High Nitrogen Fertilization Decreases Seed Weight but Increases Longevity in Tomato Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Treatments
2.3. Measurements
2.3.1. Fruit and Seed Weight
2.3.2. Standard Germination
2.3.3. Physiological Germination
2.3.4. Seed Longevity
2.4. Statistical Analysis
3. Results
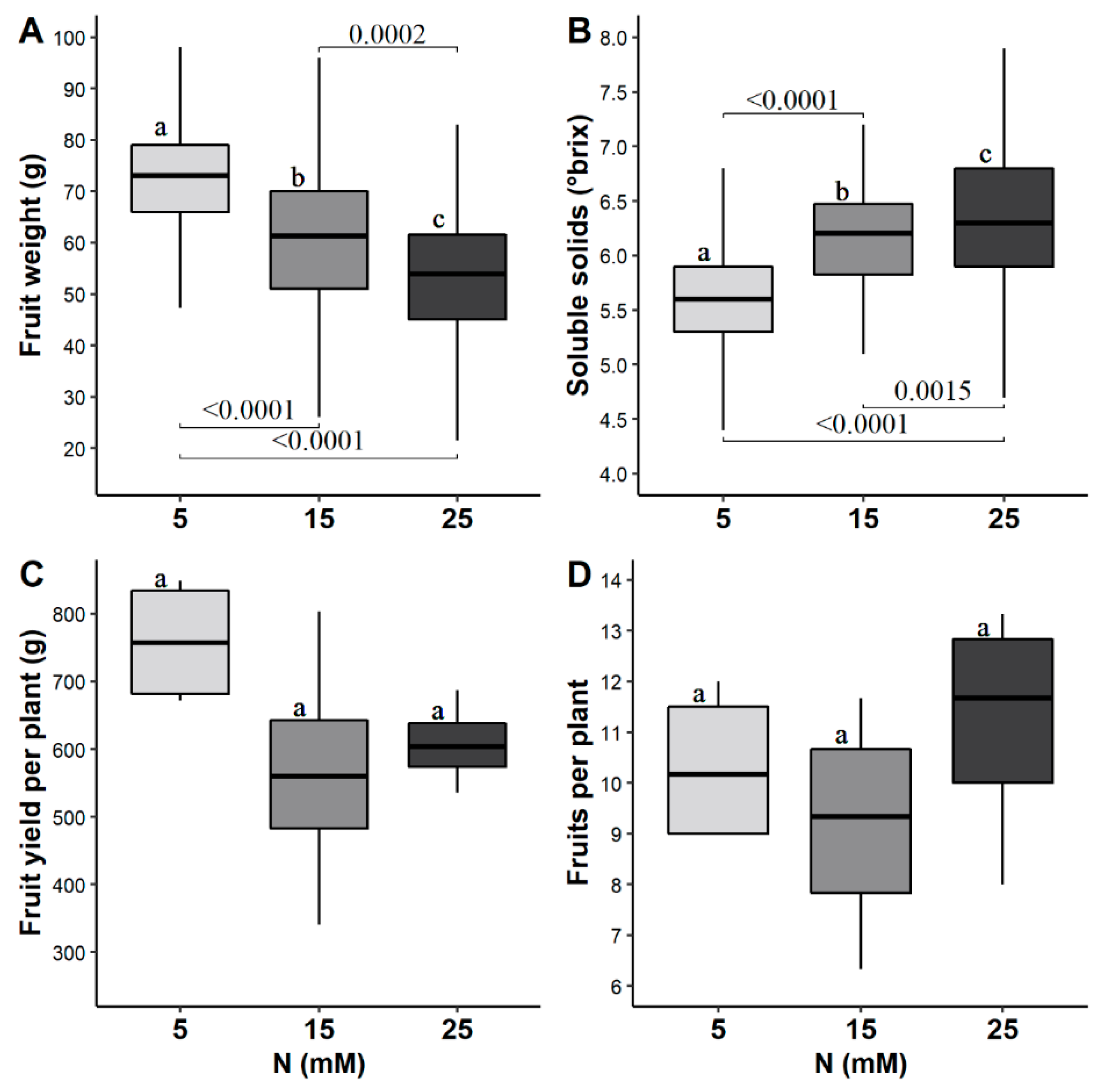
3.1. Fruit Yield and Quality
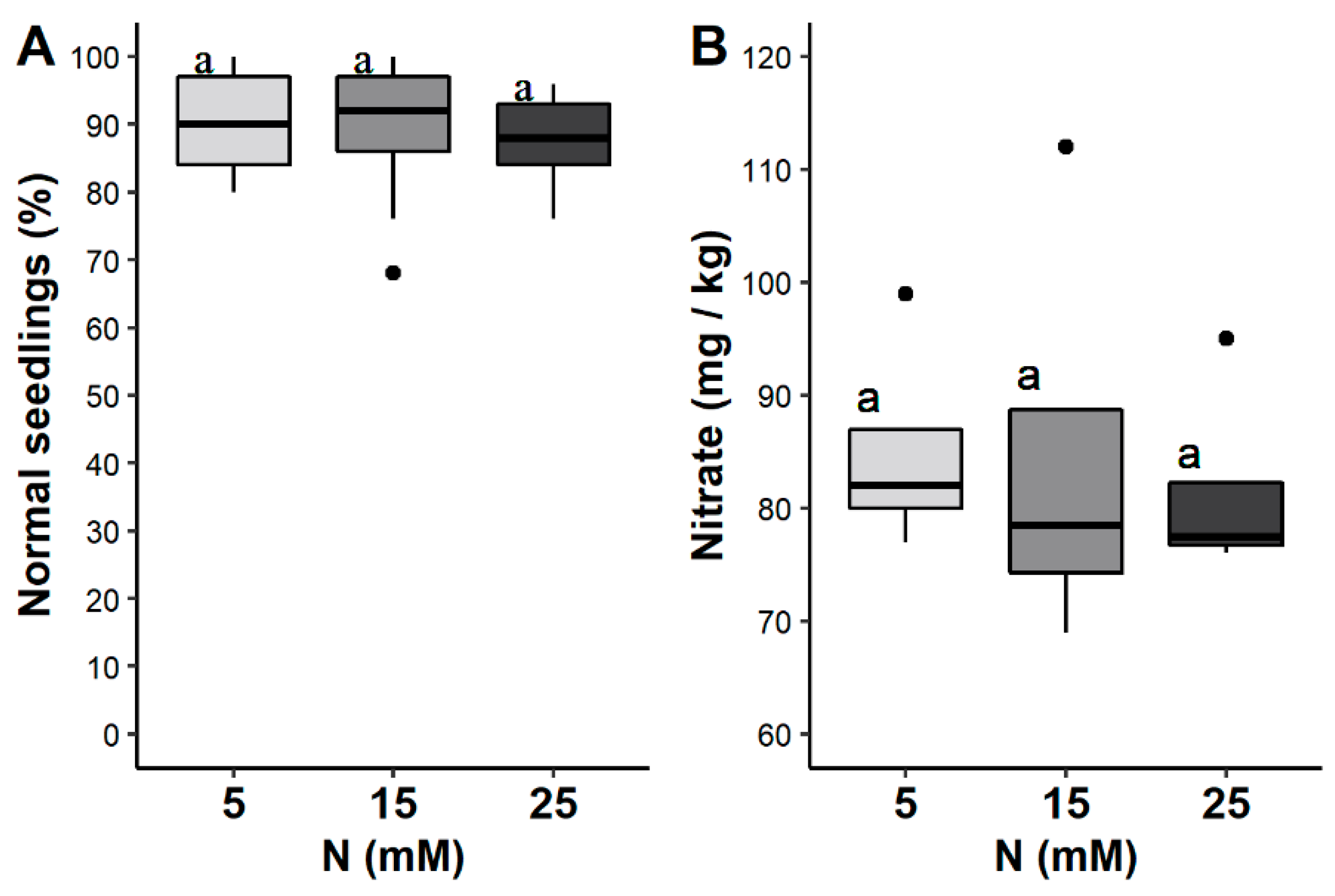
3.2. Seed Yield and Nitrate Content
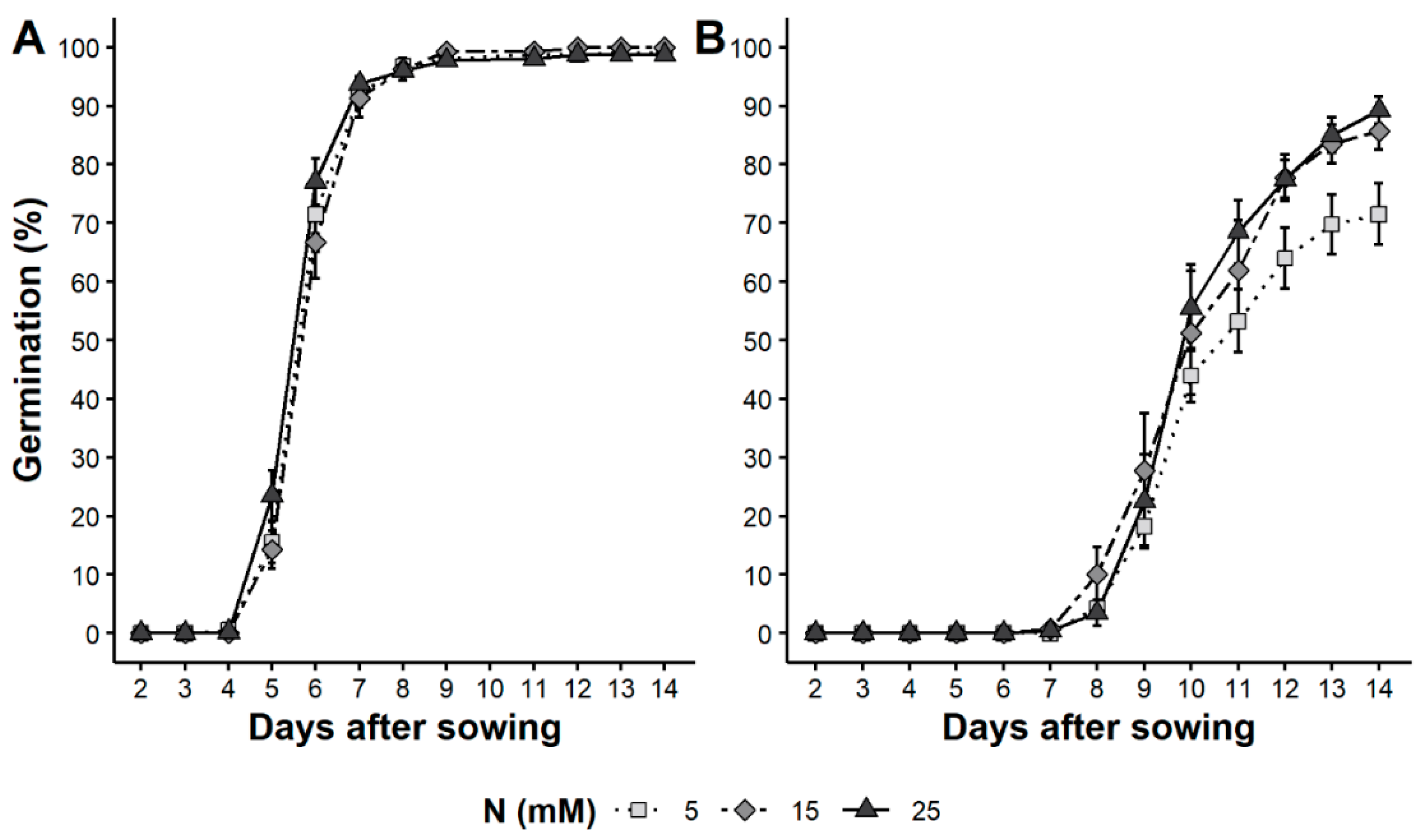
3.3. Standard and Physiological Germination
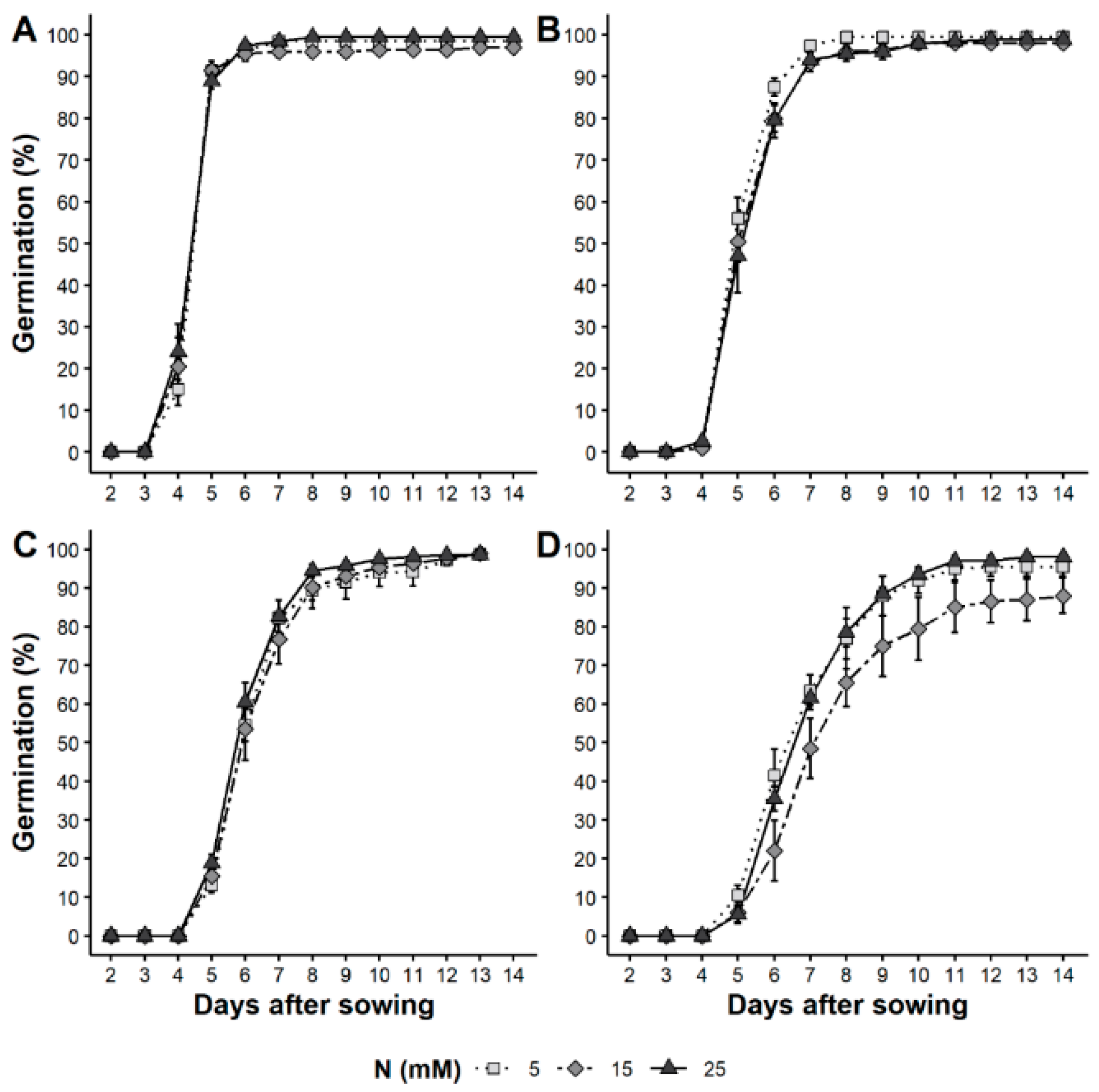
3.4. Longevity
4. Discussion
4.1. Fruit Yield and Quality
4.2. Seed Yield and Weight
4.3. Seed Physiological Quality
4.4. Dormancy and Nitrate Content
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cheng, M.; Wang, H.; Fan, J.; Xiang, Y.; Tang, Z.; Pei, S.; Zeng, H.; Zhang, C.; Dai, Y.; Li, Z.; et al. Effects of nitrogen supply on tomato yield, water use efficiency and fruit quality: A global meta-analysis. Sci. Hortic. 2021, 290, 110553. [Google Scholar] [CrossRef]
- Parisi, M.; Burato, A.; Pentangelo, A.; Ronga, D. Towards the optimal mineral N fertilization for improving peeled tomato quality grown in Southern Italy. Horticulturae 2022, 8, 697. [Google Scholar] [CrossRef]
- George, R.A.T. Vegetable seed production. In Vegetable Seed Production; George, R.A.T., Ed.; CABI: Oxfordshire, UK, 2009; pp. 104–115. [Google Scholar]
- Reed, R.C.; Bradford, K.J.; Khanday, I. Seed germination and vigor: Ensuring crop sustainability in a changing climate. Heredity 2022, 128, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Varis, S.; George, R.A.T. The influence of mineral nutrition on fruit yield, seed yield and quality in tomato. J. Hortic. Sci. 1985, 60, 373–376. [Google Scholar] [CrossRef]
