Abstract
Cremastra appendiculata var. variabilis (Blume) I.D. Lund), also known as single-leaf cremastra (SLC), is a rare and threatened species native to Korea, and it has the potential to be grown as a beautiful flowering pot or garden plant. There is still no reliable strategy to multiply SLC. Thus, an effective method for propagating single-leaf cremastra was needed for its conservation and mass production. In the present study, we examined the effects of culture media, seed age, activated charcoal, and plant growth regulators on in vitro asymbiotic seed germination, secondary protocorm induction, and seedling formation. Asymbiotic seed germination of SLC was influenced by culture media, seed age, and their interaction. The addition of activated charcoal (500 mg/L) to the Murashige and Skoog (MS) medium increased the rate of germination. The seeds were best germinated (91.9%) by culturing on MS medium supplemented with activated charcoal (500 mg/L), α-naphthaleneacetic acid (3 µM), and kinetin (1 µM). The highest number (28.9) of secondary protocorms were produced when protocorms were cultured on MS medium containing 6-benzyladenine (4 µM) and kinetin (2 µM). When the protocorms were cultivated in a medium containing gibberellic acid (1 µM), they were able to transform into SLC with the highest success rate (78.7%). The propagation protocol described here may be helpful for SLC restoration programs and large-scale production.
1. Introduction
Cremastra appendiculata var. variabilis (Blume) I.D. Lund, commonly known as single-leaf or warty lip cremastra, is a terrestrial orchid belonging to the genus Cremastra that possesses pseudobulbs. Single-leaf cremastra (SLC) is distributed in central and southern China, Korea, southern Sakhalin and Kuril Islands, Japan, Thailand, and northern Vietnam. It occurs in mountain streams, hillsides, and forests [1]. SLC has variegated leaves and beautiful fragrant flowers [2] and can be used as an ornamental pot and garden plant. It is one of the rare, threatened species in Korea, and the natural population of SLC has decreased recently due to deforestation, environmental condition (climate change), urbanization, and illegal collection [3]. The conventional methods (sexual and asexual) used in the propagation of SLC are inefficient due to the low seed germination rate and the shortage of pseudobulbs (tubers). Thus, alternative propagation methods are required for its conservation and commercial production.
In vitro propagation methods have been used to multiply endangered, rare, threatened, and commercial plants [4,5]. There are several reports detailing the in vitro propagation of Cremastra appendiculata [6,7,8]. Zhang et al. [8] studied the impact of endophyte extract and plant growth regulators (PGR) on protocorm initiation using apical buds. The protocorms of C. appendiculata obtained on Murashige and Skoog [9] (MS) medium containing 10.0 mg/L endophyte extract, 2.0 mg/L 6-Benzyle adenine (BA) and 0.5 mg/L 2,4-dichlorophenoxyacetic acid (2,4-D) were sprouted and rooted best on half-strength MS medium containing 10.0 mg/L endophyte extract, and 0.5 mg/L of each α-naphthaleneacetic acid (NAA) and indole-3-butyric acid (IBA). Gao et al. [7] tested the influence of symbiotic fungus on in vitro seed germination of C. appendiculata. Mycorrhizal fungus (Coprinellus disseminatus) promoted higher germination (71.61%) of C. appendiculata seeds as compared with asymbiotic germination (9.68%). On the other hand, Yang et al. [8] obtained a high frequency (76.42%) of asymbiotic germination when the C. appendiculata seeds were cultured in MS medium with 1.0 mg/L NAA and 75 g/L potato extract. However, there are no reported protocols for the propagation of SLC (Cremastra appendiculata var. variabilis) using in vitro culture techniques.
Asymbiotic in vitro-seed germination method has been extensively used for the mass production of terrestrial orchids such as Bletilla striata [10], Cyrtopodium paludicolum [11], and Spathoglottis plicata [12]. Numerous orchid species, including C. appendiculata (D. Don). Makino [7,8] has also been multiplied by symbiotic or asymbiotic in vitro-seed germination [13,14,15,16]. Thus, asymbiotic germination of SLC seeds in vitro permits higher multiplication rates than conventional propagation methods. Indeed, the rate of asymbiotic seed germination in orchids is associated with the age of the seeds [17], culture media [18], activated charcoal (AC) [19], organic extracts [20], and PGR [21,22]. In this study, we described an in vitro propagation protocol of SLC. We investigated the effect of seed age, culture media, AC, and PGR on asymbiotic seed germination, protocorm multiplication (secondary protocorm formation), and SLC development. The optimized method described here will be helpful in the large-scale propagation of SLC.
