Abstract
Vaccinium oldhamii Miq. is an edible berry; however, little is known about its seed dormancy-break and germination. Therefore, the aim of this study was to elucidate the seed-dormancy break and germination characteristics of V. oldhamii. The results showed that the length and width of the seeds of V. oldhamii were 2.4588 ± 0.0273 mm and 1.7028 ± 0.0248 mm, respectively, the filled percentage was 49.0 ± 3.0%, and the 1000-seed weight was 0.9453 g. Additionally, water imbibition test showed that the seed weight of V. oldhamii increased by more than 70% after 24 h, indicating the absence of physical dormancy. Embryo length measured at dispersal and just before germination did not differ significantly. There was no significant difference in E:S (Embryo:Seed) ratio in length at the time of seed dispersal (0.6780 ± 0.0258) and before germination (0.7370 ± 0.0469), indicating the absence of morphological dominance. Temperature treatments showed that the germination of the seed was 0, 6.0, 6.0, 20.0, and 0% under 15, 20, 25, 25/15, and 15/6 °C, respectively. However, treatment with 250 ppm of GA3 increased the germination of the seeds to 22.0, 36.0, 32.0, 40.0, and 1.0%, respectively. Additionally, treatment with 500 ppm of GA3 increased the germination to 34.6, 40.0, 40.0, 46.0 %, respectively. These results indicated that the seeds of V. oldhamii exhibited Nondeep physiological dormancy (Nondeep PD). Overall, the findings of this study showed that treatment with 500 ppm of GA3 at 25/15 °C can effectively break the dormancy of V. oldhamii seeds, which could facilitate further research on the species.
1. Introduction
Vaccinium oldhamii Miq. is a deciduous shrub in the heath family (Ericaceae) (1–3 m in height), and it grows in the mountainous areas of Gyeongbuk, South Chungcheong, and the west coast (Chungnam, Hwanghae-do) of South Korea. V. oldhamii produces pale reddish yellow-green or reddish-brown bisexual flowers in clusters of 5–15 at the tip of new branches between May and June, and fruits (spherical black/purple berries; 4–6 mm in diameter) between September and October [1]. V. oldhamii is endemic to Korea, the eastern part of China, and Japan [2]. Species of the Vaccinium that grow wild in Korea, include V. japonicum Miq., V. bracteatum Thunb., V. hirtum Thunb. var. koreanum (Nakai) Kitam., V. uliginosum L., and V. vitis-idaea L.
The antibacterial and antioxidant activity and anthocyanin and total phenol content of Vaccinium have been extensively studied in this species [3,4,5]. V. oldhamii possesses medicinal and ornamental value. Additionally, as a native species, it is designated as an “Out of the country transportation permit required species”, and approval must be obtained from government before it can be exported abroad [6].
The seeds of wild plants exhibit different kinds of dormancy [7], occurring in more than 50% of wild plants. Dormancy is a crucial plant trait that prevents germination during unfavorable conditions, and it serves as insurance for seed survival [8,9]. Accurate classification of species dormancy can provide comprehensive understanding of the early stages of the plant life history and facilitate efficient seed propagation [10]). Baskin and Baskin [9] classified seed dormancy into five categories based on a combination of physiological and morphological factors: physiological dormancy, morphological dormancy, physical dormancy, and combinational dormancy. A poor consideration of the dormancy and germination traits of seeds in restoration planning often contributes to the failure of plant establishment [11]. However, studies on the germination and dormancy characteristics of the seeds of V. oldhamii, a wild berry, are limited. Moreover, it is necessary to examine crop wild relatives (CWR) owing to their potential genetic values.
CWR contain several genes of potential value for plant breeding, among which are genes regulating traits that are relevant for climate change adaptation. Some of these genes are responsible for pest and disease resistance [12]. CWR are distributed across a wide range of habitats, including mountains, deserts, grasslands, salt marshes, and rainforests, and they have evolved survival strategies for these diverse climatic conditions [12]. V.oldhamii is a CWR of blueberries and is highly adapted to the climatic conditions of Korea. The genetic traits that allow CWR species to thrive in different, and sometimes extreme, habitats represent a valuable resource for plant breeding in the context of climate change. However, it is necessary to understand the dormancy and germination characteristics of CWR, such as V. oldhamii, to facilitate breeding efforts.
