Abstract
Lilium davidii var. unicolor is an important variety of lily with high economic, ornamental, edible, and medicinal values. Up to now, the research on polysaccharide as the main active substance is not vast, especially the effect of potassium on lily polysaccharide. Here, we investigated the effects of potassium fertilizer application on the growth and development, polysaccharide accumulation in the bulb, and differential metabolites in L. davidii var. unicolor. It was treated with fixed amounts of nitrogen (N) and phosphorus (P), and four K concentrations comprising K0 (0 mg·L−1), K1 (447.6 mg·L−1), K2 (671.4 mg·L−1), or K3 (895.2 mg·L−1). The growth indexes were determined for L. davidii var. unicolor under different K concentrations in different growth stages. The polysaccharide contents of the bulbs were determined using the sulfuric acid−phenol method. An analysis of the differential polysaccharide metabolites was also conducted. The application of potassium promoted the growth and increased the bulb polysaccharide content of L. davidii var. unicolor, and the most suitable K concentration of 671.4 mg·L−1 had the most significant effects. Non-targeted metabolomics analysis screened 37 differential polysaccharide metabolites under K0 and K2, where 25 were significantly upregulated and 12 were significantly downregulated. Three metabolic pathways were enriched in polysaccharide-related differential metabolites, i.e., the galactose metabolism, amino sugar and nucleotide sugar metabolism, and starch and sucrose metabolism. The results could provide a theoretical basis for an improved fertilization management and the high-quality cultivation of L. davidii var. unicolor.
1. Introduction
The Lilium davidii var. unicolor is an important economic crop; it is the only edible sweet lily in China and its high levels of polysaccharides can be used as potential bulb quality biomarkers [1]. The L. davidii var. unicolor is mostly found in Lanzhou city, Gansu Province, China, where it is mainly cultivated through the bulb propagation method. It is commonly known as the “sweet lily” because of its edible, nutritious, and medicinal properties [2]. The bulbs of L. davidii var. unicolor are regularly consumed as food for their distinctive taste and are common ingredients in soups, stir-fries, and stew-like dishes such as a “hot pot”. It has been used as a herb in Chinese folk medicine as a remedy for insomnia and dreamful sleep. The bulbs are rich in starch, protein, and dietary fiber, as well as bioactive polysaccharides [3]. Polysaccharides have a polycarbohydrate structure with at least 10 monosaccharides linked by glycosidic bonds. During hydrolysis, polysaccharides produce a series of intermediate products before ultimately yielding monosaccharides through the complete hydrolysis [4]. In addition, pharmacological studies have shown that the polysaccharides have antioxidant [5,6], immune [7], antitumor [8,9,10,11], and hypoglycemic properties [12,13,14], and thus it is known as “vegetable ginseng”.
Ornamental species, especially flowering plants, demand high amounts of K because this element is directly related to the flowering process [15]. In calla lily plants, K is the most important essential nutrient [16,17]. The demand of potassium plants for potassium is greater than that of nitrogen and phosphorus. A single application of nitrogen and phosphorus fertilizer can promote the growth of lily plants, but its increase is lower than that of potassium fertilizer. The seedlings of L. davidii var. unicolor also exhibit a strong but inconsistent response to the K treatment: whereas an intermediate K supply increases the seedling height, basal shoot diameter, leaf number, and leaf area, a high K supply decreases or has little effect on these growth characteristics [18]. The polysaccharide, reducing sugar contents, and nine mineral elements were studied in the bulbs of four types of edible lily [19], and the highest polysaccharide content was 14.58 mg·g−1 in L. davidii and the lowest was 9.74 mg·g−1 in Lilium lancifolium. In another study, the phenol−sulfuric acid method and aluminum blue colorimetric method were used to determine the polysaccharide contents of Lilium under different fertilization treatments [20], where potassium fertilizer had the greatest influence on the quality of Lilium and the polysaccharide content was significantly higher than the average level after a foliar fertilizer treatment in the later stage. Integrated metabolic profiling and transcriptome analysis using petal tissues from the lily cultivar “Vivian” [21] detected metabolites in different regions using a wide targeting metabolomics method, and 23 differentially accumulated metabolites were found. The levels of cinnamic acid and coumarinic acid were higher in the bud stage in “Vivian” petal samples than pigmented petal samples. In recent years, the expansion of the cultivation area, seed degeneration, and quality declines have resulted in the decreased accumulation of active ingredients in Lilium, thereby affecting the edible and medicinal properties [22].
Previous studies mainly focused on the polyphenol contents of lily bulbs after fertilization and the optimal fertilizer ratio, whereas few have considered the accumulation of polysaccharides, metabolic pathways for polysaccharides, and differential metabolites during the growth and development of lily bulbs in Lanzhou. Therefore, in this study, we investigated the effects of different potassium concentrations on the growth and development of L. davidii var. unicolor, and the accumulation of polysaccharides in the bulb. Metabolomics analysis was conducted to determine the bulb polysaccharide-related metabolites and metabolic pathways after the potassium application in order to provide a reference for an improved fertilization management and the development of medicinal health resources for L. davidii var. unicolor.
