New Insights into the Role of Alternating Temperatures and Cyanide in the ROS-Mediated Cardoon Seed Dormancy Termination
Abstract
1. Introduction
2. Materials and Methods
2.1. Achenes Collection
2.2. General Procedures for Germination Tests
2.3. Effect of Cyanide on Germination
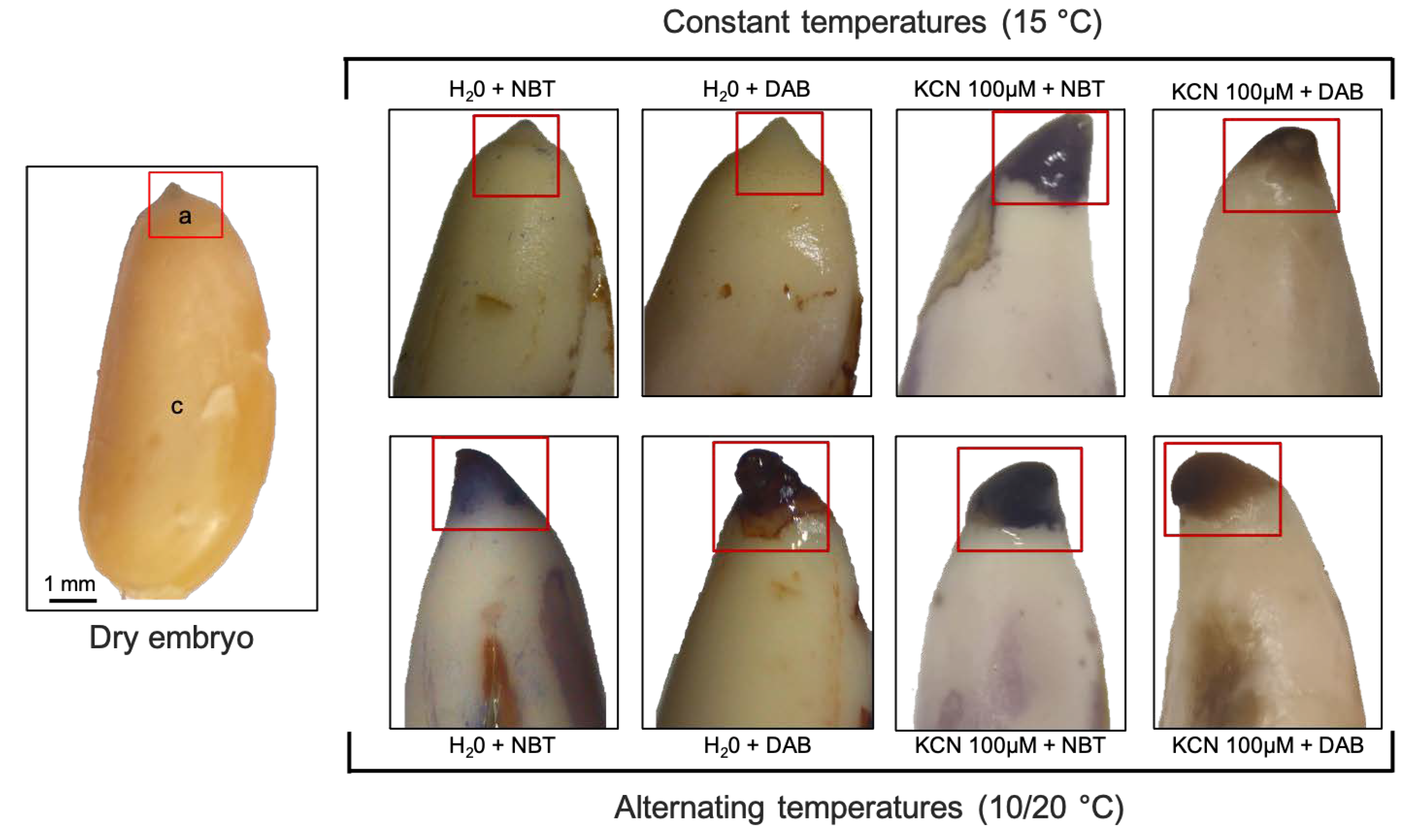
2.4. Localization O2 and H2O2
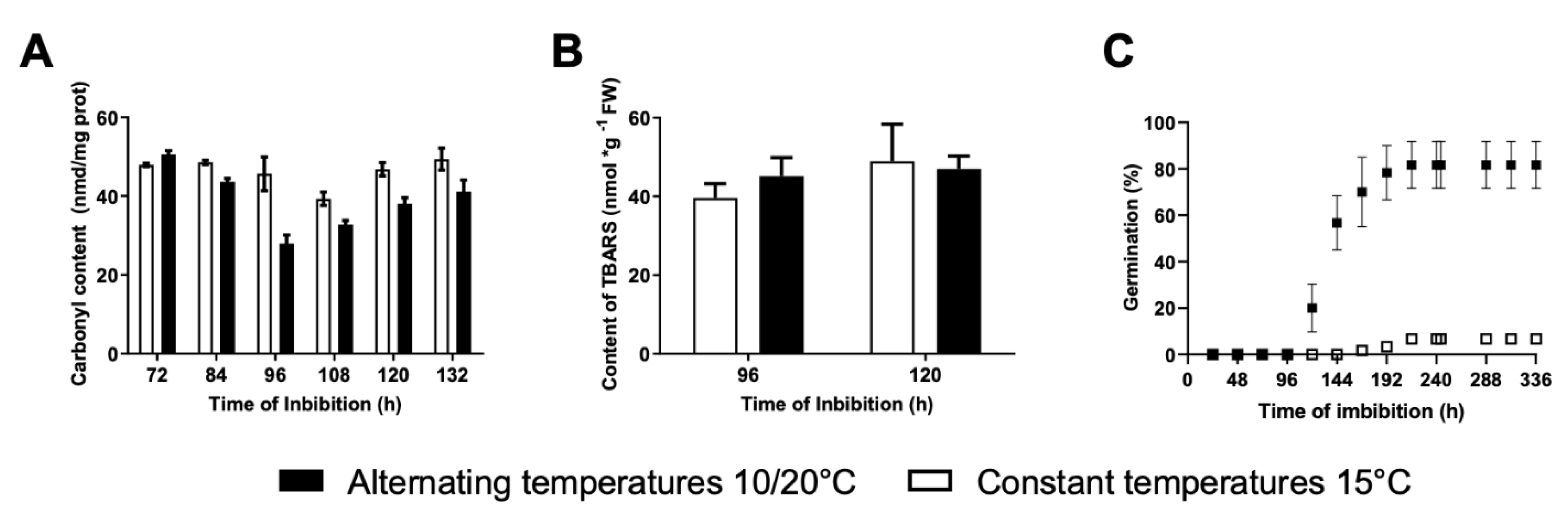
2.5. Determination of Carbonyl Content in Proteins
2.6. Determination of Thiobarbituric Acid Reactive Substances (TBARS)
2.7. Transcriptome Analysis
3. Results
3.1. Cyanide Treatment
3.2. Comparison of ROS Localization in Embryos Isolated from Seeds Exposed to Cyanide or Alternating Temperatures
3.3. Effects of Alternating Temperatures on Protein and Lipid Oxidation
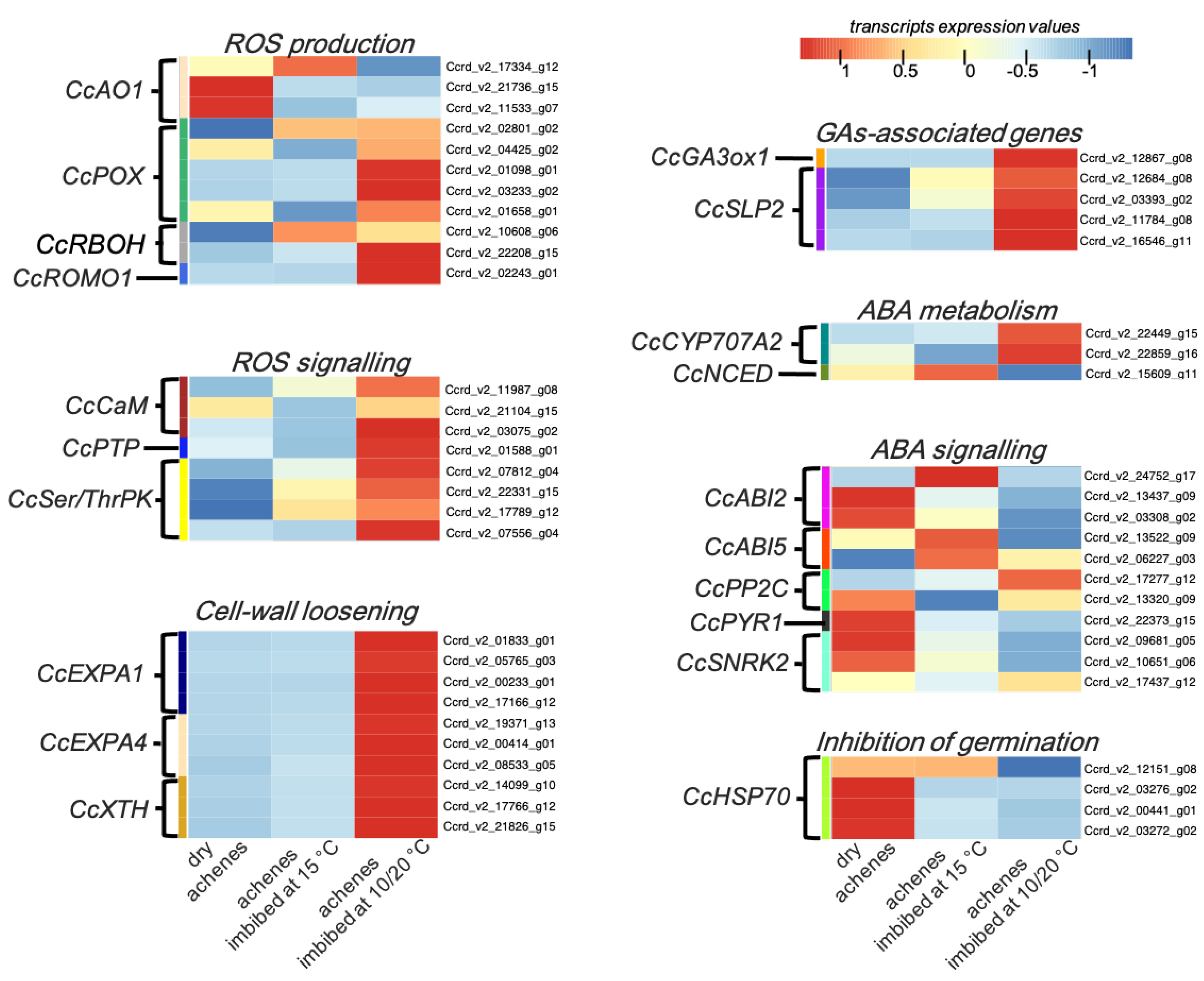
3.4. In Silico Expression Pattern Analysis of Genes Associated with Alternating or Constant Temperatures
3.4.1. ROS Production and Signalling
3.4.2. Cell-Wall Loosening
3.4.3. GAs-Associated Genes
3.4.4. ABA Metabolism and Signalling
3.4.5. Inhibition of Germination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Batlla, D.; Malavert, C.; Farnocchia, R.B.F.; Footitt, S.; Benech-Arnold, R.L.; Finch-Savage, W.E. A quantitative analysis of temperature-dependent seasonal dormancy cycling in buried Arabidopsis thaliana seeds can predict seedling emergence in a global warming scenario. J. Exp. Bot. 2022, 73, 2454–2468. [Google Scholar] [CrossRef]
- Holdsworth, M.J.; Bentsink, L.; Soppe, W.J.J. Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytol. 2008, 179, 33–54. [Google Scholar] [CrossRef] [PubMed]
- Finch-Savage, W.E.; Footitt, S. To germinate or not to germinate: A question of dormancy relief not germination stimulation. Seed Sci. Res. 2012, 22, 243–248. [Google Scholar] [CrossRef]