Therefore, the aim of this study was to elucidate the seed-dormancy break and germination characteristics of V. oldhamii. It is believed that the findings of this study could provide basic information for the preservation of the genetic resources of V. oldhamii.
2. Materials and Methods
2.1. Plant Material
The seeds used in this study were harvested from plants grown at Buyeo-gun, Chungnam-do, Republic of Korea on 14 September 2021. After the seeds were dried for 2 weeks (15 °C, RH15%), they were sealed in an aluminum bag with silica gel and stored at −20 °C for about 3 months until the beginning of the experiment.
2.2. Basic Characteristics
V. oldhamii seed were examined to determine seed characteristics, including seed length, width, and 1000-seed weight. Four replicates of 100 seeds were weighted using an electronic balance (ML204/01, Mettle Toledo, Columbus, OH, USA). Seed length, width, and width:length (W:L) ratio were calculated using a seed scanner (VideometerLab4, Videometer, Copenhagen, Denmark). To determine embryo phenology, V. oldhamii seeds were incubated at 25/15 °C, and seeds were harvested every week from 10 October 2021, for analysis. Briefly, the seeds were dissected, photographed using a digital microscope (DVM6, Leica Microsystems GmbH, Wetzler, Germany), and measured using ImageJ software (Java., LOCI, University of Wisconsin, WI, USA). Embryo length to seed length (E:S) ratio was calculated using the formula described by Vandelook et al. [13].
2.3. Water Uptake Test
Water absorption test was performed to determine if the seed coat of V. oldhamii is permeable or impermeable to water. Accurately weighed seeds were placed on a filter paper moistened with distilled water in petri dishes (90 × 15 mm). Thereafter, the weight of the seeds was determined after 0, 3, 6, 9, 12, 24, and 72 h using an electronic balance (ML204/01, Mettler Toledo, Columbus, OH, USA). Water absorption was calculated using the formula below:
where Ws = percentage increase in seed weight, Wh = weight of imbibed seed, Wi = initial seed weight.
% Ws = [(Wh − Wi)/Wi] × 100
2.4. Seed Germination
Before the germination test, 25 seeds were randomly x-rayed using EMT-F70 (Softex Co., Ltd., Ebina-shi, Kanagawa, Japan) to determine the percentage of filled seeds. All measurements were repeated four times, making a total of 100 seeds. The filled percentage was calculated as shown below.
Filled percentage (%) = filled seeds/analyzed seeds (100 seeds) × 100
For the germination test, the seeds were grown in petri dishes containing 1% agar medium. All germination experiments were performed in temperature-controlled germination chambers (TGC-130H; Espec Mic Co., Aichi, Japan). The seeds were sown in petri dishes in the germination chambers at constant temperatures of 15, 20, and 25 °C, and alternate temperatures of 25/15 and 15/6 °C, under a 12 h light/12 h dark photoperiod for 12 weeks. Seed germination was based on the protrusion of more than 1 mm of the root. A total of 25 seeds was used for the germination experiment and the experiment was repeated four times.
2.5. Gibberellic Acid (GA3) Treatment
The effect of 250 and 500 ppm GA3 solution (≥90%; Sigma-Aldrich, St. Louis, MO, USA) on germination of the seeds was examined. Briefly, the seeds were sown in petri dishes (SPL Life Sciences Co., Ltd., Pocheon, Korea) containing 1% agar medium supplemented with either 250 or 500 ppm of GA3 solution at constant temperatures of 15, 20, and 25 °C and alternate temperatures of 25/15 and 15/6 °C under a 12 h light/12 h dark photoperiod for 10 weeks. A total of 25 seeds was used for the germination experiment, and the experiment was repeated four times. Seed germination was based on the protrusion of more than 1 mm of the root.