2. Materials and Methods
2.1. Sampling
The test materials comprised L. davidii var. unicolor bulbs with a circumference of 12−15 cm purchased from Huamu Company, Lanzhou city, Gansu Province, China. The experiment was conducted in a glass greenhouse at the agricultural training base of Ningxia University in China. The average indoor temperature was 24 °C and natural illumination was available for more than 10 h per day. The relative humidity was 70−80%.
2.2. Chemicals and Reagents
Pure analytical reagents comprising phenol, sulfuric acid, and methanol were purchased from Bodi Chemical Co., Ltd. (Tianjin, China). Methanol, HPLC, 67-56-1, Fisher Chemical; Acetonitrile, HPLC, 75-05-8, Fisher Chemical; Formic Acid, HPLC, 64-18-6, CNW; Water, LC-MS, 7732-18-5, Fisher Chemical; 2-Propanol, HPLC, 67-63-0, Merck; 2-Chloro-L-Phenylalanine, ≥98%, 103616-89-3, Adamas-beta.
2.3. Experimental Design
A single factor completely randomized design was used to fix the nitrogen and phosphorus contents in Hoagland nutrient solution, and four potassium concentration gradients were set: K0, nutrient solution containing no K+; K1, standard Hoagland nutrient solution; K2, 1.5× Hoagland nutrient solution; and K3, 2× Hoagland nutrient solution. (Since the N element in the Hoagland formulation comes from more than two substances, ammonium bicarbonate (N), potassium chloride (K), and sodium dihydrogen phosphate (P) were used in this test under the condition of ion balance with the Hoagland nutrient solution. The concentrations after the balance are shown in Table 1). There were 4 treatments in the experiment and 20 pots for each treatment, reaching a total of 400 pots. A 20 cm × 20 cm plastic flowerpot was selected and the pot was filled with a substrate (peat:perlite = 2:1) of 3 kg for lily planting. Each pot was planted with a bulb, with a depth of about 10 cm. The pots of different treatments were digitally marked and placed on a movable seedbed in turn, watered with deionized water every 3–5 days. The seedlings of L. davidii var. unicolor (on 8 April) were irrigated with different concentrations of potassium nutrient solution, and each pot received an amount of irrigated nutrient solution of 400 mL. Samples were taken every 15 days after the potassium application, and five pots were randomly selected from each treatment for mixed sampling and sent back to the laboratory for cleaning, drying at 50 °C, crushing, and screening.

Table 1.
Different potassium concentrations applied to the seedlings of L. davidii var. unicolor (mg·L−1).
2.4. Determination of Growth Indicators
The plant height was measured as the distance from the plant base to the top with a steel tape. The stem diameter was measured at the thickest part of the stalk base by using Vernier calipers at internodes on the stem base. The effective blade number was counted on the plants from the bulb base. The chlorophyll contents were measured with a chlorophyll meter for the blades growing at the top, middle, and bottom of the plant. The bulbs were weighed, and the bulb circumference was measured with a ruler to circle the bulb.
2.5. Extracts and Determination of Polysaccharide Contents
The lily bulb extracts for polysaccharide analysis were prepared using the following methods. The lily bulbs used in the experiment were cleaned, dried in an oven, crushed by a grinder, and ground to make a dry lily bulb powder. The dry lily bulb powder was prepared and 1.00 g was placed into a 50 mL centrifuge tube, before adding 10 mL of methanol solution. After sealing, an ultrasonic extraction was conducted at 50 °C for 30 min, before centrifugation at 4 °C for 10 min (12,000 r·min−1). The supernatant was collected in a 15 mL centrifuge tube and stored in a refrigerator in the dark at 4 °C to determine the polysaccharide contents.
The phenol–sulfuric acid method was used to determine the polysaccharide contents [23] and the results were expressed as the glucose mass (mg) per gram of the fresh lily bulb sample. Next, 0.02 mL of Lanzhou lily bulb extract was placed in a 10 mL test tube, before adding 1.98 mL of methanol and 1 mL of 5% phenol reagent. The mixture was shaken and 5 mL of the concentrated sulfuric acid was added, before shaking again for 2 min and heating in a boiling water bath for 15 min. The tube was then immediately placed in a cold water bath to cool, before adding 2 mL of distilled water, shaking, and measuring the absorbance at 490 nm. Three replicates were analyzed for each treatment.
2.6. HPLC Analysis for Polysaccharide
The sample extraction: 50 mg of lily bulb was placed in a 2 mL centrifuge tube, before adding 6 mm diameter grinding beads and 400 μL of the extract (the extract was methanol:water = 4:1 (v:v), containing 0.02 mg·mL−1 L-2-chlorophenylalanine). Under the condition of −10 °C, 50 Hz, it was put into the freeze grinding machine for grinding over a course of 6 min. At 5 °C, with a 40 KHz low temperature ultrasonic extraction at 30 min, the extract was placed under the condition of −20 °C for 30 min and then centrifuged for 15 min. Finally, the supernatant was removed to the sample bottle for intubated machine analysis.
