Abstract
With increasing nitrogen application in soil, the problem of nitrate accumulation in soil and vegetable bodies has become increasingly serious. In this study, Bacillus subtilis (B. subtilis) Strain11 was isolated and studied for its effects in combination with Molybdenum (Mo) on the growth of Chinese cabbage and soil nitrate content. The results showed that the strain effectively increased the growth, height, and moisture content of Chinese cabbage by 27% and 2.5%, respectively, compared to the control. Mo application alone reduced soil nitrate accumulation and increased root length, height, chlorophyll content, and moisture content of Chinese cabbage, with an optimal rate of 0.8–1.2 mg/kg soil. The application of Mo fertilizer in combination with the Bacillus strain was the most effective in increasing plant height and root length of Chinese cabbage, which were 1.49 times and 1.68 times that of the control, respectively. The proportion of dry matter, the average fresh weight, and the dry weight under this treatment were 9.01%, 5.83 g/plant, and 0.53 g/plant, respectively, higher than the rest. At harvest, the highest ammonium-nitrogen content in this treatment group was 8.56 mg/kg, and the soil nitrate reduction reached 40.68%. In conclusion, Mo fertilizer at a rate of 0.8 mg Mo/kg soil, in combination with B. subtilis Strain11, was recommended for the remediation of nitrate-contaminated soils.
1. Introduction
In recent years, excessive application of nitrogen fertilizers has increased soil nitrate pollution in China, which has a dangerous and negative impact on the ecological environment and human health [1]. However, the promotion of domestic fertilizers does not focus on trace-element fertilizers, and people are still willing to believe in the role of nitrogen, phosphorus, and potassium fertilizers. To solve the problems of production reduction and environmental pollution caused by excessive application of chemical fertilizers, microbial agents and microbial fertilizers are adopted as green environmental protection fertilizers. Microbial fertilizers can promote crop growth in various ways [2,3,4,5,6]. Bacillus subtilis (B. subtilis) is a typical beneficial bacterium used to make microbial agents. Numerous studies have found that the application of B. subtilis biofertilizer can reduce farmland soil nitrogen loss, improve nitrogen use efficiency and yield, reduce nitrification, increase denitrification [7], promote plant growth and disease control, and improve crop yield [8]. Molybdenum (Mo) is an essential nutrient for plants [9] and a component of nitrate reductase, which promotes the reduction of nitrate in plants [10,11]. Previous studies have shown that the appropriate application of Mo can improve the quality of vegetables [12]. Mo affects the structure and function of soil microorganisms by participating in the synthesis and metabolism of various Mo-containing enzymes [13]. The appropriate application of Mo can effectively increase the relative existence of beneficial microorganisms [14,15,16], and excessive application of Mo fertilizer inhibits its activity. Theoretically, by controlling the content of Mo in vegetables, plants can be used to increase the nitrate reduction rate, thereby reducing the nitrate content in soil [17]. However, few studies have explored the effects of molybdenum on nitrogen transformation in plant root-soil, and none has revealed the effects of interaction between molybdenum and microorganisms on plants and soil.
In this experiment, a B. subtilis strain was isolated and identified by 16S rDNA sequence analysis. Four-season Chinese cabbage was used as a model plant to investigate the effects of the strain on cabbage growth. In addition, the effect of Mo fertilizer on the growth of Chinese cabbage and the reduction of soil nitrate content was explored. Finally, the synergetic effects of B. subtilis strain and Mo fertilizer on the growth of Chinese cabbage and the reduction of soil nitrate were studied.
2. Materials and Methods
2.1. Isolation of B. subtilis Strain11
The test soils were collected from the experimental base of Shatou Town, Guangling District, Yangzhou City, Jiangsu Province (119°53′ E, 32°31′ N).
1 g of fresh soil was mixed with 9 mL of sterile water and shaken thoroughly. The soil solution was diluted sequentially with sterile 0.9% NaCl to an achieved concentration of 10−1–10−8. One hundred microliters of each dilution for inoculation on LB medium used spreading plate techniques. The plates were incubated at 28 °C for 2–3 days. The individual colony was picked and transferred till morphologically homogeneous single colonies were obtained. The purity of the cells was confirmed by microscopy.
2.1.1. Physiological Identification of Strains
The cellulose utilization, inorganic phosphorus dissolving, organic phosphorus decomposition, silicate utilization, as well as nitrogen fixation were tested according to standard detection procedures [18]. The colonies were transferred to the corresponding medium and inoculated at 28 °C for 2–6 days to observe the growth status and products.
The growth rate was studied through cell counting. The strain was inoculated into a liquid LB medium, and samples were taken every 3 h for cell counting under a microscope.
The siderophore-producing capacity was detected using the CAS method, according to Chen, S. [19].