- Fernández-Pascual, E.; Seal, C.E.; Pritchard, H.W. Simulating the germination response to diurnally alternating temperatures under climate change scenarios: Comparative studies on Carex diandra seeds. Ann. Bot. 2015, 115, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Baskin, J.M.; Baskin, C.C.; Bu, H.; Du, G.; Ma, M. Effect of Diurnal Fluctuating versus Constant Temperatures on Germination of 445 Species from the Eastern Tibet Plateau. PLoS ONE 2013, 8, e69364. [Google Scholar] [CrossRef] [PubMed]
- Arana, M.V.; Tognacca, R.S.; Estravis-Barcalá, M.; Sánchez, R.A.; Botto, J.F. Physiological and molecular mechanisms underlying the integration of light and temperature cues in Arabidopsis thaliana seeds. Plant Cell Environ. 2017, 40, 3113–3121. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, N.; Curadi, M.; Picciarelli, P.; Martelloni, L.; Sbrana, C.; Giovannetti, M. Globe artichoke as a functional food. Med. J. Nutrition Metab. 2010, 3, 197–201. [Google Scholar] [CrossRef]
- Leonardi, C.; Pappalardo, H.; Genovese, C.; Puglia, G.; Bua, G.D.; Dugo, G.; Raccuia, S.A. Mechanisms of phytoextraction in Cynara cardunculus L. Growing under cadmium and arsenic stress. Acta Hortic. 2016, 1147, 139–144. [Google Scholar] [CrossRef]
- Pappalardo, H.D.; Toscano, V.; Puglia, G.D.; Genovese, C.; Raccuia, S.A. Cynara cardunculus L. as a Multipurpose Crop for Plant Secondary Metabolites Production in Marginal Stressed Lands. Front. Plant Sci. 2020, 11, 240. [Google Scholar] [CrossRef]
- Huarte, H.R.; Luna, V.; Pagano, E.A.; Zavala, J.A.; Benech-Arnold, R.L. Fluctuating temperatures terminate dormancy in Cynara cardunculus seeds by turning off ABA synthesis and reducing ABA signalling, but not stimulating GA synthesis or signalling. Seed Sci. Res. 2014, 24, 79–89. [Google Scholar] [CrossRef]