The chromatographic conditions: UPLC was conducted using an ACQUITY UPLC HSS T3 chromatographic column (Waters, Milford, USA, with a column temperature of 40 °C, mobile phase A comprising 95% water + 5% acetonitrile (containing 0.1% formic acid), and mobile phase B comprising 47.5% acetonitrile + 47.5% isopropanol + 5% water (containing 0.1% formic acid)). The mobile phase elution gradient: the flow rate was set to 0.4, 0.5, and 0.6 mL. At min−1 3.5~5.5 min, A decreased from 75.5% to 0% and B increased from 24.5% to 100%; at 7.4~7.6 min, A increased from 0% to 48.5% and B decreased from 100% to 51.5%; and at 7.8~10 min, A was 100% and B was 0%.
2.7. Data Analysis
All experiments were repeated three times and the results were expressed as the mean ± standard deviation. Excel 2010 was used for the data processing, SPSS 19.0 software was used to conduct the analysis of variance (ANOVA), and Duncan’s multiple comparison test was applied to detect the significance differences at p < 0.050. Pearson’s correlation coefficients were calculated to detect the correlations. Origin 2018 software was used for mapping. A multivariate statistical analysis (Python v1.0.0 and R language ropls package) was used for the chemometric analyses, i.e., partial least squares-discriminant analysis (PLS-DA).
3. Results and Analysis
3.1. Effects of Potassium Application on Growth and Development of L. davidii var. unicolor
The effects of the potassium application on the growth and development of L. davidii var. unicolor are shown in Table 2, which demonstrates that the potassium application obtained a better plant growth compared with the no potassium treatment (K0). From 60 days to 75 days after the potassium application, the plant height, stem diameter, and chlorophyll content increased initially and then decreased as the potassium concentration increased. The overall L. davidii var. unicolor plant height was highest in this growth period compared with other treatments, and the maximum plant height, stem diameter, and chlorophyll content were obtained under K2 with 74.56 cm, 9.09 mm, and 71.47, respectively. From 30 days to 45 days after the fertilization, the chlorophyll content of the L. davidii var. unicolor leaves was positively correlated with the K concentration in the budding stage, and the chlorophyll content increased with the K concentration. Under different K concentrations, the bulb weight and bulb circumference increased initially but then decreased as the K concentration increased, where they were higher under all of the K treatments compared with K0. In particular, the bulb weight and bulb circumference were highest under K2. Thus, adding an appropriate K concentration could increase the bulb weight and bulb circumference, maintain the bulb growth, and continuously support the aboveground growth. During the whole growth period, compared with K0, the potassium application promoted the growth and development of L. davidii var. unicolor, and the growth parameters varied significantly under the different treatments and in the different growth stages.

Table 2.
Effects of potassium application on growth and development of L. davidii var. unicolor.
3.2. Effect of Potassium Application on Polysaccharide Content in Bulbs of L. davidii var. unicolor
As shown in Figure 1, during the whole growth period, except for 30 days of potassium application, K3 reduced the polysaccharide content compared with the K0 treatment. At 60 days, the polysaccharide content of the K3, K2, and K1 treatments was lower than that of the K0 treatment. At 15 days, 45 days, and 75 to 135 days, the potassium application increased the polysaccharide content in the bulb compared with the K0 (no potassium application). Within 45 days of the potassium fertilizer application, the polysaccharide contents increased gradually in the bulbs as the potassium application rate increased, and the polysaccharide contents were higher than those in the later growth period. After the fertilization at the 60 days mark, with the increase in the potassium concentration, the content of polysaccharide decreased first and then increased, but it was lower than that of the K0 treatment. Similarly, a previous study on potassium fertilizer application showed that the polysaccharide content was significantly higher in the early stage of the medicinal lily cultivation than the average level. Thus, potassium fertilizer is beneficial for the accumulation of the active substances in lily plants.
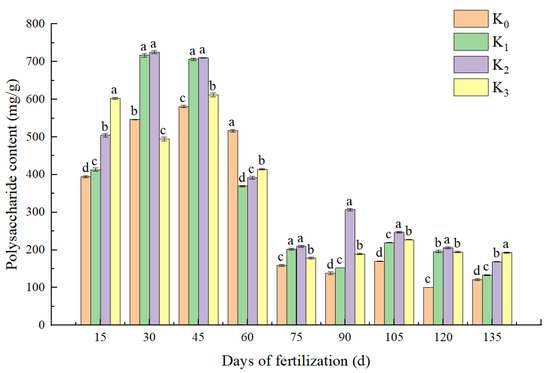
Figure 1.
Effects of potassium application on polysaccharide contents of L. davidii var. unicolor bulbs. The same letter indicates no significance between treatments, and different letters indicate significance, K concentrations comprising K0 (0 mg·L−1), K1 (447.6 mg·L−1), K2 (671.4 mg·L−1), or K3 (895.2 mg·L−1). Samples were taken every 15 days after potassium application.