2.1.2. Systematic Taxonomic Identification of Strains
The strain was phylogenetically identified. First, the 16S rRNA gene was amplified using the universal bacterial primers 27F (5′-AGTTTGATCMTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′). The reaction system was (25 μL): 10× Buffer 2.5 μL, dNTPs (2.5 mmol/L each) 1.0 μL; 27F (10 μg/mL) 0.5 μL; 1492R (10 μg/mL) 0.5 μL; Template (genomic DNA 20–50 ng/μL) 0.5 μL; Taq enzyme (5 U/μL) 0.2 μL; Ultrapure water 19.8 μL. The reaction conditions were as follows 94 °C 4 min; 30 cycles of 94 °C 45 s, 55 °C 45 s, 72 °C 1 min followed by a final extension at 72 °C for 10 min [20]. The PCR product was purified and sequenced (Shanghai Shenggong). The sequences were quality controlled before aligning to the GenBank database using the Blastn search tools. The Mega software (https://www.megasoftware.net/ (accessed on 12 November 2021) was used to generate the phylogenetic tree.
2.2. Experimental Design of the Growth and Development of Chinese Cabbage
2.2.1. Effects of Strain11 on the Growth of Chinese Cabbage
Chinese cabbage was grown in glass beakers filled with quartz sand, with Hogland solution as a nutrient supply. 1 mL bacterial solution (concentration of approximately 1 × 108 cells/mL) was added to each beaker. As a control, 1 mL of water instead of the bacterial solution was added. The beakers were covered with an Al foil to protect the roots from light. Three replicates were prepared for each treatment, and a total of 9 replicates were used.
The height of Chinese cabbage was measured every 4 days. The biomass was measured at harvest (20 days).
2.2.2. Effects of Mo Fertilizer on the Growth of Chinese Cabbage and Soil Nitrate
The test soil was collected from a strawberry plantation greenhouse of a farmer in the Hanjiang District, Yangzhou City, Jiangsu Province (119°36′ E, 32°34′ N). The basic physiochemical properties of the soil were determined using the method described in a soil analytical manual [21]. The physical and chemical properties of the soil are listed in Table 1.

Table 1.
Physicochemical properties of soil.
The fertilizer required for the experiment were nitrogen, phosphorus, and potassium (CH₄N₂O 50 g, KH₂PO 50 g, and K2SO4 25 g), Mg-Zn-Mn-B fertilizer (MgSO4 20 g, ZnSO₄ 2 g, MnSO₄ 1 g, and H3BO3 0.5 g), CaCl2 fertilizer (CaCl2 30 g), and EDTA·FeNa 5 g. They were prepared as complete fertilizer (500 mL) according to the calculation. Mo fertilizer was prepared using (NH₄)₂MoO₄. (NH₄)₂MoO₄ solution is calculated according to the needs of different treatments. The soil was dried and ground, and each pot contained 3 kg of soil. Ten milliliters of each fertilizer solution were added and fully mixed with the soil after loading the pot (plastic container, size 50 × 40 × 20 cm). One hundred Chinese cabbage seeds (100–200 g) were sown in each pot. During the experiment, each pot was watered with 50 mL water every day to keep the soil moist.
Six treatments were prepared, including T1 (no fertilization), T2 (complete fertilizer), T3 (complete fertilizer + 0.4 mg/kg Mo fertilizer), T4 (complete fertilizer + 0.8 mg/kg Mo fertilizer), T5 (complete fertilizer + 1.2 mg/kg Mo fertilizer), T6 (complete fertilizer + 1.6 mg/kg Mo fertilizer).
This experiment was set up in 3 parallels and a total of 3 replicates.
2.2.3. Synergetic Effect of Mo Fertilizer with B. subtilis Strain11 on the Growth of Chinese Cabbage and Soil Nitrate Content
A mixture of KNO3, NaNO3, Ca(NO3)2, and Mg(NO3)2 (1/4 each) was added to the soil to obtain a final soil nitrate concentration of 1000 mg N/kg soil (contaminated soil). A mixture of CaHPO₄ and KH₂PO (1/2 each) was added to the soil to obtain 100 mg P/kg soil. MgSO4 was added to the soil to obtain a soil concentration of 50 mg S/kg soil. Na2B4O7 was added to the soil to obtain 2 mg B/kg soil. ZnSO₄ was added to the soil to obtain 3 mg of Zn/kg soil. (NH₄)₂MoO₄ was added to the soil to obtain a soil concentration of 0.8 mg Mo/kg soil.
Five treatments were prepared, including M1 (original soil), M2 (contaminated soil), M3 (contaminated soil + Mo fertilizer, 0.8 mg Mo/kg soil), M4 (contaminated soil + B. subtilis Strain11, 5 mL/kg soil, cell concentration was 108 cells/mL), M5 (contaminated soil + Mo, 0.8 mg Mo/kg + B. subtilis Strain11, 5 mL/kg).
This experiment was set up in 3 parallels and a total of 3 replicates.
2.3. Sample Analyses
Soil samples (0–20 cm) were collected using a soil auger (10 mm in diameter). Soil samples from five points in each pot were randomly taken and mixed. The plant roots and debris were removed. The soils were air-dried, ground and sieved (20 mesh aperture) before the determination of physiochemical properties. Sampling was performed every 10 days from the beginning of the experiment.
After removing the marginal effect, the whole Chinese cabbage plant was collected. Plant height and root length were measured, leaf chlorophyll was determined using a chlorophyll analyzer (Konica Minolta), and soil moisture content was determined by drying method; soil pH was determined using the pH meter, soil electrical conductivity (EC) was determined using an EC analyzer following the standard procedure, and nitrate content was determined using salicylic acid [21].