2.6. Cold Stratification Treatment
To examine the effect of cold stratification treatment on dormancy break, 25 seeds were sown on 1% agar medium, and cold stratification was performed at 5 °C in the dark for 0, 4, 8, and 12 weeks. After cold stratification treatment, seeds were moved to 20 and 25/15 °C under a 12 h light/12 h dark photoperiod and monitor for 10 weeks.
2.7. Seed Germination Charateristics
Germination, T50 (days spent to reach 50% germination) and MGT (mean germination time) were calculated using the formulas below:
where Ng = number of final germination and Nt = total number of seeds sown
where T is the time in days from day 1 to the final day of germination test, and S is the total number of germinated seeds on day T
where N = total number of seeds germinated until the deadline for germination investigation, Nj = total number of seeds germinated immediately after 50% of N, Ti = germination period until Ni, Tj = germination period until Nj.
Germination = Ng/Nt × 100
MGT(days) = ∑(T × S)/∑S
T50 = Ti + {(N + 1)/2 − Ni} × {Tj − Ti}/(Nj − Ni)
2.8. Data Analysis
Data of water absorption, germination (G), and GA3 treatment were analyzed for significance difference between treatments using SPSS (IBM Corporation, Armonk, NY, USA), followed by multiple mean comparison using Duncan’s multiple range test. Means were considered statistically significant at p < 0.05. The E:S ratio at dispersal point and before germination were compared using t-test. All graphs were prepared using Sigma plot 10.0 software (Systat Software Inc., San Jose, CA, USA).
3. Results
3.1. Basic Characteristics of Vaccinium oldhamii Miq.
V. oldhamii is a berry, and the seeds are reddish-brown (Figure 1). The mean length and width of the seeds were 2.4588 ± 0.0273 mm and 1.7028 ± 0.0248 mm, respectively (Table 1), giving it an obovoid shape. The surface of the seed exhibited a reticulate-foveate structure. Additionally, the 1000-seed weight of V. oldhamii was 0.9453 g.
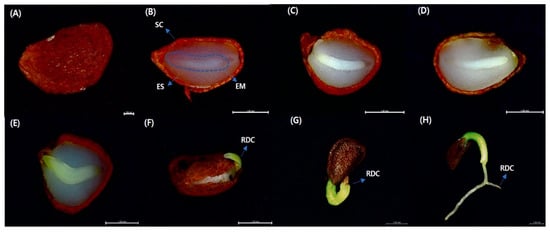
Figure 1.
Embryo growth, radicle emergence, and seedling emergence of Vaccinium oldhamii Miq. (A) V. oldhamii seed (B) 7, (C) 14, (D) 21, (E) 28, (F) 35, (G) 42, and (H) 49 d into the germination process. SC = seed coat, EM = embryo, ES = endosperm, RDC = radicle. Scale bar = 1 mm.

Table 1.
Seed characteristics of Vaccinium oldhamii Miq.
The water absorption test showed that the seed weight of V. oldhamii increased by more than 70% after 24 h, but plateaued after 48 h (Figure 2). Additionally, microscopic examination showed that the embryo was axial-linear (straight) in shape. There was no significant difference in E:S ratio at the time of seed dispersal (0.6780 ± 0.0258) and before germination (0.7370 ± 0.0468) (Figure 3).
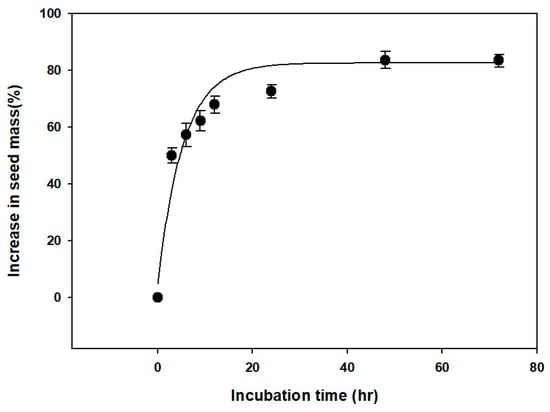
Figure 2.
