Screening of the Volatile Composition of Moroccan Olive Oils by Using SPME/GC-MS-FID over a Two-Year Period: A Pedoclimatic Discrimination
Abstract
1. Introduction
2. Materials and Methods
2.1. Olive Oil and Soil Samples
2.2. The Study Area
2.3. Chemical and Reagents
2.4. Sample Preparation
2.5. SPME-Extraction Conditions
2.6. GC-MS and GC-FID Analyses
2.7. Soil Analysis
2.8. Statistical Analysis
3. Results and Discussion
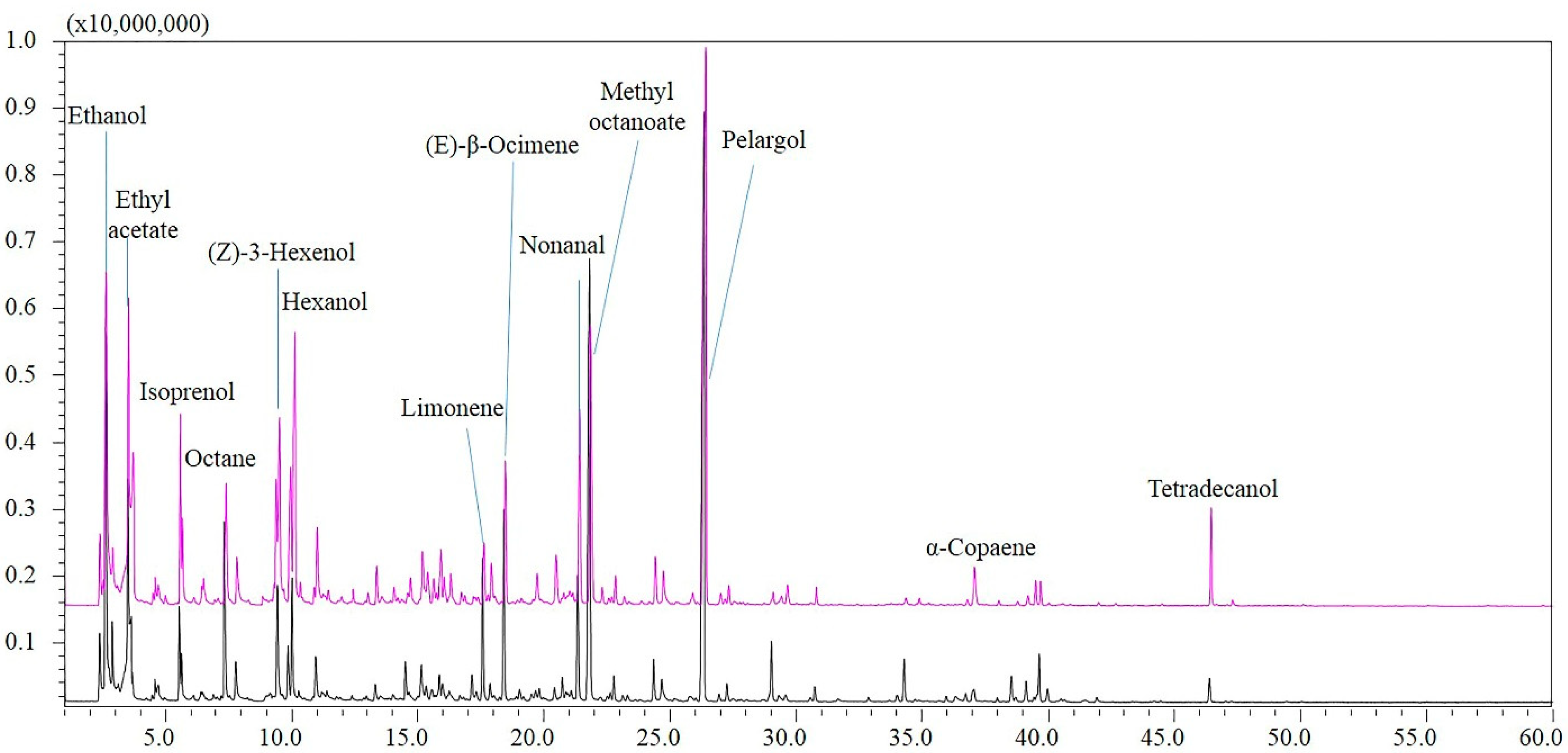
3.1. Volatile-Fraction Analysis
| Components | Geographical Area | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sp1 | Sp2 | Sp3 | Sp4 | Sp5 | Sp6 | Sp7 | Sp8 | Sp9 | Sp10 | Sp11 | Sp12 | Sp13 | Sp14 | Sp15 | Sp16 | Sp17 | Sp18 | Sp19 | ||
| Alcohols | 2018 | 34.54 hi ± 1.12 | 30.44 ab ± 0.61 | 32.01 ah ± 1.04 | 31.68 ah ± 1.03 | 36.30 fi ± 1.18 | 36.95 fi ± 1.20 | 41.13 de ± 1.34 | 27.7 b ± 0.90 | 34.69 hi ± 1.13 | 29.20 ab ± 0.95 | 45.44 c ± 1.48 | 42.50 cde ± 1.38 | 40.84 de ± 1.33 | 38.98 df ± 1.27 | 42.79 ce ± 1.24 | 43.90 ce ± 1.43 | 50.48 g ± 1.24 | 51.38 g ± 1.50 | 46.05 c ± 1.39 |
| 2019 | 39.22 dh ± 1.31 | 32.62 ac ± 1.09 | 40.11 dfh ± 1.34 | 46.91 gij ± 1.57 | 54.52 b ± 1.82 | 39.98 dfh ± 1.34 | 40.97 efh ± 1.37 | 38.87 dh ± 1.30 | 45.34 egij ± 1.52 | 28.37 a ± 0.50 | 56.92 b ± 1.90 | 36.50 cd ± 1.22 | 43.06 efgh ± 0.40 | 53.01 b ± 1.77 | 56.32 b ± 1.88 | 54.66 b ± 1.57 | 47.54 ij ± 1.59 | 48.50 i ± 1.62 | 44.02 efgj ± 1.22 | |