- Raccuia, S.A.; Puglia, G.; Pappalardo, H.; Argento, S.; Leonardi, C.; Calderaro, P.; Melilli, M.G. Dormancy-related genes isolation in Cynara cardunculus var. sylvestris. Acta Hortic. 2016, 1147, 315–322. [Google Scholar] [CrossRef]
- Argento, S.; Puglia, G.; Pappalardo, H.; Pulvirenti, M.; Melilli, M.G.; Raccuia, S.A. Seed germination responses to salt stress in wild and cultivated Sicilian cardoon genotypes. Acta Hortic. 2016, 1147, 9–14. [Google Scholar] [CrossRef]
- Huarte, H.R.; Benech-Arnold, R.L. Hormonal nature of seed responses to fluctuating temperatures in Cynara cardunculus (L.). Seed Sci. Res. 2010, 20, 39–45. [Google Scholar] [CrossRef]
- Oracz, K.; Bouteau, H.E.M.; Farrant, J.M.; Cooper, K.; Belghazi, M.; Job, C.; Job, D.; Corbineau, F.; Bailly, C. ROS production and protein oxidation as a novel mechanism for seed dormancy alleviation. Plant J. 2007, 50, 452–465. [Google Scholar] [CrossRef]
- Oracz, K.; El-Maarouf-Bouteau, H.; Kranner, I.; Bogatek, R.; Corbineau, F.; Bailly, C. The mechanisms involved in seed dormancy alleviation by hydrogen cyanide unravel the role of reactive oxygen species as key factors of cellular signaling during germination. Plant Physiol. 2009, 150, 494–505. [Google Scholar] [CrossRef]
- Yu, L.L.; Liu, C.J.; Peng, Y.; He, Z.Q.; Xu, F. New insights into the role of cyanide in the promotion of seed germination in tomato. BMC Plant Biol. 2022, 22, 1–18. [Google Scholar] [CrossRef]
- Huarte, H.R.; Borlandelli, F.; Varisco, D.; Batlla, D. Understanding dormancy breakage and germination ecology of Cynara cardunculus (Asteraceae). Weed Res. 2018, 58, 450–462. [Google Scholar] [CrossRef]
- Huarte, H.R.; Puglia, G.D.; Varisco, D.; Pappalardo, H.; Calderaro, P.; Toscano, V.; Raccuia, S.A. Effect of reactive oxygen species on germination of Cynara cardunculus (L.) cultivars. Acta Hortic. 2020, 1284, 33–39. [Google Scholar] [CrossRef]