Water uptake by Vaccinium oldhamii Miq. seeds incubated at 20 °C on filter paper moistened with distilled water for 0–72 h. Error bars indicate mean ± standard error (n = 4).
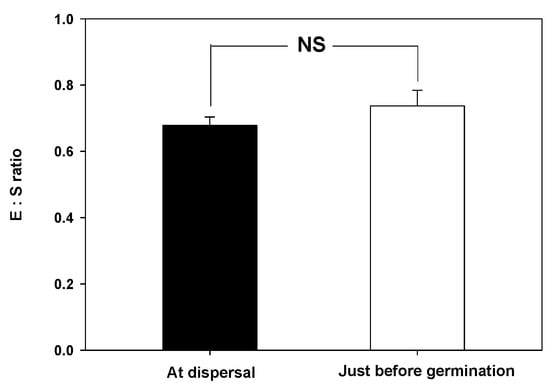
Figure 3.
Embryo to seed (E:S) ratio of Vacinnium oldhamii at dispersal and before germination, Error bars indicate the mean ± standard error (n = 10). Each E:S ratio at dispersal and prior to germination was compared using a paired t-test (NS: no significant).
3.2. Seed Germination
The results of the x-ray analysis showed that the filled percentage of the seed was 49.00 ± 3.0%. It can be confirmed that the progression pattern is being developed through Figure 1.
Additionally, the germination of the seeds at 15, 20, 25, 25/15, and 15/6 °C was 0.0, 6.0, 6.0, 20.0, and 0.0%, respectively (Figure 4), indicating that 25/15 °C temperature was most favorable for seed germination. Moreover, the MGT at 20, 25, and 25/15 °C were 28.5, 46.08, and 41.18 d, respectively (Table 2). The T50 at 20, 25 and 25/15 °C were 28.35, 45.94, and 37.5 d, respectively.
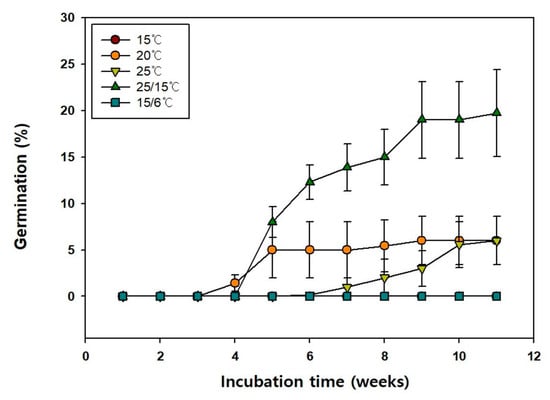
Figure 4.
Germination of Vaccinium oldhamii seeds incubated at alternate and constant temperature. Seeds collected in 2021 were used for this study. Vertical bars represent Standard error (n = 4).

Table 2.
Germination characteristics of Vaccinium oldhamii Miq. seeds under different germination temperature and Gibberellic acid (GA3) treatments.