- Eryuce, N.; Aydin, S. The effects of different nitrogen, phosphorus, potassium fertilizer application on tomato seed properties. In Optimization of Plant Nutrition; Fragoso, M.A.C., Van Beusichem, M.L., Houwers, A., Eds.; Springer: Dordrecht, The Netherlands, 1993; pp. 435–438. [Google Scholar]
- Geshnizjani, N.; Sarikhani Khorami, S.; Willems, L.A.J.; Snoek, B.L.; Hilhorst, H.W.M.; Ligterink, W. The interaction between genotype and maternal nutritional environments affects tomato seed and seedling quality. J. Exp. Bot. 2019, 70, 2905–2918. [Google Scholar] [CrossRef] [PubMed]
- Albornoz, F.; Vilches, I.; Contreras, S. Managing lettuce seed quality through nitrogen nutrition in soilless production. Sci. Hortic. 2019, 252, 169–175. [Google Scholar] [CrossRef]
- Sano, N.; Poll, A. ABA metabolism and homeostasis in seed dormancy. Int. J. Mol. Sci. 2021, 22, 5069. [Google Scholar] [CrossRef] [PubMed]
- Groot, S.P.C.; Karssen, C.M. Dormancy and germination of abscisic acid-deficient tomato seeds: Studies with the sitiens mutant. Plant Phys. 1992, 99, 952–958. [Google Scholar] [CrossRef]
- Duermeyer, L.; Khodapanahi, E.; Yan, D.; Krapp, A.; Rothstein, S.J.; Nambara, E. Regulation of seed dormancy and germination by nitrate. Seed Sci. Res. 2018, 28, 150–157. [Google Scholar] [CrossRef]
- Hilhorst, H.W.M.; Karssen, C.M. The Role of Light and Nitrate in Seed Germination. In Recent Advances in the Development and Germination of Seeds; Taylorson, R.B., Ed.; Springer: Boston, MA, USA, 1989; pp. 191–205. [Google Scholar]
- Lara, T.S.; Lira, J.M.S.; Rodrigues, A.C.; Rakocevic, M.; Alvarenga, A.A. Potassium Nitrate Priming Affects the Activity of Nitrate Reductase and Antioxidant Enzymes in Tomato Germination. J. Agric. Sci. 2014, 6, 72. [Google Scholar] [CrossRef]