2. Materials and Methods
2.1. Plant Materials and Surface Sterilization
Seed capsules collected from hand-pollinated greenhouse-grown SLC plants at three distinct ages (132, 159, and 210 days) were thoroughly washed in running tap water and then rinsed with distilled water (DH2O). After air drying, capsules of SLC were sterilized in ethanol (EtOH; 70%) for 2.5 min, sodium hypochlorite (NaClO; 3.0%) for 16 min, and mercuric chloride (HgCl2; 0.2%) for 12 min. After being treated with EtOH, NaClO, and HgCl2, the capsules of SLC were washed with autoclaved DH2O 2, 4, and 3 times respectively.
2.2. In Vitro Seed Germination
Seeds extracted from the surface-sterilized SLC capsules were placed on Murashige and Skoog (MS) [9], half-MS, Knudson C [23] (KC), and Hyponex [(N-P-K, 6.5-6-19) 3 g/L (Hyponex Japan Corp. Ltd., Osaka, Japan) with peptone (2 g/L), banana (30 g/L), and apple (20 g/L)] media contained sucrose (30 g/L) and plant agar (8 g/L) to examine the effect of media on seed germination. To study the usefulness of activated charcoal (AC) in improving asymbiotic germination, the seeds of SLC were placed on a culture medium (MS contained 30 g/L sucrose and 8 g/L plant agar) with 0–2000 mg/L AC. To examine the effectiveness of PGRs in improving in vitro germination, the seeds of SLC were placed on a culture medium with 500 mg/L AC and 0–10 µM indole-3-acetic acid (IAA), indole-3-butyric acid (IBA), or α-naphthaleneacetic acid (NAA) and then supplemented with 0–8 µM kinetin (KIN) and 3.0 µM NAA. Before autoclaving at 123 °C for 23 min, the pH of all seed germination medium was adjusted to 5.7–5.8 using potassium hydroxide (0.1 N) or hydrochloric acid (0.1 N). The SLC seed containers (500 mL bottles) were maintained for 90 days at 23 ± 2 °C under 5 µmol m–2 s–1 for 12 h with white light emitting diodes (WLED) light. Seeds of SLC randomly removed from each culture bottle were fixed and observed under a light microscope. For each treatment, ten 500 mL culture bottles (more than 1500 seeds placed in each bottle) were used, and the experiment was repeated twice. The seed germination percentage was calculated after 90 days as the number of germinated seeds/total number of seeds × 100 [24].
2.3. Secondary Protocorm Formation and Conversion
The protocorms developed from SLC seeds were placed on a culture medium containing 2, 4, or 8 µM 6-benzyladenine (BA) combined with 1, 2, or 4 µM KIN and maintained for 60 days at 23 ± 2 °C under 30 µmol m–2 s–1 for 16 h with WLED light. For each treatment, fifteen 500 mL culture bottles (5 protocorms placed in each bottle) were used, and the experiment was repeated twice. The number of secondary SLC protocorms and their conversion rate were recorded after 60 days.
2.4. Protocorm Conversion and SLC Development
For conversion and SLC development, the protocorms were placed on a culture medium containing 0–3 µM gibberellic acid (GA3) and maintained under 10 µmol m–2 s–1 for 12 h with WLED light for 45 days and then placed under 45 µmol m–2 s–1 for 16 h with WLED light for 45 days at 23 ± 2°C. Five protocorms were placed in each of the fifteen 500 mL culture bottles for each treatment, and the experiment was repeated twice. The protocorm conversion rate was recorded after 90 days.
2.5. Statistical Analysis
Analysis of variance (ANOVA) of the collected data was done using the SAS (SAS Institute, NC, USA) statistical software release 9.4. The averages were separated by Duncan’s multiple range test (DMRT) at the 5% level of significance.