3.3. Correlations between Agronomic Traits and Polysaccharide Contents of L. davidii var. unicolor
The correlations between the agronomic traits and bulb polysaccharide contents were analyzed based on the different indexes analyzed for L. davidii var. unicolor. The results showed that the factors were correlated at p < 0.05, and the correlation coefficients ranged between −0.051 and 0.82 (Figure 2). The polysaccharide contents were significantly negatively correlated with the plant height (a correlation coefficient of −0.44) and significantly positively correlated with the leaf number and chlorophyll contents, obtaining correlation coefficients of 0.45 and 0.56, respectively. The bulb circumference was positively correlated with the stem diameter and bulb weight, but the correlation with the bulb weight was highest at 0.82.
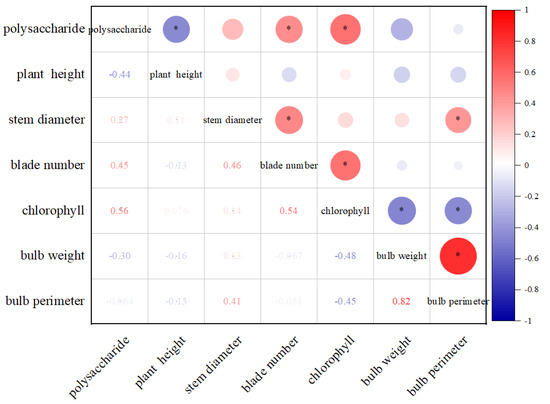
Figure 2.
Correlations between agronomic traits and polysaccharide contents of L. davidii var. unicolor. * represents correlation (p ≤ 0.05): red is positive correlation, blue is negative correlation. K concentrations comprising K0 (0 mg·L−1), K1 (447.6 mg·L−1), K2 (671.4 mg·L−1), or K3 (895.2 mg·L−1). Samples were taken every 15 days after potassium application.
3.4. Metabolomics Analysis of Polysaccharides in Bulb Extract of L. davidii var. unicolor
During the harvest period (on August 24), the metabolomics analyses were conducted to determine the polysaccharide contents under K0 and K2.
3.4.1. PLS-DA Results
After the PCA, a supervised PLS-DA was conducted to identify the different metabolites that contributed to the clusters obtained by the PCA data. According to the PLS-DA score plot (Figure 3), the data under K2 and K0 were clearly separated, thereby demonstrating that the changes in the small molecular metabolites were obvious during the growth and development of L. davidii var. unicolor after the potassium fertilizer application. In the permutation test, the two groups of R2Y > Q2Y, and the intercept is less than 0.05, indicating that the PLS-DA model is stable and reliable, and no fitting has occurred, which can explain the differences between the samples in each group. Thus, the data interpretation and prediction capacities of the PLS-DA model were good, so it could be used to further screen differential metabolites.
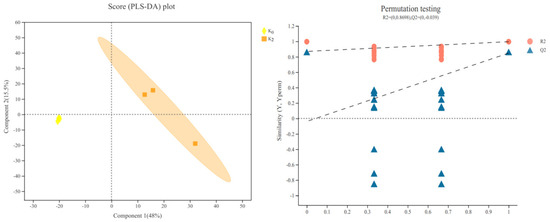
Figure 3.
PLS-DA score plot and model validation. K concentrations: K0 (0 mg·L−1), K2 (671.4 mg·L−1). The greater the degree of separation of the two groups of samples in the figure, the more significant the classification effect. The abscissa represents the permutation retention of the permutation test. The point with a permutation retention of 1 is the R2 and Q2 values of the original model. The ordinate represents the values of the R2 and Q2 permutation tests. The two dashed lines represent the regression lines of R2 and Q2, respectively.
3.4.2. Composition of Polysaccharides and Related Compounds
Thus, a cluster analysis was used to determine the metabolic patterns of the metabolites under different experimental conditions. Metabolites with similar metabolic patterns had similar functions or participated in the same metabolic process or cell pathway. A hierarchical cluster analysis was performed based on all of the identified differential metabolites and the relative quantitative values for the differential metabolites were normalized and clustered. Different color regions represent the differences in the clustering grouping information. The metabolic expression patterns in the same group were similar, where they had similar functions or participated in the same biological process. As shown in Figure 4, 37 carbohydrate metabolites were screened from bulbs of L. davidii var. unicolor treated under K0 and K2, and the types and contents of each metabolite differed. Compared with the different metabolites under K0, the different metabolites under K2 varied significantly, and the sugar content was higher under K2.
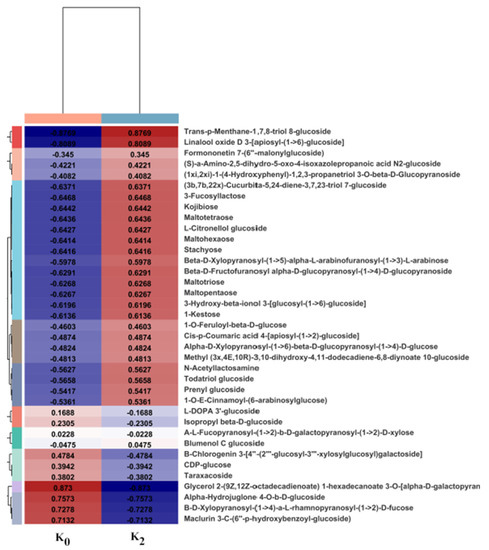
Figure 4.