2.4. Data Analysis
All experimental data were organized and plotted using Microsoft Excel 2010. SPSS 22.0 software was used for one-way ANOVA analysis of differences between different treatments, significance tests were performed by LSD method (p < 0.05), and all resulting data were expressed in the form of mean ± standard deviation.
3. Results
3.1. Characterization of B. subtilis Strain11
The isolated strains appeared rough, opaque, dilated, stained white or slightly yellowish under the microscope. They could utilize cellulose, inorganic phosphorus, organophosphorus, nitrogen-fixing, and non-decomposable silicates. The above results were consistent with the physiological and biochemical characteristics of Bacillus spp. The sequencing results were compared to the homology of the sequences using the Blastn search database in GenBank, which revealed that the strain was B. subtilis (Figure 1a). The closest species in systematic taxonomy is B. subtilis.

Figure 1.
Phylogenetic tree ((a), the word marked in red is Strain11), CAS test results of siderophore production ability (b), and growth curve (c) of Strain11.
Strain11 was inoculated onto the CAS detection medium, and after 5 days of incubation, a distinct orange-yellow halo appeared around the strain (Figure 1b), indicating that the strain has the ability to produce iron chelators that took iron from the CAS complex, which turned orange.
The strain had a lag phase of 20 h when cell proliferation was not obvious. The 20 to 33 h was the logarithmic growth period, the growth rate was 6 × 107 cells/mL/h, and the maximum growth density was 7.8 × 108 cells/mL. The number of the strain decreased steadily from 35 to 43 h after a short stationary phase. The growth rate tended to stabilize after 43 h, and the cell density was 4.1 × 108 cells/mL (Figure 1c).
3.2. Effects of Different Treatments on the Growth and Development of Chinese Cabbage
3.2.1. Effects of B. subtilis Strain11 on the Growth and Development of Chinese Cabbage
The height of Chinese cabbage was measured every 4 days. For better comparison, increasing height (comparing the present height to the height 4 days prior) was used to represent the growth rate of Chinese cabbage. The height of Chinese cabbage treated with the Strain11 was higher than that of the control, and with an increase in culture time, the growth height gradually increased. After 16 days of cultivation, the cabbage in bacterial treatment was 27% higher than that of the control (Figure 2a).
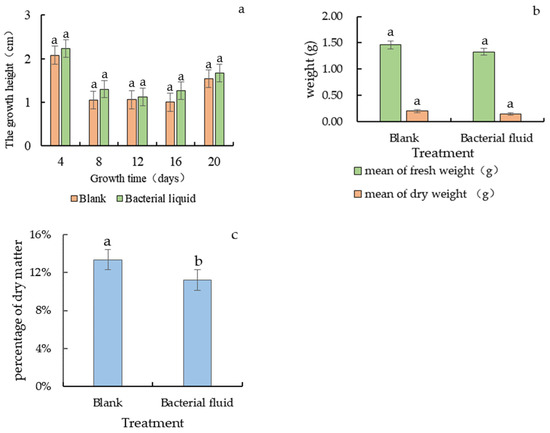
Figure 2.
Plant height of Chinese cabbage at different growth times (a), biomass (b) and percentage of dry matter (c) of Chinese cabbage at harvest time (20 days). Different letters in the same column indicate statistically significant differences among the samples based on one-way ANOVA (LSD, p < 0.05).
Figure 2b shows the average weight and dry matter ratio of cabbage after 20 days of cultivation. Inoculation with B. subtilis Strain11 reduced the amount of dry matter in cabbage plants and increased the water content. However, this reduction and increase were not statistically significant.
The proportion of dry matter (Figure 2c) of Chinese cabbage reached 13.39% in the control, significantly higher than that of bacterial treatment (11.23%).
3.2.2. Effect of Mo Fertilizer Addition on the Growth Index of Chinese Cabbage
The root length of Chinese cabbage in different treatments varied (Table 2) Significantly. With the increase of Mo fertilizer application, the root length increased first and then decreased, following a parabolic curve. The highest root length was observed in T4 (0.8 mg Mo/kg soil) or T5 (1.2 mg Mo/kg soil) at all sample times. The promotion effect tended to decrease when applying Mo fertilizer at a rate higher than 1.2 mg Mo/kg.

Table 2.
Root length (cm) of Chinese cabbage at different treatments (see Section 2.2.2) and periods *.
Table 3 shows that with the increase of Mo fertilizer application, the aboveground height of Chinese cabbage showed a parabolic trend and reached the highest in treatment T5 (1.2 mg Mo/kg soil). When the amount of Mo continued to increase, the ground height of Chinese cabbage decreased. T6 was even lower than T1 (unfertilized contaminated soil) at 30 days. At harvest, the maximum height of Chinese cabbage in treatment T5 was 8.64 cm.

Table 3.
Above-ground height (cm) of Chinese cabbage at different treatments (see Section 2.2.2) and periods *.