- Pill, W.G.; Frett, J.J.; Morneau, D.C. Germination and Seedling Emergence of Primed Tomato and Asparagus Seeds under Adverse Conditions. HortScience 1991, 26, 1160–1162. [Google Scholar] [CrossRef]
- Alboresi, A.; Gestin, C.; Leydecker, M.T.; Bedu, M.; Meyer, C.; Truong, H.N. Nitrate, a signal relieving seed dormancy in Arabidopsis. Plant Cell Environ. 2005, 28, 500–512. [Google Scholar] [CrossRef]
- Degwale, A.; Tesfa, T.; Meseret, B.; Fantaw, S. Seed extraction methods affect the physiological quality of tomato seed and developing seedlings. Int. J. Veg. Sci. 2022, 1–9. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing; The International Seed Testing Association: Bassersdorf, Switzerland, 2022. [Google Scholar]
- Michel, B.E. Evaluation of the water potentials of solutions of polyethylene glycol 8000 both in the absence and presence of other solutes. Plant Phys. 1983, 72, 66–70. [Google Scholar] [CrossRef]
- Jianhua, Z.; McDonald, M.B. The saturated salt accelerated aging test for small-seeded crops. In Seed Science and Technology; The Internatinal Seed Testing Association: Bassersdorf, Switzerland, 1997. [Google Scholar]
- Greenspan, L. Humidity fixed points of binary saturated aqueous solutions. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1977, 81, 89. [Google Scholar] [CrossRef]
- Ellis, R.H.; Roberts, E.H. Improved equation for the prediction of seed longevity. Ann. Bot. 1980, 45, 13–30. [Google Scholar] [CrossRef]
- Parisi, M.; Giordano, I.; Pentangelo, A.; D’Onofrio, B.; Villari, G. Effects of different levels of nitrogen fertilization on yield and fruit quality in processing tomato. Acta Hortic. 2006, 700, 129–132. [Google Scholar] [CrossRef]