- Huarte, H.R.; Puglia, G.D.; Prjibelski, A.D.; Raccuia, S.A. Seed transcriptome annotation reveals enhanced expression of genes related to ros homeostasis and ethylene metabolism at alternating temperatures in wild cardoon. Plants 2020, 9, 1225. [Google Scholar] [CrossRef]
- Bailly, C. Active oxygen species and antioxidants in seed biology. Seed Sci. Res. 2004, 14, 93–107. [Google Scholar] [CrossRef]
- Liu, J.; Xia, C.; Dong, H.; Liu, P.; Yang, R.; Zhang, L.; Liu, X.; Jia, J.; Kong, X.; Sun, J. Wheat male-sterile 2 reduces ROS levels to inhibit anther development by deactivating ROS modulator 1. Mol. Plant 2022, 15, 1428–1439. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. The signalling role of ROS in the regulation of seed germination and dormancy. Biochem. J. 2019, 476, 3019–3032. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ye, N.; Liu, R.; Chen, M.; Zhang, J. H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. J. Exp. Bot. 2010, 61, 2979–2990. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Kumari, A.; Thakur, M.; Koul, A. Hydrogen peroxide signaling integrates with phytohormones during the germination of magnetoprimed tomato seeds. Sci. Rep. 2019, 9, 8814. [Google Scholar] [CrossRef]
- Bazin, J.; Langlade, N.; Vincourt, P.; Arribat, S.; Balzergue, S.; El-Maarouf-Bouteau, H.; Bailly, C. Targeted mRNA oxidation regulates sunflower seed dormancy alleviation during dry after-ripening. Plant Cell 2011, 23, 2196–2208. [Google Scholar] [CrossRef]
