 12 | 344 | 6.41 | 37,407.22 |
| MiCHS20 | mango023633.t1 | chr 14 | 391 | 6.13 | 42,833.43 |
| MiCHS21 | mango025592.t1 | chr 15 | 391 | 6.42 | 42,755.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, H.; Shi, B.; Zhu, W.; Zheng, B.; Zhou, K.; Qian, M.; Wu, H. Genome-Wide Identification, Characterization and Expression Analysis of Mango (Mangifera indica L.) chalcone synthase (CHS) Genes in Response to Light. Horticulturae 2022, 8, 968. https://doi.org/10.3390/horticulturae8100968
Hu H, Shi B, Zhu W, Zheng B, Zhou K, Qian M, Wu H. Genome-Wide Identification, Characterization and Expression Analysis of Mango (Mangifera indica L.) chalcone synthase (CHS) Genes in Response to Light. Horticulturae. 2022; 8(10):968. https://doi.org/10.3390/horticulturae8100968
Chicago/Turabian StyleHu, Haofeng, Bin Shi, Wencan Zhu, Bin Zheng, Kaibing Zhou, Minjie Qian, and Hongxia Wu. 2022. "Genome-Wide Identification, Characterization and Expression Analysis of Mango (Mangifera indica L.) chalcone synthase (CHS) Genes in Response to Light" Horticulturae 8, no. 10: 968. https://doi.org/10.3390/horticulturae8100968
APA StyleHu, H., Shi, B., Zhu, W., Zheng, B., Zhou, K., Qian, M., & Wu, H. (2022). Genome-Wide Identification, Characterization and Expression Analysis of Mango (Mangifera indica L.) chalcone synthase (CHS) Genes in Response to Light. Horticulturae, 8(10), 968. https://doi.org/10.3390/horticulturae8100968