3. Results
3.1. Influence of Culture Medium and Seed Age on Asymbiotic SLC Germination
The surface disinfection method of SLC seed capsules generated a 100% of aseptic seed culture. The sterile seeds obtained from SLC capsules placed on a germination medium (Figure 1a) were swollen after ten days of culture (Figure 1b). After 90 days of incubation of the SLC seed culture, yellowish-green protocorms with rhizoids were formed (Figure 1c,d). The cultivation media (F value: 71.08), seed age (F value: 102.45) and interaction of cultivation media and seed age (F value: 6.10) had a significant (p < 0.0001) effect on SLC seed germination (Table 1). Among the four SLC seed cultivation media tested, a high frequency of germination was obtained on MS (25.6%) medium followed by half-strength MS (19.6%), KC (16.1%), and Hyponex (6.8%) media. The mean asymbiotic germination of 159-day-old SLC seeds was higher (25.8%) than that obtained for 132-day-old (15.8%) and 210-day-old (9.6%) SLC seeds. A maximum germination rate (39.1%) was obtained when 159-day-old SLC seeds were cultivated on the MS medium (Table 1). Thus, MS medium and 159-day-old SLC seeds were selected for further asymbiotic germination studies.
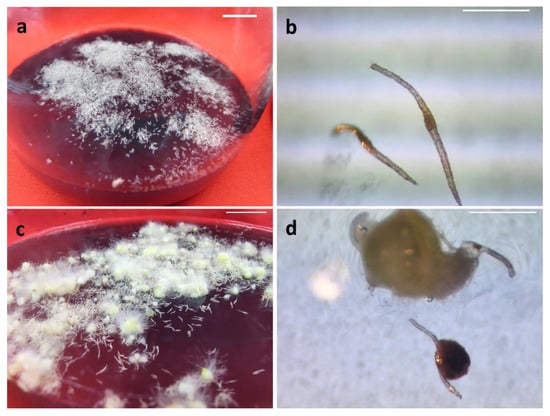
Figure 1.
In vitro asymbiotic SLC seed germination: (a) Seeds extracted from SLC capsules were placed on culture media (0-day); (b) Light micrograph of SLC seeds (10-day); (c) Induction of protocorms (90-day); (d) Light micrograph of protocorm formation (60-day). Scale bar. (a,c) 10 mm; (b,d) 0.1 mm.

Table 1.
Effect of media and seed age on seed germination.
3.2. Impact of AC on Asymbiotic SLC Seed Germination
Asymbiotic germination of SLC is influenced significantly (p < 0.0001) by AC. Low doses of AC in the MS medium improved the rate of SLC seed germination. The mean germination rates of SLC were 39.1%, 41.9%, 48.7%, 35.7%, and 27.3% for AC concentrations of 0, 250, 500, 1000, and 2000 mg/L, respectively (Table 2).

Table 2.
Effect of activated charcoal on seed germination.
3.3. Impact of Auxins on Asymbiotic SLC Seed Germination
Auxin (F value: 16.30), concentration (F value: 117.25) and their interaction (F value: 14.05) had a significant (p < 0.0001) effect on SLC seed germination (Table 3). Of the three auxins tested, NAA (57.1%) was more effective in enhancing SLC seed germination than IAA (52.1%) and IBA (50.5%). Among the different concentrations (1.5–10.0 µM) of auxins tested, 3.0 µM resulted in a higher SLC seed germination rate (64.4%) than 5.0 µM (55.2%), 1.5 µM (54.6%), and 10.0 µM (38.7%). About 48.7% of SLC seeds germinated on the control (auxin-free) medium. The mean germination rates of SLC were 52.1%, 54.7%, 58.3%, and 43.3% for IAA concentrations of 1.5, 3.0, 5.0, and 10.0 µM, respectively (Table 3). The average germination rates of SLC were 56.2%, 65.1%, 46.8%, and 33.9% for IBA concentrations of 1.5, 3.0, 5.0, and 10.0 µM, respectively. The germination rate of SLC seeds on a culture medium with 1.5–10.0 M NAA was between 38.9% to 73.4%. Increasing the concentration of NAA in the culture medium from 1.5–5.0 µM enhanced the SLC germination percentage. However, the germination rate (38.9%) decreased in the presence of 10.0 µM NAA. On a culture medium containing 3.0 µM NAA, the highest seed germination rate (73.4%) was observed (Table 3).

Table 3.
Effect of auxins on seed germination.