| Terpenes | 2018 | 28.47 j ± 0.93 | 22.77 i ± 0.68 | 14.18 g ± 0.46 | 18.49 ac ± 0.60 | 12.96 fg ± 0.42 | 17.82 abce ± 0.58 | 20.44 d ± 0.67 | 17.6 abce ± 0.57 | 16.22 e ± 0.53 | 18.28 abc ± 0.59 | 18.94 ad ± 0.62 | 17.06 bce ± 0.56 | 12.07 f ± 0.39 | 19.34 ad ± 0.63 | 16.58 be ± 0.54 | 19.11 ad ± 0.62 | 13.46 fg ± 0.54 | 13.98 g ± 0.45 | 25.94 h ± 0.84 |
| 2019 | 31.94 k ± 1.03 | 18.60 hi ± 0.60 | 18.34 dh ± 0.59 | 20.53 i ± 0.66 | 17.49 cdh ± 0.57 | 16.67 bcdh ± 0.59 | 24.67 aj ± 0.80 | 26.57 j ± 0.86 | 15.76 bc ± 0.51 | 23.37 a ± 1.04 | 16.56 bcd ± 0.54 | 39.09 e ± 1.26 | 15.15 bf ± 0.67 | 12.99 g ± 0.42 | 15.98 bc ± 0.52 | 16.38 bcd ± 0.40 | 12.67 g ± 0.41 | 13.31 fg ± 0.43 | 15.77 bc ± 0.51 | |
| Hydrocarbons | 2018 | 7.26 c ± 0.24 | 12.44 h ± 0.40 | 2.96 g ± 0.10 | 4.91 ef ± 0.16 | 3.01 g ± 0.10 | 6.67 bc ± 0.22 | 3.13 g ± 0.10 | 18.0 i ± 0.59 | 9.62 a ± 0.31 | 8.89 a ± 0.29 | 9.66 a ± 0.31 | 6.65 bc ± 0.22 | 5.24 de ± 0.17 | 5.98 bd ± 0.19 | 4.22 f ± 0.14 | 6.07 b ± 0.20 | 7.15 c ± 0.14 | 2.89 g ± 0.09 | 5.91 b ± 0.19 |
| 2019 | 6.82 lj ± 0.23 | 3.28 hm ± 0.11 | 2.85 m ± 0.10 | 9.16 n ± 0.31 | 5.29 de ± 0.18 | 7.20 j ± 0.10 | 3.69 gh ± 0.12 | 3.00 m ± 0.10 | 4.48 fl ± 0.15 | 10.36 a ± 0.35 | 6.07 bc ± 0.20 | 5.67 bd ± 0.19 | 5.04 ef ± 0.17 | 5.62 bfe ± 0.19 | 3.82 gh ± 0.13 | 6.88 ij ± 0.23 | 8.26 k ± 0.28 | 6.33 ci ± 0.21 | 4.06 gl ± 0.14 | |
| Esters | 2018 | 3.86 h ± 0.13 | 2.57 d ± 0.08 | 9.83 bcf ± 0.32 | 10.68 ae ± 0.35 | 7.27 j ± 0.24 | 8.37 g ± 0.27 | 4.22 h ± 0.14 | 4.27 h ± 0.14 | 11.08 e ± 0.36 | 10.26 abc ± 0.33 | 2.74 d ± 0.09 | 10.46 abe ± 0.34 | 9.10 fg ± 0.30 | 9.54 cf ± 0.31 | 9.39 f ± 0.31 | 9.70 bcf ± 0.32 | 3.54 h ± 0.31 | 6.14 i ± 0.20 | 5.50 i ± 0.18 |