Cluster heat maps of polysaccharides and related compounds in lily bulb extracts. K concentrations: K0 (0 mg·L−1), K2 (671.4 mg·L−1). Each column represents a sample and each row represents a metabolite. The colors in the figure represent the relative expression levels of metabolites in a group of samples. The left-hand side shows the metabolite clustering tree and the right-hand side shows the names of the metabolite. The expression of two metabolites is closer when their branches are closer.
3.4.3. Differential Metabolites of Polysaccharides and Related Compounds
Based on the variable importance projection and fold change (FC) differences according to the PLS-DA, we screened the differential metabolites of polysaccharides and the related compounds in lily bulb extracts treated with different potassium concentrations (Table 3). We identified 37 different metabolites of polysaccharides under different potassium concentrations, where 25 were significantly upregulated and 12 were significantly downregulated, including B-D-Xylopyranosyl-(1->4)-a-L-rhamnopyranosyl-(1->2)-D-fucose.

Table 3.
Differential metabolites of polysaccharides and related compounds.
3.4.4. Analysis of Differential Metabolites Using Venn Diagrams
The differential metabolites screened after the pairwise comparisons of the bulb samples of L. davidii var. unicolor treated with K0 (0 mg·L−1) and K2 (671.4 mg·L−1) were analyzed using Venn diagrams. Clearly, K0 and K2 shared common polysaccharides and specific polysaccharides. As shown in Figure 5, 29 polysaccharide metabolites were screened under K0 and 37 polysaccharide metabolites were screened under K2, with 29 common polysaccharide metabolites and eight different polysaccharide metabolites: maltotetraose, (3b,7b,22x)-cucurbita-5,24-diene-3,7,23-triol 7-glucoside, L-citronellol glucoside, (1xi,2xi)-1-(4-hydroxyphenyl)-1,2,3-propanetriol 3-O-beta-D-glucopyranosid, cis-p-coumaric acid 4-[apiosyl-(1->2)-glucoside], 1-O-E-cinnamoyl-(6-arabinosylglucose), maltohexaose, and (S)-a-amino-2,5-dihydro-5-oxo-4-isoxazolepropanoic acid N2-glucoside.
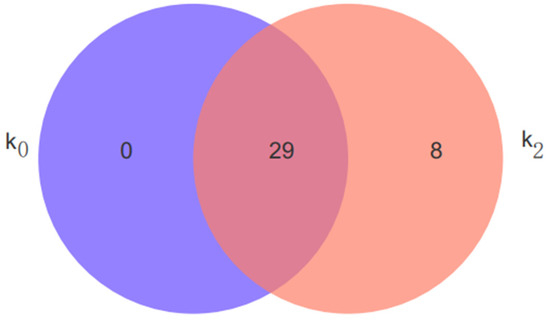
Figure 5.
Venn diagram of differential metabolites under K0 and K2. K concentrations: K0 (0 mg·L−1), K2 (671.4 mg·L−1). The overlapping portions in the figure represent the number of metabolites shared by multiple metabolic sets, the non-overlapping portions represent the number of metabolites unique to the metabolic set, and the numbers represent the number of corresponding metabolites.
3.4.5. Kyoto Encyclopedia of Genes and Genomes (KEGG) Classifications and Functional Pathways
The KEGG database analysis was conducted to classify the compounds found in the samples to annotate the related metabolic pathways (Figure 6). Eight KEGG classifications comprising vitamins and cofactors, peptides, nucleic acids, hormones and transmitters, steroids, organic acids, lipids, and carbohydrates were assigned to 45 compounds. The KEGG metabolic pathway annotations were obtained for 154 compounds, where they were mainly assigned to metabolism, human diseases, genetic information processing, and environmental information processing.
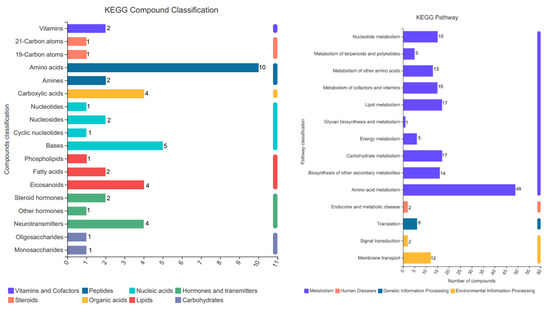
Figure 6.
KEGG classifications and functional pathways. During the harvest period (August 24), metabolomics analyses were conducted. K concentrations: K0 (0 mg·L−1), K2 (671.4 mg·L−1). The ordinate is the KEGG compound classification, and the abscissa is the number of compounds annotated to the class; the color representation of the bar belongs to the compound primary category.