- Albornoz, F. Crop responses to nitrogen overfertilization: A review. Sci. Hortic. 2016, 205, 79–83. [Google Scholar] [CrossRef]
- Chang, T.; Zhang, Y.; Xu, H.; Shao, X.; Xu, Q.; Li, F.; Yu, L.; Zhang, Z. Osmotic adjustment and up-regulation expression of stress-responsive genes in tomato induced by soil salinity resulted from nitrate fertilization. Int. J. Agric. Biol. Eng. 2018, 11, 126–136. [Google Scholar] [CrossRef]
- Elia, A.; Conversa, G. Agronomic and physiological responses of a tomato crop to nitrogen input. Eur. J. Agron. 2012, 40, 64–74. [Google Scholar] [CrossRef]
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2017, 217, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Agius, C.; Tucher, S.; Rozhon, W. The effect of salinity on fruit quality and yield of cherry tomatoes. Horticulturae 2022, 8, 59. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Gong, X.; Li, S.; Pang, J.; Chen, Z.; Sun, J. Optimizing irrigation and nitrogen management strategy to trade off yield, crop water productivity, nitrogen use efficiency and fruit quality of greenhouse grown tomato. Agric. Water Manag. 2021, 245, 106570. [Google Scholar] [CrossRef]
- Sun, S.; Gao, X.; Lu, Z. Effects of different nitrogen fertilization levels on quality of tomato cultivated in solar greenhouse. North. Hortic. 2011, 11, 36–37. [Google Scholar]
- Silva, A.; Melo, S.; Umbelino, B.; Sa, F.; Dias, N.; Ferreira, M. Cherry tomato production and seed vigor under irrigation with saline effluent from fish farming. Rev. Bras. Eng. Agric. Ambient. 2021, 25, 380–385. [Google Scholar] [CrossRef]
- Alvarez Medina, A.; Martínez Solís, J.; Rodríguez-Pérez, J.E.; Peña Ortega, M.G. Relationship between the physiological quality tests of tomato (Solanum lycopersicum L.). Rev. Chapingo Serie Hort. 2011, 17, 57–62. [Google Scholar] [CrossRef]