3.3. GA3 Treatment
GA3 has been shown to effectively break seed dormancy, improve embryo development, and enhance seed germination [7]. Generally, treatment with 250 and 500 ppm of GA3 improved the germination of the seeds at 15, 20, 25, 25/15, and 15/6 °C compared with the nontreated group (Figure 5). Specifically, treatment with 250 ppm of GA3 increased the germination of the seeds to 22.0, 36.0, 32.0, 40.0, and 1.0% under 15, 20, 25, 25/15, and 15/6 °C, respectively. The MGT at 15, 20, 25, and 25/15, 15/6 °C; were 52.44, 45.37, 49.67, 45.17 and 64.00 d respectively (Table 2). Additionally, the T50 at 15, 20, 25, and 25/15 °C were 53.75, 43.19, 49.0, and 38.30 d, respectively. Treatment with 500 ppm of GA3 increased the germination to 26.0, 40.0, 40.0, 46.0, and 16.0% under 15, 20, 25, 25/15, and 15/6 °C, respectively. The MGT at 15, 20, 25, 25/15, and 15/6 °C; were 37.08, 43.34, 48.89, 40.04, and 65.33 d, respectively. Additionally, the T50 at 15, 20, 25, 25/15, and 15/6 °C were 36.38, 42.0, 47.31, 39.15, and 65.64 d, respectively (Table 2). There was a decrease in the MGT of seeds in the 500 ppm GA3 group compared with those of seeds in the 250 ppm GA3 group (Table 2). Regardless of the treatment, seeds at 25/15 °C had the highest germination. Taking the seed quality (Filled percentage; G/FP) into consideration, seeds in 250 ppm GA3, and 500 ppm GA3 groups exhibited 81.63, and 93.88% germination under 25/15 °C. As a result, it is judged that V. oldhamii is affected by temperature and hormone.
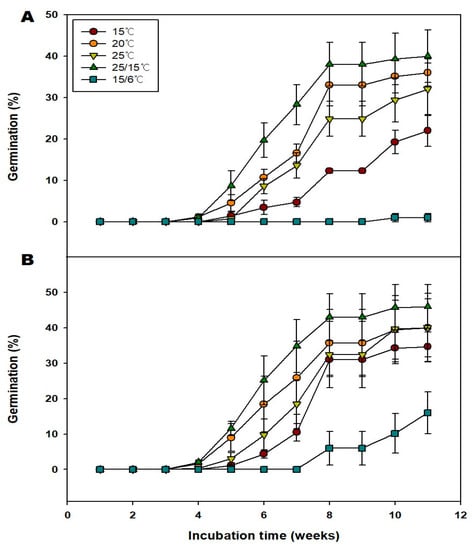
Figure 5.
Effects of the 250 and 500 ppm GA3 treatments with alternate and constant temperatures on the germination of the Vaccinium oldhamii Miq. seeds. (A); 250 ppm GA3, (B); 500 ppm GA3. Seeds collected in 2021 were used for this study. Vertical bars indicate standard error (n = 4).
3.4. Cold Stratification Treatments
To examine the effect of cold stratification treatment on the dormancy of V. oldhamii seed, the germination of the seeds was observed after cold stratification treatment for 4, 8, and 12 weeks. However, germination did not occur in all treatment groups after cold stratification.
4. Discussion
The results of the present study showed that 15 and 15/6 °C and cold stratification treatment did not promote germination of V. oldhamii seeds, as evidenced by the 0% germination observed at 20 and 25/15 °C and after cold stratification treatment. However, cold stratification has previously been shown to decrease the temperature requirements for V. myrtillus and V. vitis-idaea seed germination [7]. Due to the characteristics of the seeds, it is difficult to collect seeds with uniform maturation. Taking the seed quality (Filled percentage; G/FP) into consideration (empty seed), the most effective treatment showed 93.88% germination at 25/15 °C in 500 ppm GA3 groups. Treatment with GA3 hormone effectively broke the seed dormancy and promoted germination under low temperature conditions, although the germination percentage was low.
Additionally, water imbibition treatment showed that the weight of V. oldhamii seeds increased by ≥ 20% during the water uptake test, indicating that the seed coat was highly permeable [14]. This result rules out the possibility of physical dormancy in the seeds; moreover, the seeds germinated within 30 days under favorable conditions. Furthermore, there was no significant difference in the E:S ratio before dispersal and before germination, indicating the absence of morphological dormancy. However, the germination of the seeds did not reach 10% within 30 days, indicating that the seeds of V. oldhamii have physiological dormancy (PD). Moreover, the dormancy of the seeds was broken by GA3 treatment, confirming the presence of physiological dormancy. Based on the physiological mechanism of germination inhibition, physiological dormancy can be divided into three levels: non-deep, intermediate, and deep [15]. Non-deep physiological dormancy can be broken by short-term warm or cold stratification or by GA3 treatment, intermediate physiological dormancy can be broken by cold stratification for at least two months and GA3 treatment, while deep physiological dormancy can be broken by cold stratification for 3–4 months but not by GA3 treatment [14]. The results of the present study showed that although cold stratification did not induce germination, GA3 treatment induced germination, indicating that the seeds exhibited non-deep physiological dormancy (Nondeep PD).