3.4.6. Analysis of Pathways for Differential Metabolites
A pathway enrichment analysis was conducted for the differential metabolites based on the KEGG database in order to understand the mechanisms associated with the changes in the differential metabolites in various metabolic pathways. As shown in Table 4, 37 differential metabolites screened under K0 and K2 were assigned to three metabolic pathways: the galactose metabolism, amino sugar and nucleotide sugar metabolism, and starch and sucrose metabolism. The main enriched differential metabolites were stachyose, maltotriose, and CDP-glucose, where CDP-glucose is involved in multiple metabolic pathways.

Table 4.
KEGG metabolic pathways assigned to lily bulb polysaccharide-related metabolites.
4. Discussion
L. davidii var. unicolor has attracted much attention because of its bioactive components, especially its polysaccharides [24]. Potassium is an important macro-nutrient for plant growth and development, and the plant requirements for K are higher than those for any other macro-nutrient after nitrogen [25,26]. In this experiment, the dynamic changes of growth and the accumulation of the polysaccharide content in bulbs of L. davidii var. unicolor under different K application rates in different sampling periods were studied, and a metabolomics analysis was carried out. The results showed that potassium could promote the development of underground bulbs and leaf growth, increase the plant height and stem diameter, increase the chlorophyll content, and make full use of light energy to enhance the photosynthetic efficiency, thus promoting the plant’s growth and development. We also found that the bulb weight and bulb circumference decreased significantly with an excessive K fertilizer application, as also found in other crops [27,28], possibly because the application of an excessive amount of K hinders the absorption of other nutrients such as N and P, thereby negating the positive effects of K fertilization [29]. Potassium is involved in the synthesis, transportation, and transformation of sugars, where it can inhibit acid invertase and promote the activity of sucrose phosphate synthase to decrease the hydrolysis of sucrose and promote the accumulation of polysaccharides [30]. The polysaccharide content decreases in the 75 to 135 d timepoints compared to the earlier timepoints. This could be between 75 and 135 days, where the aboveground part began to gradually age, the nutrients transported downward, the underground bulbs expanded, more nutrients were accumulated, the bulb K content increased, and an excessive potassium concentration inhibited the accumulation of the polysaccharide content. As the correlation analysis shows, the stem diameter was positively correlated with the polysaccharide content, as also found in a previous study of L. davidii var. unicolor [31]. A recent review showed that many of the compounds related to the medicinal functions of L. davidii var. unicolor have not yet been identified. Therefore, it is necessary to classify and identify the important compounds found in L. davidii var. unicolor [32]. Non-targeted metabolomics was used to detect and identify the polysaccharides in the bulbs of L. davidii var. unicolor. There were differences in the composition and content of the polysaccharides in the bulbs of L. davidii var. unicolor treated with different potassium concentrations. We identified 37 different metabolites of polysaccharides, of which 25 polysaccharides and its related compounds were significantly upregulated, and 12 were significantly downregulated. Metabolite B-D-Xylopyranosyl- (1->4) -a-L-rhamnopyranosyl-(1->2)-D-fucose has an important role in salt tolerance [33]. Through a hierarchical cluster analysis, compared with the different metabolites under K0, the different metabolites under K2 varied significantly, and the sugar content was higher under K2. The glucose content in monochrome corm was very high. This was consistent with a previous analysis of 13 endogenous free sugars in different lily samples [34]. The common differential metabolites of polysaccharides were found in the bulbs of L. davidii var. unicolor with different potassium application rates, and they were enriched into multiple metabolic pathways, the galactose metabolism, amino sugar and nucleotide sugar metabolism, and starch and sucrose metabolism, which indicated that the nutrients in the bulbs were constantly metabolized after the potassium application, and the types and quantities of the polysaccharides metabolites involved were increasing, which are consistent with the glycometabolism pathway in Grifola frondosa [35]. Galactose is a polysaccharide component in many primary cell walls and its metabolism is closely related to cell wall expansion during growth [36].
5. Conclusions
In this study, the application of potassium at a concentration of 671.4 mg·L−1 effectively promoted the growth and development of L. davidii var. unicolor and the accumulation of polysaccharides in the bulb compared with the no potassium treatment (K0). Non-targeted metabolomics analysis screened out 37 differential polysaccharide metabolites under K0 and K2. The metabolic pathways enriched for polysaccharide-related differential metabolites comprised the galactose metabolism, amino sugar and nucleotide sugar metabolism, and starch and sugar metabolism. This experiment can further determine transcriptomics, combined with metabolomics, to study the mechanism of potassium fertilizer regulating the polysaccharide accumulation and metabolism.
Author Contributions
X.S.: conceptualization; data curation; formal analysis; investigation; supervision; and writing—original draft. P.Z.: conceptualization; investigation; project administration; resources; formal analysis; and supervision. Y.Y.: investigation; supervision; formal analysis; and project administration. H.B.: investigation; formal analysis; data curation; and supervision. Y.M.: investigation; formal analysis; data curation; and supervision. L.J.: conceptualization; investigation; formal analysis; resources; funding acquisition; supervision; and writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Chunhui Program of the Chinese Ministry of Education (2016), the West First Discipline Fund (No. NXYLXK2017B03).
Informed Consent Statement
Not applicable.