- Marcos-Filho, J. Seed vigor testing: An overview of the past, present and future perspective. Sci. Agric. 2015, 72, 363–374. [Google Scholar] [CrossRef]
- Saeed, S.; Ullah, A.; Ullah, S.; Noor, J.; Ali, B.; Nauman, M.; Hashem, M.; Mostafa, Y. Validating the impact of water potential and temperature on seed germination of wheat (Triticum aestivum L.) via hydrothermal time model. Life 2022, 12, 983. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Bewley, J.D., Bradford, K.J., Hilhorst, H.W.M., Nonogaki, H., Eds.; Springer: Dordercht, The Netherlands, 2013; pp. 247–297. [Google Scholar]
- Sano, N.; Rajjou, L.; North, H.M.; Debeaujon, I.; Marion-Poll, A.; Seo, M. Staying alive: Molecular aspects of seed longevity. Plant Cell Physiol. 2016, 57, 660–674. [Google Scholar] [CrossRef]
- Nigam, M.; Mishra, A.; Salehi, B.; Kumar, M.; Sahrifi-Rad, M.; Coviello, E.; Iriti, M.; Sharifi-Rad, J. Accelerated ageing induces physiological and biochemical changes in tomato seeds involving MAPK pathways. Sci. Hortic. 2019, 248, 20–28. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, F.; Luo, X.; Dai, Y.; Yang, Y.; Zheng, C.; Yang, W.; Shu, K. A matter of life and death: Molecular, physiological, and environmental regulation of seed longevity. Plant Cell Environ. 2020, 43, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Zinsmeister, J.; Leprince, O.; Buitink, J. Molecular and environmental factors regulating seed longevity. Biochem. J. 2020, 477, 305–323. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Kafkafi, U.; Wolf, S.; Sugimoto, Y. Mother plant nutrition and growing condition affect amino and fatty acid composition of hybrid sweet pepper seeds. J. Plant Nutr. 2002, 8, 1645–1665. [Google Scholar] [CrossRef]