3.4. Impact of KIN and NAA on Asymbiotic SLC Seed Germination
The addition of 1–4 µM KIN to a culture medium containing 3 µM NAA significantly enhanced (p < 0.0001) the rate of SLC seed germination (Table 4). However, the addition of 8 µM KIN decreased germination by 63.8% in SLC seeds. The highest rate (91.9%) of seed germination was obtained on a culture medium containing 2 µM KIN and 3 µM NAA.

Table 4.
Effect of KIN (1–8 µM) and NAA (3.0 µM) on seed germination.
3.5. Effects of BA and KIN on Secondary Protocorm Formation and Conversion
The primary protocorms from SLC seeds on a PGR-free media failed to develop secondary protocorms. Including varied concentrations (2–8 µM) of BA in combination with 1–4 µM KIN in the culture medium promoted secondary protocorm induction (Figure 2a,b). After 14 days of incubation, the explants produced secondary protocorms, and after 45 days, the secondary protocorms developed rhizoids and shoot primordia (Figure 2c). However, significant (p < 0.05) differences were observed in the number of secondary protocorms and their conversion rate. Of the various BA and KIN combinations tested, the highest number of secondary protocorms (28.9) was produced on a culture medium with 4 µM BA and 2 µM KIN (Table 5, Figure 2d). About 18% of secondary protocorms on this medium were converted to SLC seedlings. On the other hand, the highest conversion rate (41.6%) was found on a culture medium containing 2 µM BA and 1 µM KIN; however, only 4.4 secondary protocorms were produced.
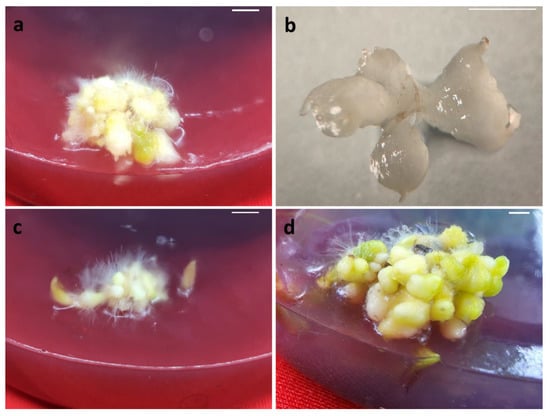
Figure 2.
Induction and conversion of SLC secondary protocorms: (a) formation of secondary protocorms (30-day); (b) light micrograph of secondary protocorms (30-day); (c) conversion of secondary protocorms (45-day); (d) secondary protocorm formation (60-day). Scale bar. (a,c,d) 10 mm; (b) 0.1 mm.

Table 5.
Effects of BA and KIN on secondary protocorm formation and conversion.
3.6. Impact of GA3 on the Conversion of SLC Protocorms
The protocorm developed shoot and roots (within 45 days) when the cultures were kept under low WLED light (Figure 3a). The well-developed SLC seedlings with pseudo bulb were obtained after 90 days of incubation under high WLED light (Figure 3b). In a GA3-free growing media, about 19.2% of protocorms were converted into SLC. The concentrations of GA3 had a significant (p < 0.05) effect on the conversion of SLC protocorms. The average conversion rates of SLC protocorms were 54.2%, 78.7%, 62.3%, and 40.6% for GA3 concentrations of 0.5, 1.0, 2.0, and 3.0 µM, respectively (Table 6).
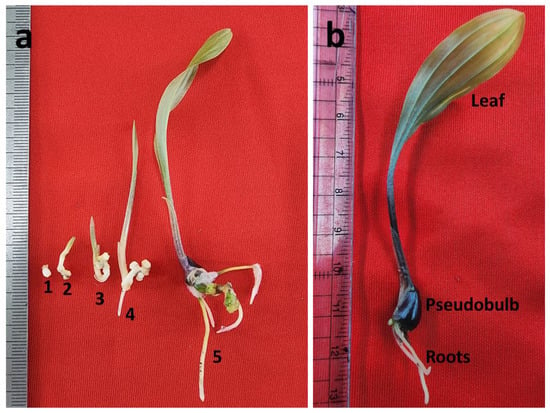
Figure 3.
Protocorm conversion and SLC development: (a) different stages of protocorm conversion (1–4) and seedling development (5); (b) SLC seedlings with pseudobulb. 1 (0-day), 2 (18-day), 3 (30-day), 4 (45-day), and 5 (90-day).

Table 6.
Effect of various concentrations of GA3 on the conversion (%) of SLC protocorms.