- Gao, F.; Rampitsch, C.; Chitnis, V.R.; Humphreys, G.D.; Jordan, M.C.; Ayele, B.T. Integrated analysis of seed proteome and mRNA oxidation reveals distinct post-transcriptional features regulating dormancy in wheat (Triticum aestivum L.). Plant Biotechnol. J. 2013, 11, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, B.; Xu, Z.; Shi, Z.; Chen, S.; Huang, X.; Chen, J.; Wang, X. Involvement of reactive oxygen species in endosperm cap weakening and embryo elongation growth during lettuce seed germination. J. Exp. Bot. 2014, 65, 3189–3200. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Building an extensible cell wall. Plant Physiol. 2022, 189, 1246–1277. [Google Scholar] [CrossRef] [PubMed]
- Esashi, Y.; Sakai, Y.; Ushizawa, R. Cyanide-sensitive and Cyanide-resistant Respiration in the Germination of Cocklebur Seeds. Plant Physiol. 1981, 67, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Esashi, Y.; Komatsu, H.; Ishihara, N.; Ishizawa, K. Dormancy and impotency of cocklebur seeds VIII. Lack of germination responsiveness in primarily dormant seeds to cyanide, azide, anoxia and chilling. Plant Cell Physiol. 1982, 23, 41–47. [Google Scholar] [CrossRef]
- Bethke, P.C.; Libourel, I.G.L.; Reinöhl, V.; Jones, R.L. Sodium nitroprusside, cyanide, nitrite, and nitrate break Arabidopsis seed dormancy in a nitric oxide-dependent manner. Planta 2006, 223, 805–812. [Google Scholar] [CrossRef]
- Siegień, I.; Bogatek, R. Cyanide action in plants—From toxic to regulatory. Acta Physiol. Plant. 2006, 28, 483–497. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, D.W.; Zhu, F.; Tang, H.; Lv, X.; Cheng, J.; Xie, H.F.; Lin, H.H. A novel role for cyanide in the control of cucumber (Cucumis sativus L.) seedlings response to environmental stress. Plant. Cell Environ. 2012, 35, 1983–1997. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Liu, Y.; Xu, F. Comparative transcriptome analysis reveals significant differences in the regulation of gene expression between hydrogen cyanide- and ethylene-treated Arabidopsis thaliana. BMC Plant Biol. 2019, 19, 92. [Google Scholar] [CrossRef]