- Gianella, M.; Doria, E.; Dondi, D.; Milanese, C.; Gallotti, L.; Börner, A.; Zannino, L.; Macovei, A.; Pagano, A.; Guzzon, F.; et al. Physiological and molecular aspects of seed longevity: Exploring intra-species variation in eight Pisum sativum L. accessions. Physiol Plant. 2022, 174, e13698. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Oliveira, R. Plant Physiological Ecology, 3rd ed.; Springer: Dordecht, The Netherlands, 2019. [Google Scholar]
- Taiz, L.; Moller, I.; Murphy, A.; Zeiger, E. Plant Physiology and Development, 7th ed.; Sinauer Associates: Sunderland, MA, USA, 2022. [Google Scholar]
- Ali, F.; Qanmber, G.; Li, F.; Wang, Z. Updated role of ABA in seed maturation, dormancy, and germination. J. Adv. Res. 2022, 35, 199–214. [Google Scholar] [CrossRef]
- Bareke, T. Biology of seed development and germination physiology. Adv. Plants Agric. Res. 2018, 8, 336–346. [Google Scholar] [CrossRef]
- Soedjana, A.A.; Hasegawa, I.; Noguchi, A.; Tanaka, H.; Tsuchiya, K.; Yazaki, J. Effects of nitrogen application on the yield and quality of F1-hybrid tomato seeds. In Plant Nutrition for Sustainable Food Production and Environment; Ando, T., Fujita, K., Mae, T., Matsumoto, H., Mori, S., Sekiya, J., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 961–962. [Google Scholar]
- Yan, A.; Chen, Z. The Control of Seed Dormancy and Germination by Temperature, Light and Nitrate. Bot. Rev. 2020, 86, 39–75. [Google Scholar] [CrossRef]
- He, H.; De Souza Vidigal, D.; Snoek, L.B.; Schnabel, S.; Nijveen, H.; Hilhorst, H.; Bentsink, L. Interaction between parental environment and genotype affects plant and seed performance in Arabidopsis. J. Exp. Bot. 2014, 65, 6603–6615. [Google Scholar] [CrossRef]