4. Discussion
Surface sterilization of plant materials is a crucial step in plant tissue culture [25]. Asymbiotic germination of orchid seed in vitro was commonly impeded by contamination of the culture medium. In this study, a method for surface sterilization of SLC seeds in order to accomplish asymbiotic germination in vitro. Frequently, the composition of asymbiotic culture medium affects the germination of terrestrial orchid seeds. In this study, the MS medium produced the highest germination rate overall. On the other hand, the germination rate of SLC seeds that were grown on half-strength MS and KC media was similar (Table 1). The MS medium can promote SLC seed germination in vitro due to its high nutrition content. Reducing the nutrients in the MS medium had a negative effect on SLC seed germination. This result indicates that SLC seeds require a high concentration of mineral nutrients in order to germinate in vitro. The total mineral nutrient concentration of MS, half-strength MS and KC media were 96.02 (mM), 48.01 (mM), and 46.72 (mM), respectively [26]. The seed germination of various terrestrial orchids such as C. appendiculata [8,27], Paphiopedilum bellatulum, Paphiopedilum godefroyae, Paphiopedilum helenae, Paphiopedilum henryanum, Paphiopedilum niveum, Paphiopedilum spicerianum [28], Pecteilis radiata [22], and Phaius tancarvilleae [29] was attained best using MS medium. The age of the seed also affects the asymbiotic germination rate of orchids [17,30]. However, the effect of seed age on non-symbiotic Cremastra germination has not been reported. In this study, 159-day-old SLC seeds showed higher germination frequency than 132-day-old and 210-day-old seeds. The result indicates that seed age is critical in enhancing the germination rate in SLC. The germination rate in 210-day-old SLC seeds significantly decreased (Table 1). Several factors may contribute to the low germinability of mature seeds of various orchids, such as the presence of chemical inhibitors [31], impermeable testa [30], and lack of germination-promoting phytohormones [32].
In some orchid species, the addition of AC to the seed-cultivation medium enhanced the germination rate [19,20,22,24]. The germination rate of SLC seedlings was increased by 500 mg/L of AC added to the medium (Table 2). It has been reported that AC absorbs chemical inhibitors and nutrients from the growth media [33], thereby improving SLC seeds’ germinability in vitro. Besides culture media, seed age, and AC, another vital factor, PGR, has also shown a significant effect on SLC seed germination (Table 3 and Table 4). In asymbiotic in vitro propagation, various PGRs have often been used to increase the seed germination rate of several orchids [14,21,22,32], including C. appendiculata [8,27]. Yang et al. [8] examined the effects of different levels of auxins (IAA, IBA, and NAA) in MS medium on the germination of C. appendiculata seeds and found that the greatest rate (76.42%) of asymbiotic germination with 5.4 µM of NAA. Similarly, NAA at 3.0 µM yielded the best (73.4%) germination rate in SLC. However, a combined treatment of KIN (2 µM) and NAA (3 µM) was most effective at enhancing (91.9%) germination of SLC seed. In contrast, Gao et al. [7] noticed a low germination rate (9.68%) when the C. appendiculata seeds were cultured in MS medium with 1.0 mol/L NAA and 1.0 mol/L BA.
Protocorms obtained from the orchid seeds are often used as explants for the mass production of seedlings. The multiplication and conversion of protocorms are often influenced by PGR [32]. This study observed protocorm multiplication only on cytokinin-containing MS medium. Similarly, cytokinin has been used to achieve protocorm multiplication in Cymbidium aloifolium [34], Gastrochilus matsuran [24], Gastrochilus japonicus [35], and Gastrodia pubilabiata [36]. Yang et al. [8] examined the effects of BA, IBA, and NAA on PLB multiplication in C. appendiculata. They found the best protocorm proliferation rate (170.07%) with a half-MS medium containing 1 mg/L each of BA and NAA and 0.2 g/L AC. In this study, the multiplication of SLC protocorm was achieved best on MS medium with 4 µM BA and 2 µM KIN, but the conversion of protocorm is low. Protocorms, on the other hand, were best converted to shoots on half-MS with 2.0 mg/L thidiazuron and 0.2 mg/L NAA. The requirement of PGR and their concentrations for the multiplication and conversion of protocorm can vary with orchid species [8,14,17,22].