A previous study reported germination of 81% and 54.66% for V. meridionale, a blueberry in Colombia, at 18 ± 2 and 6 ± 2 °C, respectively [16]. Additionally, Hudson et al. [17] observed a rapid and uniform increase in the germination of V. myrtilloides seed stratified for 8 weeks. Moreover, physiological dormancy has been observed in 20 species of Vaccinium, indicating a similar dormancy type in the genus across different climatic zones globally. However, the seed germination characteristics of species of the genus differed according to the location and climate conditions of the native habitat (Table 3).

Table 3.
Seed dormancy classes in Vaccinium taxa.
Presently, most blueberries grown in Korea are imported from the United States. Therefore, the findings of the present study can aid in the development of varieties suitable and well adapted to the climatic and environmental conditions of Korea. In the future, additional research is needed to determine how germination proceeds after receiving low temperatures in the natural state, and whether it is possible to increase the germination under relatively low temperature conditions.
Author Contributions
Conceptualization, D.H.L. and W.G.P.; methodology, D.H.L. and C.S.N.; formal analysis, D.H.L.; investigation, C.Y.P., Y.H.J., J.H.K., S.H.P., H.J.S. and W.G.P.; data curation, D.H.L.; writing-original draft preparation, D.H.L.; writing-review and editing, D.H.L. and C.Y.P.; resources, H.J.S.; project administration, C.S.N.; funding acquisition, C.S.N.; supervision, W.G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was carried out with the support of “R&D Program for Forest Science Technology (Project No. ‘2021400B10-2125-CA02’ provided by Korea Forest Service (Korea Forestry Promotion Institute))”.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, T.Y.; Kim, J.S. Woody Plants of Korea Peninsula. Paju-si. Gyeongii-do, Korea. 2018. Available online: https://florakorea.myspecies.info/en (accessed on 15 July 2022).
- Plants of the World Online. Available online: www.powo.science.kew.org (accessed on 18 July 2022).
- Chae, J.W.; Jo, B.S.; Joo, S.H.; Ahn, D.H.; Chun, S.S.; Cho, Y.J. Biological and antimicrobial activity of Vaccinium oldhami fruit. J. Food Sci. Nutr. 2012, 41, 1–6. [Google Scholar] [CrossRef][Green Version]
- Tsuda, H.; Kunitake, H.; Kawasaki-Takaki, R.; Nishiyama, K.; Yamasaki, M.; Komatsu, H.; Yukizaki, C. Antioxidant activities and anti-cancer cell proliferation properties of Natsuhaze (Vaccinium oldhamii Miq.), Shashanbo (V. bracteatum Thunb.) and Blueberry cultivars. Plants 2013, 2, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, M.S.; Kim, S.H.; Yun, K.W.; Song, J.H. Analysis of total phenolic content and antioxidant activity from fruits of Vaccinium oldhamii Miq. J. Korean For. Soc. 2013, 102, 566–570. [Google Scholar] [CrossRef][Green Version]
- National institute of Biological Resource. List Indigenous/Endemic Species of Korea; National institute of Biological Resource: Incheon, Korea, 2019. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Donohue, K.; Rubio de Casas, R.; Burghardt, L.; Kovach, K.; Willis, C.G. Germination, post germination adaptation, and species ecological ranges. Annu. Rev. Ecol. Evol. Systamatics. 2010, 14, 293–319. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 160. [Google Scholar] [CrossRef]
- Jang, G.H.; Chung, J.M.; Rhie, Y.H.; Lee, S.Y. Seed dormancy class and ecophysiological Features of Veronicastrum sibiricum (L.) Pennell (Scrophulariaceae) Native to the Korea Peninsula. Plants 2022, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Kildisheva, O.A.; Dixon, K.W.; Silveira, F.A.O.; Chapman, T.; Di Sacco, A.; Mondoni, A.; Turner, S.R.; Cross, A.T. Dormancy and germination: Making every seed count in restoration. Restor. Ecol. 2020, 28 (Suppl. S3), S256–S265. [Google Scholar] [CrossRef]
- Crop Wild Relatives. Available online: www.cwrdiversity.org (accessed on 18 July 2022).