- Hilhorst, H.W.M.; Downie, B. Primary dormancy in tomato (Lycopersicon esculentum cv. Moneymaker): Studies with the sitiens mutant. J. Exp. Bot. 1996, 47, 89–97. [Google Scholar] [CrossRef]


| Treatment (N Concentration) | Fertilizer Concentration (mM) | EC (dS m−1) |
|---|---|---|
| 5 mM | 4 KCl, 2 CaCl2, 2.5 Ca(NO3)2 × 4H2O, 1KH2PO4, 2 MgSO4 × 7H2O | 2.2 |
| 15 mM | 5 NH4NO3, 4 KCl, 2 CaCl2, 2.5 Ca(NO3)2 × 4H2O, 1 KH2PO4, 2 MgSO4 × 7H2O | 3.0 |
| 25 mM | 10 NH4NO3, 4 KCl, 2 CaCl2, 2.5 Ca(NO3)2 × 4H2O, 1 KH2PO4, 2 MgSO4 × 7H2O | 3.8 |
| Parameter | Evaluation Time | Water Potential (MPa) | |||
|---|---|---|---|---|---|
| 0 | −0.10 | −0.15 | −0.20 | ||
| Germination (%) | 5 months | 99.3 a A | 82.2 b B | - | - |
| 8 months | 98.3 a A | 98.8 a A | 99.3 a | 93.8 b | |
| MGT (days) | 5 months | 6.2 b A | 10.5 a A | - | - |
| 8 months | 4.9 d B | 5.7 c B | 6.6 b | 7.3 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, J.; Albornoz, F.; Contreras, S. High Nitrogen Fertilization Decreases Seed Weight but Increases Longevity in Tomato Seeds. Horticulturae 2022, 8, 942. https://doi.org/10.3390/horticulturae8100942
Sánchez J, Albornoz F, Contreras S. High Nitrogen Fertilization Decreases Seed Weight but Increases Longevity in Tomato Seeds. Horticulturae. 2022; 8(10):942. https://doi.org/10.3390/horticulturae8100942
Chicago/Turabian StyleSánchez, Javier, Francisco Albornoz, and Samuel Contreras. 2022. "High Nitrogen Fertilization Decreases Seed Weight but Increases Longevity in Tomato Seeds" Horticulturae 8, no. 10: 942. https://doi.org/10.3390/horticulturae8100942
APA StyleSánchez, J., Albornoz, F., & Contreras, S. (2022). High Nitrogen Fertilization Decreases Seed Weight but Increases Longevity in Tomato Seeds. Horticulturae, 8(10), 942. https://doi.org/10.3390/horticulturae8100942