Auxins, cytokinins, GA3, and chemical additives have been found to convert protocorms into seedlings [10,14,22,24,32,35]. In this study, SLC-protocorm converted best (78.7%) when the medium contained 1.0 µM GA3. GA3 has been shown to promote in other orchids, such as Cattleya tigrine [37] and Thrixspermum japonicum [38]. However, when significant levels of GA3 are added to the medium, the conversion rate decreases. High GA3 levels have also been shown to have adverse effects on protocorm conversion in C. tigrine [37] and T. japonicum [38].
5. Conclusions
In this study, we investigated the effects of seed age, culture medium, AC, and PGR on the asymbiotic seed germination of SLC, a rare, threatened Korean ornamental plant. We found that seed age and culture medium had a significant effect on SLC seed germination. The optimal seed age and culture media for asymbiotic germination were 159-days and MS medium, respectively. The addition of AC and PGR in the culture medium increased the rate of SLC seed germination. The in vitro-derived protocorms multiplied best in cytokinin-containing media. Protocorm conversion, seedlings development, and pseudobulb were observed when the secondary protocorms were grown in the presence of 1.0 µM GA3. Acclimatization and reintroduction of SLC seedlings to their natural environment will be the subject of future research.
Author Contributions
Conceptualization, K.W.K. and I.S.; methodology, P.K.S., K.W.K., and I.S.; resources, P.K.S. and K.W.K.; data curation, I.S.; writing—original draft preparation, I.S.; writing—review and editing, M.F. and I.S.; funding acquisition, I.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All relevant data are within the article.
Acknowledgments
This article was supported by the KU Research Professor Program of Konkuk University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chung, M.Y.; Lopez-Pujol, J.; Moon, M.O.; Maki, M.; Yukawa, T.; Sugiura, N.; Chung, M.G. Population history of the terrestrial orchid Cremastra appendiculata var. variabilis from Korea, inferred from levels and distribution of genetic diversity. Bot. J. Linn. Soc. 2013, 173, 721–732. [Google Scholar] [CrossRef][Green Version]
- Sugiura, N. Pollination biology of Cremastra appendiculata var. variabilis (Orchidaceae). Plant Species Biol. 1996, 11, 185–187. [Google Scholar] [CrossRef]
- Chung, M.Y.; López-Pujol, J.; Son, S.; Suh, G.U.; Yukawa, T.; Chung, M.G. Patterns of genetic diversity in rare and common orchids focusing on the Korean peninsula: Implications for conservation. Bot. Rev. 2018, 84, 1–25. [Google Scholar] [CrossRef]
- Park, H.Y.; Kim, D.H.; Sivanesan, I. Micropropagation of Ajuga species: A mini review. Biotechnol. Lett. 2017, 39, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kim, K.-S.; Ak, G.; Zengin, G.; Cziáky, Z.; Jekő, J.; Adaikalam, K.; Song, K.; Kim, D.H.; Sivanesan, I. Establishment of a rapid micropropagation system for Kaempferia parviflora wall. Ex Baker: Phytochemical analysis of leaf extracts and evaluation of biological activities. Plants 2021, 10, 698. [Google Scholar] [CrossRef]
- Zhang, M.S.; Wu, S.J.; Jie, X.J.; Zhang, L.X.; Jiang, X.H.; Du, J.C.; Qi, J.L.; Liu, Z.; Yang, Y.H. Effect of endophyte extract on micropropagation of Cremastra appendiculata (D. Don) Makino (Orchidaceae). Propag. Ornam. Plants 2006, 6, 83–89. [Google Scholar]
- Gao, Y.Y.; Peng, S.J.; Hang, Y.; Xie, G.F.; Ji, N.; Zhang, M.S. Mycorrhizal fungus Coprinellus disseminatus influences seed germination of the terrestrial orchid Cremastra appendicualta (D Don). Makino. Sci. Hortic. 2022, 293, 110724. [Google Scholar] [CrossRef]