- Puglia, G.; Carta, A.; Bizzoca, R.; Toorop, P.; Spampinato, G.; Raccuia, S.A. Seed dormancy and control of germination in Sisymbrella dentata (L.) O.E. Schulz (Brassicaceae). Plant Biol. 2018, 20, 879–885. [Google Scholar] [CrossRef]
- Sun, W.Q.; Leopold, A.C. The Maillard reaction and oxidative stress during aging of soybean seeds. Physiol. Plant. 1995, 94, 94–104. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Puglia, G.D.; Prjibelski, A.D.; Vitale, D.; Bushmanova, E.; Schmid, K.J.; Raccuia, S.A. Hybrid transcriptome sequencing approach improved assembly and gene annotation in Cynara cardunculus (L.). BMC Genomics 2020, 21, 317. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq-A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Krasuska, U.; Gniazdowska, A. Nitric oxide and hydrogen cyanide as regulating factors of enzymatic antioxidant system in germinating apple embryos. Acta Physiol. Plant. 2012, 34, 683–692. [Google Scholar] [CrossRef]
- Desikan, R.; A.-H.-Mackerness, S.; Hancock, J.T.; Neill, S.J. Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol. 2001, 127, 159–172. [Google Scholar] [CrossRef]
- Rentel, M.C.; Lecourieux, D.; Ouaked, F.; Usher, S.L.; Petersen, L.; Okamoto, H.; Knight, H.; Peck, S.C.; Grierson, C.S.; Hirt, H.; et al. OXI1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis. Nature 2004, 427, 858–861. [Google Scholar] [CrossRef]
- Diaz-Vivancos, P.; Barba-Espín, G.; Hernández, J.A. Elucidating hormonal/ROS networks during seed germination: Insights and perspectives. Plant Cell Rep. 2013, 32, 1491–1502. [Google Scholar] [CrossRef]
- Steinbrecher, T.; Leubner-Metzger, G. The biomechanics of seed germination. J. Exp. Bot. 2017, 68, 765–783. [Google Scholar] [CrossRef]