3.2.3. Synergetic Effect of Mo Fertilizer and B. subtilis Strain on the Growth of Chinese Cabbage
As shown in Figure 3a, at harvest, the plant height difference between each group of treatments was significant. The plant height of Chinese cabbage in the original soil M1 was the shortest, only 8 cm, and the highest was the contaminated soil treated with M5 Mo fertilizer and bacterial solution, up to 11.97 cm, which was 1.49 times that of M1 original treatment. The cabbage height in treatment with Mo or B. subtilis strain only was significantly lower than that of the M5 treatment.
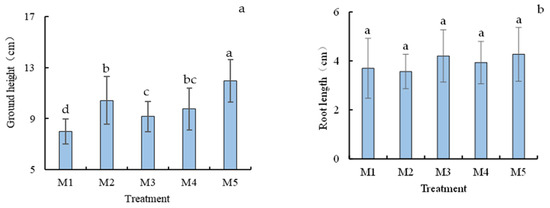
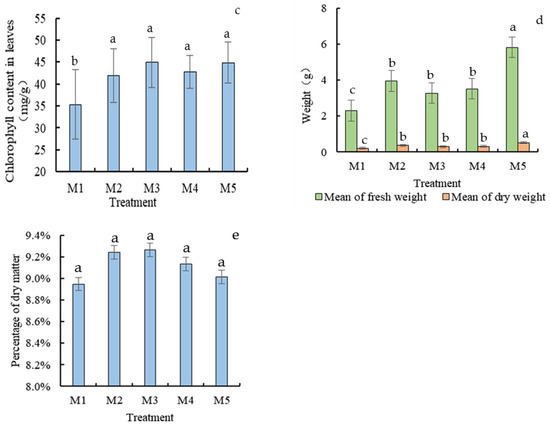
Figure 3.
Ground height (a), root length (b), Chlorophyll content (c), biomass (d) and percentage of dry matter (e) of Chinese cabbage under different treatments at harvest (60 days). Different letters in the same column indicate statistically significant differences among the samples based on one-way ANOVA (LSD, p < 0.05).
Similarly, the highest root length was observed in treatment with M5 at harvest, with an average root length of 4.27 cm. The lowest root length, 3.57 cm, was found in M2. However, these differences were not statistically significant (Figure 3b).
As to the chlorophyll content of cabbage (Figure 3c), the lowest (35.33 mg/g) was detected in the M1 treatment, significantly lower than the rest (41–45 mg/g). Among them, the chlorophyll content of cabbage treated with only Mo fertilizer in M3 and Mo fertilizer in M5 combined with a bacterial solution was higher, which was 44.93 mg/g and 44.84 mg/g, respectively.
Figure 3d and 3e show that M2-contaminated soil significantly increased the proportion of dry matter in Chinese cabbage, which was not conducive to water accumulation. The lowest dry matter ratio of Chinese cabbage, observed in M1, was 8.95%, in which the average fresh weight and dry weight were 2.29 g/plant and 0.21 g/plant, respectively. The dry matter proportion in M5 (Mo fertilizer combined with bacterial treatment) was 9.01%, and the fresh weight and dry weight were 5.83 g/plant and 0.53 g/plant, respectively. The weights of cabbage plants in the treatments of M3 and M4 were similar and significantly lower than that of the M5 treatment.
3.3. Effects of Different Treatments on Soil Properties
3.3.1. Effect of Mo Fertilizer on Soil Properties
Throughout the whole experiment, the soil pH was between 6.6–7.0, and EC was between 0.26–0.89 mS/cm (Figure 4a,b). According to the line chart in Figure 4, soil pH was slightly different in the early stages. In the later stage, with the increase in planting time, the two indices of each treatment tended to be unified, similar to EC. With cultivation time, the soil pH gradually stabilized at around 6.87, and the EC gradually stabilized at around 0.5 mS/cm. Only the pH and EC of treatment T1 were different from those of the other treatments, and the variation in each period was large without obvious regularity. Thus, the use of Mo fertilizer can improve and stabilize soil properties.
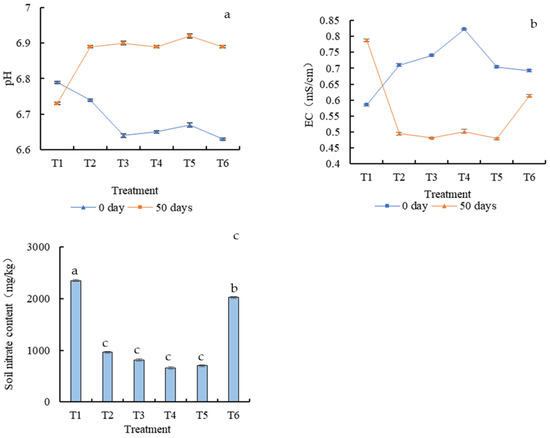
Figure 4.
Soil pH (a) and EC (b) change at different growth times. Soil nitrate content at 50 days (c). Different letters in the same column indicate statistically significant differences among the samples based on one-way ANOVA (LSD, p < 0.05).
With the application of Mo fertilizer, soil nitrate gradually decreased (Figure 4c). The lowest soil nitrate content (659.72 mg/kg) was detected in T4, while in T1, it was 2349.89 mg/kg. When the Mo content was further increased, soil nitrate increased again, which was 2025.58 mg/kg in T6, where the Mo application rate was 1.6 mg/kg, significantly higher than other fertilizer treatments. Excessive Mo fertilizer application might not be helpful in reducing soil nitrate pollution.