- Vandelook, F.; Bolle, N.; Van Assche, J.A. Seed dormancy and germination of the European Chaerophyllum temulum (Apiaceae), a member of a trans-Atlantic genus. Ann. Bot. 2007, 100, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Nikolaeva, M.G. Factors controlling the seed dormancy pattern. In The Physiology and Biochemistry of Seed Dormancy and Germination, Khan, A.A., Ed.; Elsevier: Amsterdam, The Netherlands, 1977; pp. 51–74. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. When breaking Seed dormancy is a problem; Try a move-along experiment. Nat. Plants J. 2003, 4, 17–21. [Google Scholar] [CrossRef]
- Castro, C.; Olarte, Y.; Rache, L.; Pacheco, J. Development of a germination protocol for blueberry seeds (Vaccinium meridionale Swartz). Agron. Colomb. 2012, 30, 196–203. [Google Scholar]
- Hudson, J.J.; Yücel, Ç.K.; Schoonmaker, A.L.; Sobze, J. Effects of cold stratification on the germination of Vaccinium myrtilloides (common blueberry) and Vaccinium vitis-idaea (bog cranberry) seeds from alberta, canada. Nat. Plants J. 2017, 18, 245–251. [Google Scholar] [CrossRef]
- Vander Kloet, S.P. On the Etymology of Vaccinium L.; Acadia University: Wolfville, NS, Canada; Nova Press: Scotia, ON, Canada, 1992; Volume 880, pp. 371–373. [Google Scholar]
- Vander Kloet, S.P.; Hill, N.M. Bacca quo vadis: Regeneration niche differences among seven sympatric Vaccinim species on headlands of Newfoundland. Seed Sci. Res. 2000, 10, 89–97. [Google Scholar] [CrossRef]
- Baskin, C.C.; Milberg, P.; Andersson, L.; Baskin, J.M. Germination studies of three dwalf shrub (Vanccinium, Ericaceae) of Northern Hemisphere coniferous forests. Can. J. Bot. 2000, 78, 1552–1560. [Google Scholar]
- Ranwala, S.M.W.; Naylor, R.E.L. Production, survival and germination of bilberry (Vaccinium mytillus L.) seeds. Bot. J. Scotl. 2004, 56, 55–63. [Google Scholar] [CrossRef]
- Emery, D.E. Seed Propagation of Native California Plants; Santa Barbara Botanic Garden: Santa Barbara, CA, USA, 1988. [Google Scholar]
- Nikolaeva, M.G.; Rasumova, M.V.; Gladkova, V.N. Reference book on dormant seed germination; Danilova, M.F., Ed.; Nauka Publisher, Leningrad Branch: Leningrad, Russia, 1985. [Google Scholar]
- Lopez, O.A.; Barney, D.L.; Shafii, B.; Price, W.J. Modeling the effects of temperature and gibberellic acid concentration on red huckleberry seed germination. Am. Soc. Hortis. Sci. 2008, 43, 223–228. [Google Scholar] [CrossRef]
- Gartner, B.L. Germination characteristics of arctic plants. In Fourth International Conference on Permafrost; Britton, M.E., Ed.; National Academy Press: Fairbanks, AK, USA; Washington, DC, USA, 1983; pp. 334–338. [Google Scholar]
- Shimono, Y.; Kudo, G. Comparisons of germination traits of alpine plants between fellfield and snowbed habitats. Ecol. Res. 2005, 20, 189–197. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).