- Morris, K.; Linkies, A.; Müller, K.; Oracz, K.; Wang, X.; Lynn, J.R.; Leubner-Metzger, G.; Finch-Savage, W.E. Regulation of seed germination in the close Arabidopsis relative Lepidium sativum: A global tissue-specific transcript analysis. Plant Physiol. 2011, 155, 1851–1870. [Google Scholar] [CrossRef]
- Layat, E.; Leymarie, J.; El-Maarouf-Bouteau, H.; Caius, J.; Langlade, N.; Bailly, C. Translatome profiling in dormant and nondormant sunflower (Helianthus annuus) seeds highlights post-transcriptional regulation of germination. New Phytol. 2014, 204, 864–872. [Google Scholar] [CrossRef]
- Ogawa, M.; Hanada, A.; Yamauchi, Y.; Kuwahara, A.; Kamiya, Y.; Yamaguchi, S. Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 2003, 15, 1591–1604. [Google Scholar] [CrossRef] [PubMed]
- Leymarie, J.; Vitkauskaité, G.; Hoang, H.H.; Gendreau, E.; Chazoule, V.; Meimoun, P.; Corbineau, F.; El-Maarouf-Bouteau, H.; Bailly, C. Role of reactive oxygen species in the regulation of Arabidopsis seed dormancy. Plant Cell Physiol. 2012, 53, 96–106. [Google Scholar] [CrossRef] [PubMed]
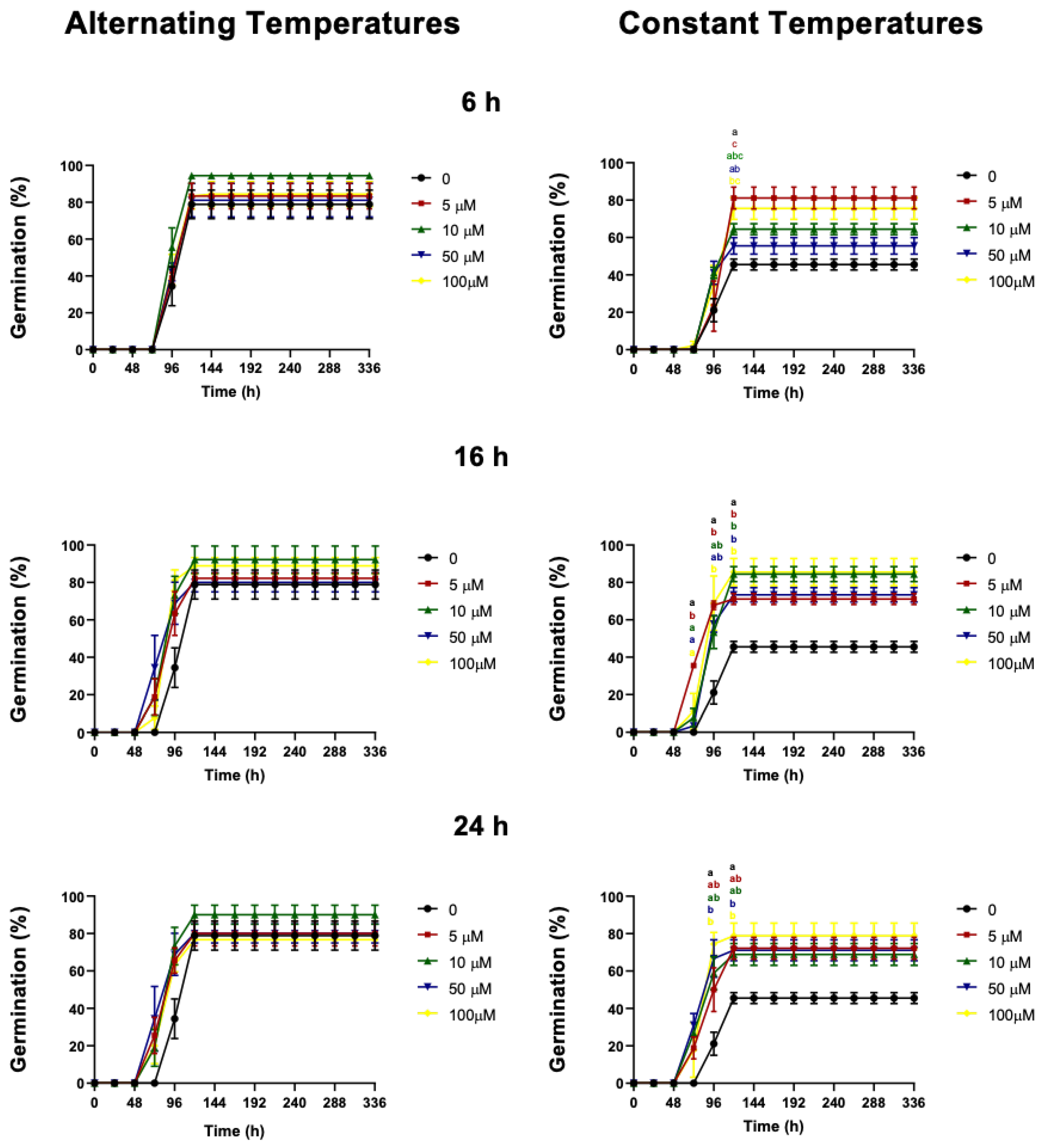
| KCN Concentration | KNC Exposure Time (h) | Imbibition Temperature | Final Germination (%) | t50 | GR (1/t50) | Tukey Test p ≤ 0.05 |
|---|---|---|---|---|---|---|
| 0 | 0 | 15 °C | 45.56 | 4.06 | 0.24 | A |
| 0 | 10/20 °C | 78.89 | 4.11 | 0.24 | BC | |
| 5 µM | 6 | 15 °C | 81.11 | 4.29 | 0.23 | BC |
| 16 | 15 °C | 71.11 | 3 | 0.33 | ABC | |
| 24 | 15 °C | 72.22 | 3.61 | 0.27 | ABC | |
| 6 | 10/20 °C | 83.33 | 4.06 | 0.24 | - | |
| 16 | 10/20 °C | 82.22 | 3.5 | 0.28 | - | |
| 24 | 10/20 °C | 80.00 | 3.36 | 0.29 | - | |
| 10 µM | 6 | 15 °C | 64.44 | 3.78 | 0.26 | ABC |
| 16 | 15 °C | 84.44 | 3.75 | 0.26 | C | |
| 24 | 15 °C | 68.59 | 4.24 | 0.23 | ABC | |
| 6 | 10/20 °C | 94.44 | 3.85 | 0.25 | - | |