3.3.2. Effects of Mo Fertilizer with B. subtilis Strain on Soil Properties
The original pH was in the range of 7.1–8.0. As shown in Figure 5a, the pH of the original treatment soil was higher than that of the others at different sampling times. The pH of the treatment with Mo fertilizer combined with a bacterial solution of (M5) increased gradually during the cultivation period and became the second highest at harvest, reaching 7.28, indicating that the combined application of Mo fertilizers and Bacillus strain can slowly restore soil pH.
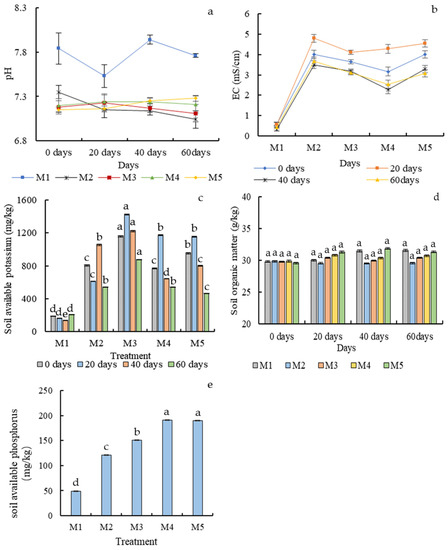
Figure 5.
Changes in soil pH (a), EC (b), available potassium (c), and organic matter (d) in different treatments at different growth times. Changes in soil available phosphorus (e) in different treatments at harvest (60 days). Different letters in the same column indicate statistically significant differences among the samples based on one-way ANOVA (LSD, p < 0.05).
Comparing the soil EC values at sowing (0 days) and harvest (60 days), in contaminated soil, each group of EC decreased differently, in which M2 contaminated soil without fertilization decreased the least (14.79%), and the EC of contaminated soil treated with M5 Mo fertilizer combined with a microbial agent decreased the most, and the salt content changed the most, so the treatment had the best desalination effect (Figure 5b).
The available potassium content was significantly lower in M1 than in the rest. At day 60, the soil’s available potassium content was 463.1 mg/kg in the treatment of M5 (Figure 5c), indicating that the longer the planting time, the more effective the treatment could reduce the salt content of the soil.
In each period, the soil organic matter content of the original treatment soil M1 and M5 treated with the Mo fertilizer combined with the bacterial solution was at a high level, and the soil organic matter content of M2–M5 showed a gradual increase (Figure 5d). Among the various treatments of contaminated soil, the soil organic matter content of the Mo fertilizer combined with bacterial liquid M5 treatment was always the highest (Figure 5d), indicating that the combined application of the two fertilizers improved soil fertility, but this improvement was not statistically significant.
The application of B. subtilis greatly improved the available phosphorus content of the soil. This was consistent with the physiochemical test that Strain11 could solubilize phosphorus. The available phosphorus content in M3 was significantly lower than those in M4 and M5. The soil available phosphorus content of M4 and M5 was 191.13 mg/kg and 190 mg/kg, respectively, which was 1.58 times that of contaminated soil.
Nitrogen transformation in the soil is complex and diverse. As shown in Figure 6a, at the beginning of the experiment, the soil total N of M1 was lower than other treatments. However, at 60 days, due to the effect of fertilizer, the total N of other treatments decreased, but the M1 became the highest. M4 was the lowest. The highest ammonium N content was detected in the M5 treatment, reaching 8.56 mg/kg (60 days). Under the condition that the ammonium nitrogen in the harvest period (60 days) of each treatment group decreased significantly compared with the sowing period (0 days), the decrease in the M5 treatment was only 5.71%. In the determination of soil nitrate nitrogen, it was found that in each treatment of contaminated soil, compared with the value of soil nitrate nitrogen at sowing time (0 days) and harvest time (60 days), each group of nitrate nitrogen had different degrees of reduction. Among them, the smallest reduction was observed in the contaminated soil without fertilization in M2 (27.69%), relying only on the role of Chinese cabbage itself, and the highest decrease was observed in the contaminated soil with M4 alone (50.81%). At the same time, the nitrate reduction in treatment M5 was also more prominent and was the second highest in each group (40.68%), which was in line with the change rule of high nitrate nitrogen conversion rate after denitrification.
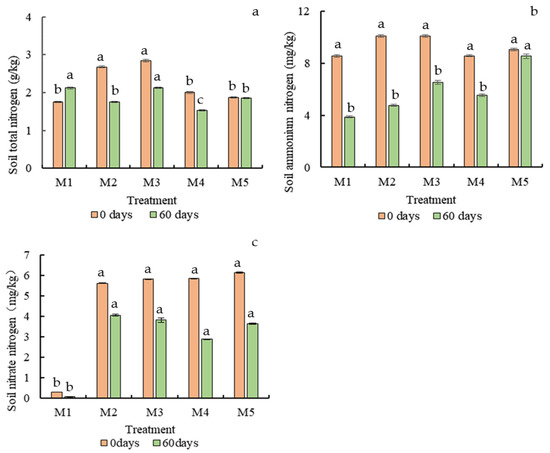
Figure 6.