- Yang, N.; Wang, D.; Gao, Y.; Hu, E.; Yu, X.; Peng, S.; Ji, J.; Zhang, M.S. An efficient micropropagation protocol, chemical components, and hypoglycemic activity for Cremastra appendiculata (D. Don) Makino pseudobulbs. In Vitro Cell. Dev. Biol. Plant 2022, 58, 213–224. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Kahraman, M.U.; Cullum, F.J. Asymbiotic Germination and Seedling Development of Terrestrial Orchid Bletilla striata Using in vitro and ex vitro Cultures. Hortic. Stud. 2021, 38, 1–14. [Google Scholar] [CrossRef]
- Ferreira, W.D.M.; Oliveira, A.M.D.; Viana, J.C.; Suzuki, R.M.; Oliveira, J.R.G.D. Asymbiotic germination, initial development in vitro and acclimatization of Cyrtopodium paludicolum Hoehne, a Brazilian Savanna orchid species. Rodriguésia 2022, 73, e01272020. [Google Scholar] [CrossRef]
- Barrientos, B.A.B.; Fang, J.Y. Influence of photoperiod and culture medium on the speed of asymbiotic seed germination and seedling development in Spathoglottis plicata. HortScience 2019, 54, 1570–1575. [Google Scholar] [CrossRef]
- Jabin Bello-Bello, J.; Zavala-Ruiz, J.; Cruz-Huerta, N.; Baltazar-Bernal, O. In vitro germination and development of the trumpetist orchid (Myrmecophila grandiflora Lindl.) using ebb-and-flow bioreactor. Propag. Ornam. Plants 2020, 20, 88–95. [Google Scholar]
- Yao, L.; Huang, J.; Zhang, S. An Improved Protocol for Asymbiotic Seed Germination and Seedling Development of Paphiopedilum tigrinum. Horticulturae 2021, 7, 298. [Google Scholar] [CrossRef]
- Arcidiacono, M.; Catalano, C.; Motisi, A.; Sajeva, M.; Carimi, F.; Carra, A. Influence of Culture Conditions on In vitro Asymbiotic Germination of Anacamptis longicornu and Ophrys panormitana (Orchidaceae). Plants 2021, 10, 2543. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Camarena, M.; Ortega-Larrocea, M.P. Mesoamerican Cypripedium: Mycorrhizal Contributions to Promote Their Conservation as Critically Endangered Species. Plants 2022, 11, 1554. [Google Scholar] [CrossRef]
- Mayo-Mosqueda, A.; García-Hernández, E.; Noguera-Savelli, E.; Cetzal-Ix, W.; Alatorre-Cobos, F. Advances in Breeding, Bioprospecting, and In vitro Culture of Laelia Orchid Species. Horticulturae 2022, 8, 103. [Google Scholar] [CrossRef]
- Kaur, S. In vitro Propagation of Vanda testacea (Lindl.) Reichb. F, a Medicinally Important Threatened Orchid. Plant Tissue Cult. Biotechnol. 2021, 31, 153–160. [Google Scholar] [CrossRef]
- Bazzicalupo, M.; Calevo, J.; Adamo, M.; Giovannini, A.; Copetta, A.; Cornara, L. Seed Micromorphology, In vitro Germination, and Early-Stage Seedling Morphological Traits of Cattleya purpurata (Lindl. & Paxton) Van den Berg. Horticulturae 2021, 7, 480. [Google Scholar]
- An, J.; Kim, P.B.; Park, H.B.; Kim, S.; Park, H.J.; Lee, C.W.; Lee, B.-D.; Kim, N.Y.; Hwang, J.E. Effects of Different Growth Media on In vitro Seedling Development of an Endangered Orchid Species Sedirea japonica. Plants 2021, 10, 1193. [Google Scholar] [CrossRef]
- Calevo, J.; Copetta, A.; Marchioni, I.; Bazzicalupo, M.; Pianta, M.; Shirmohammadi, N.; Cornara, L.; Giovannini, A. The use of a new culture medium and organic supplement to improve in vitro early stage development of five orchid species. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2022, 1, 143–151. [Google Scholar] [CrossRef]
- Kim, D.H.; Kang, K.W.; Enkhtaivan, G.; Jan, U.; Sivanesan, I. Impact of activated charcoal, culture medium strength and thidiazuron on non-symbiotic in vitro seed germination of Pecteilis radiata (Thunb.) Raf. S. Afr. J. Bot. 2019, 124, 144–150. [Google Scholar] [CrossRef]