Acknowledgments
This project is supported by the Chunhui Program of the Chinese Ministry of Education (2016). We also thank all trial participants for their contributions to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, C.L.; Hou, X.M.; Qi, N.N.; Li, C.X.; Luo, Y.Y.; Hu, D.L.; Li, Y.H.; Liao, W.B. An optimized method to obtain high-quality RNA from different tissues in Lilium davidii var. unicolor. Sci. Rep. 2022, 12, 2825. [Google Scholar] [CrossRef] [PubMed]
- Li, W.M.; Wang, Y.J.; Wei, H.L.; Zhang, Y.B.; Guo, Z.H.; Qiu, Y.; Wen, L.R.; Xie, Z.K. Structural characterization of Lanzhou lily (Lilium davidii var. unicolor) polysaccharides and determination of their associated antioxidant activity. J. Sci. Food Agr. 2020, 100, 5603–5616. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.A.; Rumbeiha, W.; Nair, M.G. Constituents in Easter lily flowers with medicinal activity. Life Sci. 2004, 76, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Huang, G.L. Preparation and immunological activity of polysaccharides and their derivatives. Int. J. Biol. Macromol. 2018, 112, 211–216. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, T.; Jin, Z.Y.; Xu, X.M.; Wang, J.H.; Zha, X.Q.; Chen, H.Q. Structural characterisation, physicochemical properties and antioxidant activity of polysaccharide from Lilium lancifolium Thunb. Food Chem. 2015, 169, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Huang, G.L. The antioxidant activities of carboxymethylated garlic polysaccharide and its derivatives. Int. J. Biol. Macromol. 2019, 140, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.P.; Ma, X.; Ma, L.; Zhang, J.; Zhang, W.M.; Song, X.P. Antioxidative and immunological activities of ophiopogon polysaccharide liposome from the root of Ophiopogon japonicus. Carbohyd. Polym. 2016, 135, 110–120. [Google Scholar] [CrossRef]
- Han, H.P.; Xie, H.C. A study on the extraction and purification process of lily polysaccharide and its anti-tumor effect. Afr. J. Tradit. Complem. 2013, 10, 485–489. [Google Scholar] [CrossRef]
- Pan, G.F.; Xie, Z.W.; Huang, S.X.; Tai, Y.L.; Cai, Q.S.; Jiang, W.; Sun, J.M.; Yuan, Y. Immune-enhancing effects of polysaccharides extracted from Lilium lancifolium Thunb. Int. Immunopharmacol. 2017, 52, 119–126. [Google Scholar] [CrossRef]
- Hou, J.; Zhu, Y.X.; Li, Y.; Zhang, B.N. Synergistic antitumor activity of Lily polysaccharide combined with metformin on human liver cancer hepG2 cells. J. Liaoning Univ. Tradit. Chin. Med. 2017, 19, 30–32. [Google Scholar] [CrossRef]
- Sun, X.; Gao, R.L.; Xiong, Y.K.; Huang, Q.C.; Xu, M. Antitumor and immunomodulatory effects of a water-soluble polysaccharide from Lilii Bulbus in mice. Carbohyd. Polym. 2014, 102, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Cui, G.; Feng, Q.; Xiao, Y. Evaluation of Hypoglycemic Activity of the Polysaccharides Extracted from Lycium Barbarum. Afr. J. Tradit. Complem. 2010, 6, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.W.; Chen, Z.Q.; Ramesh, K.S.; Xu, L.L.; Gao, X.D.; Ma, Q.Q.; Xue, Z.H.; Chen, H.X. Hypoglycemic effects of polysaccharides from corn silk (Maydis stigma) and their beneficial roles via regulating the PI3K/Akt signaling pathway in L6 skeletal muscle myotubes. Int. J. Biol. Macromol. 2018, 121, 981–988. [Google Scholar] [CrossRef]
- Zhu, M.D.; Luo, J.; Lv, H.W.; Kong, L.Y. Determination of anti-hyperglycaemic activity in steroidal glycoside rich fraction of lily bulbs and characterization of the chemical profiles by LC-Q-TOF-MS/MS. J. Funct. Foods. 2014, 6, 585–597. [Google Scholar] [CrossRef]
- Furtini, A.E.N.; Boldrin, K.V.F.; Mattson, N.S. Nutrion and quality in ornamental plants Ornam Hortic. J. Hortic. Sci. Biotechnol. 2015, 21, 139–150. [Google Scholar]
- Carneiro, D.N.M.; Almeida, E.F.A.; Paiva, P.D.D.; Frazao, J.E.M.; Santos, F.H.D.; Carneiro, L.F. Carneiro Development and dry mass accumulation in calla lily at the initial cultivation stage. Sci. Agric. Technol. 2011, 35, 1085–1092. [Google Scholar] [CrossRef]
- Carneiro, D.N.M.; Coelho, L.L.; Paiva, P.D.O. Carneiro Evaluation of macronutrient demand in calla lily (Zantedeschia aethiopica). Aust. J. Crop Sci. 2015, 9, 761–766. [Google Scholar]