Soil total nitrogen (a) (g/kg), ammonium nitrogen (b) (mg/kg) and nitrate nitrogen (c) (mg/kg) changes at different growth times. Different letters in the same column indicate statistically significant differences among the samples based on one-way ANOVA (LSD, p < 0.05).
4. Discussion
4.1. Bacterial Isolation and Identification and Its Effect on Chinese Cabbage
In this study, a strain was isolated and purified from straw-returning soils. Based on identification and sequencing, it was identified as B. subtilis. According to the number, the test strain was B. subtilis Strain11. The characteristics of the strain corresponded to those described for B. subtilis in the Common Bacterial System Identification Manual. According to the above physiological and biochemical characteristics of Strain11, it was inferred that it might promote the absorption and utilization of nutrients by regulating soil nutrients. In fact, previous studies have shown that B. subtilis has excellent effects in promoting plant growth, controlling soil-borne diseases, and increasing production and efficiency. Xu, W. [22], Zhou, D. [23], Sood, G. [24], Xu, W. [25] and other studies have found that the B. subtilis can be used as a potential biological control agent and growth promoter for cucumber Fusarium wilt. It not only promotes the growth and development of pepper, enhances disease resistance, and alleviates environmental problems such as drought encountered by maize but also biologically controls crown rot and promotes wheat growth. Previous studies have shown that B. subtilis is widely considered an environmentally friendly and effective way to promote crop growth and improve quality. This is consistent with our findings.
In this study, the effect of B. subtilis Strain11 on the growth and development of Chinese cabbage was analyzed using ecological experiments. The addition of the extracted B. subtilis solution had a great influence on the growth height of the plant, and the growth height gradually became obvious with an increase in growth time. The authors speculated that this was due to a lag in the effect of microbial agents on plants. Its mode of action is the production of its products and the regulation of growth-promoting hormones, so the effect is not immediate. Previous studies have shown that B. subtilis has the potential to increase biological nitrogen fixation, fungal resistance, and plant growth [26,27]. The effect of B. subtilis Strain11 on Chinese cabbage was consistent with previous research conclusions.
4.2. Effects of Mo Fertilizer Application on the Growth of Chinese Cabbage and Soil Nitrate Content
This study explored the effects of different Mo concentrations on root length and height. The distribution of plant roots and heights in soil is influenced by many factors, such as the physical and chemical properties of the soil [28]. In this study, the changes in root length, height, and chlorophyll content of Chinese cabbage were similar to those reported in previous studies [29,30]. However, it is clear that excessive Mo plays a relatively weak role in improving the growth and development of Chinese cabbage. The changes in soil pH and EC values showed that the soil acidity decreased, the nitrate state of the soil decreased, the electrical conductivity decreased, and the soil condition was healthier after Chinese cabbage was planted with fertilizer. The effect of 0.8 mg/kg Mo was the best. Different concentrations of Mo had different effects on the nitrate nitrogen content of the Chinese cabbage soil. Among them, the 0–1.2 mg/kg Mo application gradient treatment significantly reduced the nitrate nitrogen content in Chinese cabbage soil, and the reduction effect was the most obvious at 50 d. In 1.2–1.6 mg/kg Mo gradient treatment, nitrate content in Chinese cabbage soil increased with increasing Mo content. This is because Mo is a trace element; excessive Mo does not promote the growth and development of Chinese cabbage and cannot promote soil nitrate reduction.
4.3. Effects of Mo Fertilizer Combined with Bacterial Liquid on Chinese Cabbage Growth and Soil Nitrate Content
In the study of Mo fertilizer with bacterial liquid, through the determination of the indicators of Chinese cabbage, especially plant height and root length, it was found that even in the case of contaminated soil, the two kinds of fertilizers improved plant height and root conditions compared to the normal environment. In each treatment group of contaminated soil, the dry matter proportion of the Mo fertilizer with the bacteria group was closest to that of the original soil and closer to the moisture content of Chinese cabbage planted in normal soil. Few studies have shown that Mo fertilizer combined with B. subtilis has a growth-promoting effect on plants, but Ma, X. [31] showed that Mo combined with methylotrophic Bacillus liquid had a growth-promoting effect on melon, which is similar to the conclusion of this study.
With regard to the physical and chemical properties of soil, Mo fertilizer combined with bacterial liquid increased the pH and EC values of soil, which was unbalanced due to nitrate pollution, making it gradually close to normal soil. Soil organic matter also showed an increasing trend, indicating that Mo fertilizer combined with bacterial liquid stabilized soil properties and increased soil nutrients. Due to the phosphate-solubilizing effect of B. subtilis, the application of Strain11 effectively increased the available phosphorus content in soil. Determination of soil ammonium nitrogen found that the ammonium nitrogen content of the Mo fertilizer treatment group was the highest at harvest while reducing the minimum. The soil nitrate nitrogen content also varied with different treatments, and the largest decrease was observed in the contaminated soil of the single application group. The decrease in nitrate nitrogen in the combined application group was also more prominent, second only to that in the best single application group. It can be speculated that Mo fertilizer combined with bacterial liquid treatment can improve the conversion efficiency of nitrate-nitrogen to ammonium-nitrogen in soil. There are few studies on the effects of Mo fertilizer combined with B. subtilis on plant growth and soil improvement, and it is not clear whether the two have mutual promotion effects. However, from the perspective that the two fertilizers can be applied in combination and play a positive role, the conclusion of this study is reasonable and can be used as a reference for others.