- Knudson, L. A new nutrient solution for germination of orchid seed. Am. Orchid. Soc. Bull. 1946, 15, 214–217. [Google Scholar]
- Kang, H.; Kang, K.W.; Kim, D.H.; Sivanesan, I. In vitro Propagation of Gastrochilus matsuran (Makino) Schltr., an Endangered Epiphytic Orchid. Plants 2020, 9, 524. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Gopal, J.; Sivanesan, I. Nanomaterials in plant tissue culture: The disclosed and undisclosed. RSC Adv. 2017, 7, 36492–36505. [Google Scholar] [CrossRef]
- Dutra, D.; Johnson, T.R.; Kauth, P.J.; Stewart, S.L.; Kane, M.E.; Richardson, L. Asymbiotic seed germination, in vitro seedling development, and greenhouse acclimatization of the threatened terrestrial orchid Bletia purpurea. Plant Cell Tissue Organ Cult. 2008, 94, 11–21. [Google Scholar] [CrossRef]
- Tian, L.; Gao, Y.Y.; Yang, N.X.; Peng, S.J.; Zhang, M.S. Effects of plant growth regulators for seed embryos development of Cremastra appendiculata. Mol. Plant Breed. 2021, 19, 3090–3095. [Google Scholar]
- Lee, Y.-I. The asymbiotic seed germination of six Paphiopedilum species in relation to the time of seed collection and seed pretreatment. Acta Hortic. 2007, 755, 381–386. [Google Scholar] [CrossRef]
- Pant, B.; Swar, S. Micropropagation of Cymbidium iridioides. Nepal. J. Sci. Technol. 2011, 12, 91–96. [Google Scholar] [CrossRef]
- Kunakhonnuruk, B.; Inthima, P.; Kongbangkerd, A. In vitro propagation of Epipactis flava Seidenf, an endangered rheophytic orchid: A first study on factors affecting asymbiotic seed germination, seedling development and greenhouse acclimatization. Plant Cell Tissue Organ Cult. 2018, 135, 419–432. [Google Scholar] [CrossRef]
- Xu, X.; Fang, L.; Li, L.; Ma, G.; Wu, K.; Zeng, S. Abscisic Acid Inhibits Asymbiotic Germination of Immature Seeds of Paphiopedilum armeniacum. Int. J. Mol. Sci. 2020, 21, 9561. [Google Scholar] [CrossRef] [PubMed]
- Kauth, P.J.; Dutra, D.; Johnson, T.R.; Stewart, S.L.; Kane, M.E.; Vendrame, V. Techniques and applications of in vitro orchid seed germination. In Floriculture, Ornamental and Plant Biotechnology: Advances and Topics Issues; da Silva, J.A.T., Ed.; Global Science Books: Isleworth, UK, 2008; pp. 375–391. [Google Scholar]
- Thomas, T.D. The role of activated charcoal in plant tissue culture. Biotechnol. Adv. 2008, 26, 618–631. [Google Scholar] [CrossRef]
- Kumar, A.; Chauhan, S.; Rattan, S.; Warghat, A.R.; Kumar, D.; Bhargava, B. In vitro propagation and phyto-chemical assessment of Cymbidium aloifolium (L.) Sw.: An orchid of pharma-horticultural importance. S. Afr. J. Bot. 2022, 144, 261–269. [Google Scholar] [CrossRef]
- Kim, D.H.; Kang, K.W.; Sivanesan, I. In vitro germination and seedling development of Gastrochilus japonicus (Makino) Schltr. Propag. Ornam. Plants 2019, 19, 61–65. [Google Scholar]
- Godo, T.; Hashimoto, T.; Nakata, M.; Miyoshi, K. The effects of illumination, temperature and 6-benzylaminoprine on asymbiotic seed germination and protocorm development in vitro in the achlorophyllous orchid Gastrodia pubilabiata Sawa. In Vitro Cell. Dev. Biol. Plant 2020, 56, 230–235. [Google Scholar] [CrossRef]
- Fritsche, Y.; Deola, F.; da Silva, D.A.; Holderbaum, D.F.; Guerra, M.P. Cattleya tigrina (Orchidaceae) in vitro regeneration: Main factors for optimal protocorm-like body induction and multiplication, plantlet regeneration, and cytogenetic stability. S. Afr. J. Bot. 2022, 149, 96–108. [Google Scholar] [CrossRef]
- Seon, K.M.; Kim, D.H.; Kang, K.W.; Sivanesan, I. Highly competent in vitro propagation of Thrixspermum japonicum (Miq.) Rchb.f., a rare epiphytic orchid. In Vitro Cell. Dev. Biol. Plant 2018, 54, 302–308. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).