- Yang, Q.Y.; Zong, J.H.; Huang, P. Growth of edible lily (Lilium davidii var. unicolor) and bulb yield responses to potassium fertiliser and plastic film mulching. J. Hortic. Sci. Biotechnol. 2015, 90, 115–120. [Google Scholar] [CrossRef]
- Mao, Y.F.; Li, Z.L.; Duan, Q.; Du, W.W.; Cui, G.F. Study on the differences of nutrients in four species of lilium. J. Yunnan Agric. Univ. 2017, 32, 366–370. [Google Scholar]
- Qu, W.H.; Zhou, R.B.; He, Y.S.; Tong, Q.Z. Efects of different manure on lily quality. J. Chin. Med. Mater. 2005, 28, 79–81. [Google Scholar] [CrossRef]
- Yin, X.J.; Lin, X.Y.; Liu, Y.X.; Irfan, M.; Chen, L.J.; Zhang, L. Integrated metabolic profiling and transcriptome analysis of pigment accumulation in diverse petal tissues in the lily cultivar “Vivian”. BMC Plant Biol. 2020, 20, 446. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.Y.; Yang, L.P.; Yang, T.J.; Fu, Y.Y.; Han, L.; Quan, J. Cultivation of a new polyploid cultivar ”Yubaihe 1”. Mol. Plant Breed. 2020, 18, 4714–4724. [Google Scholar] [CrossRef]
- Chen, Z.G.; Zhu, Q.; Wang, F. Purification and molecular mass determination of lily polysaccharides. Food Sci. 2013, 34, 1–4. [Google Scholar]
- Song, S.; Liu, X.Y.; Zhao, B.T. Effects of Lactobacillus plantarum Fermentation on the Chemical Structure and Antioxidant Activity of Polysaccharides from Bulbs of Lanzhou Lily. ACS Omega 2021, 6, 29839–29851. [Google Scholar] [CrossRef] [PubMed]
- Bishwoyog, B.; Swarnima, K. Effect of potassium on quality and yield of potato tubers-A Review. Int. J. Agric. Environ. 2016, 3, 7–12. [Google Scholar]
- Slameto; Leni, N.; Indri, F.; Riza, Y.; Kacung, H. Effect of potassium fertilizer on growth, capsaicin and ascorbic acid content of local and hybrid chili (Capsicum annum L.). Plant Cell Biotechnol. Mol. Biol. 2021, 22, 337–345. [Google Scholar]
- Soliman, S.S.; Alebidia, A.I.; Al-Obeed, R.S.; Al-Saif, A.M. Effect of potassium fertilizer on fruit quality and mineral composition of fig (Ficus carica L. cv. Brown Turky). Pak. J. Bot. 2018, 50, 1753–1758. [Google Scholar]
- Wang, M.M.; Ye, Y.L.; Chu, X.; Zhao, Y.A.; Zhang, S.H.; Chen, H.; Qin, W.; Wang, Y. Responses of garlic quality and yields to various types and rates of potassium fertilizer applications. Hortscience 2022, 57, 72–80. [Google Scholar] [CrossRef]
- Huu, N.H.; Maneepong, S.; Suranin, P.P. Effects of potassium, calcium, and magnesium ratios in soil on their uptake and fruit quality of pummelo. J. Agr. Sci. Tech.-Iran. 2017, 9, 110. [Google Scholar] [CrossRef]
- Li, Q. Effects of Combined Application of Potassium Fertilizer and Organic Fertilizer on Soil Enzyme Activity, Soil Nutrients and Yield of Edible Lily; Gansu Agricultural University: Gansu, China, 2018. [Google Scholar]
- Zhu, H.; Wang, Z.J.; Wu, Y.L.; Jiang, H.T.; Zhou, F.; Xie, X.H.; Wang, R.L.; Hua, C. Untargeted metabonomics reveals intervention effects of chicory polysaccharide in a rat model of non-alcoholic fatty liver disease. Int. J. Biol. Macromol. 2019, 128, 363–375. [Google Scholar] [CrossRef]
- Zhou, J.; An, R.F.; Huang, X.F. Genus Lilium: A review on traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2021, 270, 113852. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Z.; Zayed, O.; Zeng, F.S.; Liu, C.X.; Zhang, l.; Zhu, P.P.; Hsu, C.C.; Tuncil, Y.E.; Tao, W.E.; Carpita, N.C.; et al. Arabinose biosynthesis is critical for salt stress tolerance in Arabidopsis. New Phytol. 2019, 224, 274–290. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Wang, H.; Lang, L.X.; Dou, X.Y.; Bai, J.R. Analysis of the contents of 13 intrinsic free sugars in different lily samples. Food Sci. 2021, 42, 249–254. [Google Scholar]
- Lei, L.; Wu, T.X.; Wang, C.N. Analysis of metabolic differences in fermentation of Grifola frondosa based on UPLC-QTOF-MS metabolomics. Mycosystema 2020, 39, 1920–1932. [Google Scholar] [CrossRef]
- Zhang, H.M.; Liu, J.Y. Molecular cloning and characterization of a beta-galactosidase gene expressed preferentially in cotton fibers. J. Integr. Plant Biol. 2005, 47, 223–232. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).