5. Conclusions
Overall, B. subtilis effectively promoted the growth and development of Chinese cabbage, Mo fertilizer alone in the range of 0.8–1.2 mg/kg promoted the growth of Chinese cabbage, and soil nitrate content could be optimized. Mo fertilizer combined with B. subtilis was more effective than Mo fertilizer alone. It improved the soil properties, reduced the soil nitrate content, and had an excellent growth-promoting effect on Chinese cabbage. Therefore, it can be recommended for use in facility soils with severe nitrate pollution. This study provides a scientific reference for the application of soil B. subtilis and Mo fertilizers, lays the foundation for the development of bacterial fertilizers, and provides data support for subsequent research.
Author Contributions
Conceptualization, J.W. and Y.M.; methodology, J.W. and Y.M.; software, Y.M., S.C. and J.W.; validation, Y.M. and J.W.; formal analysis, Y.M. and S.C.; investigation, Y.M.; resources, Y.M.; data curation, Y.M. and S.C.; writing—original draft preparation, J.W. and Y.M.; writing—review and editing, J.W. and Y.M.; visualization, Y.M.; supervision, S.Z., J.H., S.C. and J.W.; project administration, J.W.; funding acquisition, J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Fund for Jiangsu Agricultural Industry Project, grant number 201-SJ-039-08-12.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bai, X.; Jiang, Y.; Miao, H.; Xue, S.; Zhou, J. Intensive vegetable production results in high nitrate accumulation in deep soil profiles in China. Environ. Pollut. 2021, 287, 117598. [Google Scholar] [CrossRef]
- Tao, C.; Li, R.; Xiong, W.; Shen, Z.; Kowalchuk, G.A. Bio-organic fertilizers stimulate indigenous soil Pseudomonas populations to enhance plant disease suppression. Microbiome 2020, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Bharucha, U.; Patel, K.; Trivedi, U.B. Optimization of Indole Acetic Acid Production by Pseudomonas putida UB1 and its effect as plant growth-promoting rhizobacteria on mustard (Brassica nigra). Agric. Res. 2013, 2, 215–221. [Google Scholar] [CrossRef]
- Buensanteai, N.; Yuen, G.Y.; Prathuangwong, S. The biocontrol bacterium Bacillus amyloliquefaciens KPS46 produces auxin, surfactin and extracellular proteins for enhanced growth of soybean plant. J. Agric. Sci. Technol. 2008, 41, 101–116. [Google Scholar]
- Rachel, B.J.; Stefan, R.; Gayathri, I.; John, L.; Dana, P.; Emily, R.; Sowmyalakshmi, S.; Donald, L.S. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Gu, L.; Bao, L.; Zhang, S.; Zhuang, X. Application of biofertilizer containing Bacillus subtilis reduced the nitrogen loss in agricultural soil. Soil Biol. Biochem. 2020, 148, 107911. [Google Scholar] [CrossRef]
- Tao, S.; Wu, Z.; Wei, M.; Liu, X.; He, Y.; Ye, B.C. Bacillus subtilis SL-13 biochar formulation promotes pepper plant growth and soil improvement. Can. J. Microbiol. 2019, 65, 333–342. [Google Scholar] [CrossRef]
- Kaiser, B.N.; Gridley, K.L.; Brady, J.N.; Phillips, T.; Tyerman, S.D. The role of molybdenum in agricultural plant production. Ann. Bot. 2005, 96, 745–754. [Google Scholar] [CrossRef]
- Jean, M.E.; Phalyvong, K.; Forest-Drolet, J.; Bellenger, J.P. Molybdenum and phosphorus limitation of a symbiotic nitrogen fixation in forests of Eastern Canada: Influence of vegetative cover and seasonal variability. Soil Biol. Biochem. 2013, 67, 140–146. [Google Scholar] [CrossRef]
- Kovacs, B.; Puskas-Preszner, A.; Huzsvai, L.; Levai, L.; Bodi, E. Effect of molybdenum treatment on molybdenum concentration and nitrate reduction in maize seedlings. Plant Physiol. Biochem. 2015, 96, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, Y.J.; Wen, D.; Zhao, P.H.; Li, F.; Li, L.; Du, R.Y.; Shi, H.Z.; Deng, T.H.; Du, Y.Q. Biochar-based molybdenum slow-release fertilizer enhances nitrogen assimilation in Chinese flowering cabbage (Brassica parachinensis). Chemosphere 2022, 303, 134663. [Google Scholar] [CrossRef] [PubMed]
- Ting, S.; Yue, P.; Wang, Z.Y.; Wang, P.L.; Gen, D.X. The effects of molybdenum and boron on the rhizosphere microorganisms and soil enzyme activities of soybean. Acta Physiol. Plant. 2013, 35, 763–770. [Google Scholar]