| 16 | 10/20 °C | 92.22 | 3.50 | 0.28 | - | |
| 24 | 10/20 °C | 90.00 | 2.30 | 0.43 | - | |
| 50 µM | 6 | 15 °C | 55.56 | 3.67 | 0.27 | AB |
| 16 | 15 °C | 73.33 | 3.61 | 0.27 | ABC | |
| 24 | 15 °C | 71.11 | 3.12 | 0.32 | ABC | |
| 6 | 10/20 °C | 81.11 | 3.96 | 0.25 | - | |
| 16 | 10/20 °C | 80.00 | 3.61 | 0.27 | - | |
| 24 | 10/20 °C | 80.00 | 3.16 | 0.31 | - | |
| 100 µM | 6 | 15 °C | 75.56 | 4.08 | 0.24 | BC |
| 16 | 15 °C | 85.56 | 3.54 | 0.28 | C | |
| 24 | 15 °C | 78.89 | 3.38 | 0.29 | BC | |
| 6 | 10/20 °C | 84.44 | 3.93 | 0.25 | - | |
| 16 | 10/20 °C | 88.69 | 3.50 | 0.28 | - | |
| 24 | 10/20 °C | 76.67 | 3.42 | 0.29 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puglia, G.D.; Balestrasse, K.; Bustos, J.S.; Huarte, H.R. New Insights into the Role of Alternating Temperatures and Cyanide in the ROS-Mediated Cardoon Seed Dormancy Termination. Horticulturae 2022, 8, 960. https://doi.org/10.3390/horticulturae8100960
Puglia GD, Balestrasse K, Bustos JS, Huarte HR. New Insights into the Role of Alternating Temperatures and Cyanide in the ROS-Mediated Cardoon Seed Dormancy Termination. Horticulturae. 2022; 8(10):960. https://doi.org/10.3390/horticulturae8100960
Chicago/Turabian StylePuglia, Giuseppe Diego, Karina Balestrasse, José Santiago Bustos, and Héctor Roberto Huarte. 2022. "New Insights into the Role of Alternating Temperatures and Cyanide in the ROS-Mediated Cardoon Seed Dormancy Termination" Horticulturae 8, no. 10: 960. https://doi.org/10.3390/horticulturae8100960
APA StylePuglia, G. D., Balestrasse, K., Bustos, J. S., & Huarte, H. R. (2022). New Insights into the Role of Alternating Temperatures and Cyanide in the ROS-Mediated Cardoon Seed Dormancy Termination. Horticulturae, 8(10), 960. https://doi.org/10.3390/horticulturae8100960