- Min, Y.; Xiao, H.C. Influences of molybdenum on nitrate reductase, glutamine synthetase and nitrogen accumulation and utilization in Mo-efficient and Mo-inefficient winter wheat cultivars. Agric. Sci. China 2010, 9, 355–361. [Google Scholar]
- Woo, J.S.; Stella, A.; Qiong, W.; Dieter, M.T.; Ryan, P.C.; Ye, D.; Jorge, L.M.R.; Samuel, G.K.A.; James, W.J.; Jizhong, Z.; et al. Tropical agricultural land management influences on soil microbial communities through its effect on soil organic carbon. Soil Biol. Biochem. 2013, 65, 33–38. [Google Scholar]
- Stanton, D.E.; Batterman, S.A.; Fischer, J.; Hedin, L.O. Rapid nitrogen fixation by canopy microbiome in tropical forest determined by both phosphorus and molybdenum. Ecology 2019, 100, e02795. [Google Scholar] [CrossRef]
- Wen, X.; Hu, C.; Sun, X.; Zhao, X.; Tan, Q. Research on the nitrogen transformation in rhizosphere of winter wheat (Triticum aestivum) under molybdenum addition. Environ. Sci. Pollut. Res. 2018, 26, 2363–2374. [Google Scholar] [CrossRef]
- Dong, X.; Cai, M. Common Bacterial System Identification Manual, 1st ed.; China Science Publishing & Media Ltd. (CSPM): Beijing, China, 2001; pp. 349–398. [Google Scholar]
- Chen, S.X. Analysis of the Biosynthesis Conditions and Characteristics of Siderophores from Pseudomonas sp. SPF-1. Master Degree Thesis, Wuhan University, Wuhan, China, 2005. [Google Scholar]
- Khalifa, A.; Aldayel, M. Isolation and characterisation of the agarolytic bacterium Pseudoalteromonas ruthenica. Open Life Sci. 2019, 14, 588–594. [Google Scholar] [CrossRef]
- Bao, S. Agricultural Chemical Analysis of Soil; China Agriculture Press: Beijing, China, 2000; pp. 25–114. [Google Scholar]
- Xu, W.; Yang, Q.; Yang, F.; Xie, X.; Goodwin, P.H.; Deng, X.; Tian, B.; Yang, L. Evaluation and genome analysis of Bacillus subtilis YB-04 as a potential biocontrol agent against Fusarium wilt and growth promotion agent of Cucumber. Front. Microbiol. 2022, 13, 885430. [Google Scholar] [CrossRef]
- Zhou, D.M.; Wang, K.P.; Liu, H.X.; Gu, C.; Guo, J.H. Field evaluation of different application methods of the mixture of Bacillus cereus strain AR156 and Bacillus subtilis strain SM21 on pepper growth and disease resistance. Biocontrol Sci. Technol. 2014, 24, 1451–1468. [Google Scholar] [CrossRef]
- Sood, G.; Kaushal, R.; Sharma, M. Alleviation of drought stress in maize (Zea mays L.) by using endogenous endophyte Bacillus subtilis in North West Himalayas. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2020, 70, 361–370. [Google Scholar] [CrossRef]
- Xu, W.; Yang, Q.; Xie, X.; Goodwin, P.H.; Deng, X.; Zhang, J.; Sun, R.; Wang, Q.; Xia, M.; Wu, C.; et al. Genomic and phenotypic insights into the potential of Bacillus subtilis YB-15 isolated from rhizosphere to biocontrol against crown rot and promote growth of wheat. Biology 2022, 11, 778. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, S.; Modin, O.; Roshanzamir, F.; Neissi, A.; Mijakovic, I. Co-culturing Bacillus subtilis and wastewater microbial community in a bio- electrochemical system enhances denitrification and butyrate formation. Chem. Eng. J. 2020, 397, 125437. [Google Scholar] [CrossRef]
- Kim, Y.K.; Hong, S.J.; Shim, C.K.; Kim, M.J.; Choi, E.J.; Lee, M.H.; Park, J.H.; Han, E.J.; An, N.H.; Jee, H.J. Functional analysis of Bacillus subtilis isolates and biological control of red pepper powdery mildew using Bacillus subtilis R2-1. Chem. Eur. J. 2012, 20, 9930–9939. [Google Scholar]
- Drescher, G.L.; Silva, L.S.D.; Sarfaraz, Q.; Roberts, T.L.; Nicoloso, F.T.; Raíssa, S.; Anderson, C.R.M. Available nitrogen in paddy soils depth: Influence on rice root morphology and plant nutrition. J. Soil Sci. Plant Nutr. 2020, 20, 1029–1041. [Google Scholar] [CrossRef]
- Imran, M.; Hu, C.; Hussain, S.; Rana, M.S.; Sun, X. Mo-induced effects on photosynthetic efficacy of winter wheat (Triticum aestivum L.) under different nitrogen sources are associated with nitrogen assimilation. Plant Physiol. Biochem. 2019, 141, 154–163. [Google Scholar] [CrossRef]
- Warner, R.L.; Kleinhofs, A. Genetics and molecular biology of nitrate metabolism in high plants. Physiol. Plant. 1992, 85, 245–252. [Google Scholar] [CrossRef]
- Ma, X. Effect of Molybdenum and Methylotrophic bacillus Agent on Yield and Quality of Muskmelon. Master Degree Thesis, Northwest A&F University, Xi’an, China, 2021. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).