| 2019 | 6.56 ej ± 0.22 | 4.72 cd ± 0.16 | 9.31 a ± 0.31 | 7.36 gj ± 0.25 | 5.09 bc ± 0.17 | 12.42 h ± 0.31 | 6.47 ej ± 0.22 | 6.44 ej ± 0.22 | 9.71 a ± 0.32 | 9.37 a ± 0.31 | 4.83 bcd ± 0.16 | 5.67 bce ± 0.19 | 25.63 f ± 0.61 | 8.23 g ± 0.28 | 7.83 g ± 0.26 | 5.70 be ± 0.19 | 4.11 d ± 0.14 | 11.72 h ± 0.39 | 16.02 i ± 0.54 | |
| Aldehydes | 2018 | 10.68 cd ± 0.35 | 17.86 i ± 0.58 | 12.70 j ± 0.41 | 10.12 cd ± 0.33 | 20.53 k ± 0.67 | 14.35 a ± 0.47 | 16.29 b ± 0.53 | 15.1 ab ± 0.49 | 7.09 fh ± 0.23 | 15.18 ab ± 0.49 | 9.92 cd ± 0.32 | 4.61 e ± 0.15 | 8.18 f ± 0.27 | 9.78 c ± 0.32 | 11.11 d ± 0.36 | 6.77 gh ± 0.22 | 10.28 cd ± 0.36 | 9.50 c ± 0.20 | 5.75 eg ± 0.19 |
| 2019 | 5.38 e ± 0.18 | 19.36 g ± 0.65 | 5.27 e ± 0.18 | 6.23 h ± 0.21 | 3.45 bd ± 0.12 | 7.05 i ± 0.18 | 9.56 j ± 0.32 | 8.52 k ± 0.28 | 4.07 bc ± 0.14 | 12.63 a ± 0.11 | 4.15 bc ± 0.14 | 3.47 bd ± 0.12 | 3.63 bd ± 0.12 | 2.93 d ± 0.10 | 3.12 d ± 0.10 | 3.21 d ± 0.11 | 5.31 e ± 0.18 | 4.49 c ± 0.15 | 11.34 f ± 0.38 | |
| Acids | 2018 | 4.11 i ± 0.13 | 4.45 gi ± 0.14 | 17.83 d ± 0.58 | 10.73 c ± 0.35 | 7.19 e ± 0.23 | 5.94 fh ± 0.19 | 2.66 b ± 0.09 | 4.44 gi ± 0.14 | 15.05 g ± 0.49 | 9.11 a ± 0.30 | 2.47 b ± 0.08 | 11.10 c ± 0.36 | 17.82 d ± 0.58 | 6.79 ef ± 0.22 | 8.58 a ± 0.28 | 8.64 a ± 0.28 | 8.90 a ± 0.28 | 10.31 c ± 0.34 | 5.18 gh ± 0.17 |
| 2019 | 3.33 b ± 0.11 | 9.63 e ± 0.32 | 17.64 g ± 0.59 | 3.54 b ± 0.12 | 7.53 dh ± 0.25 | 8.05 h ± 0.59 | 2.19 f ± 0.07 | 2.41 f ± 0.08 | 13.32 i ± 0.45 | 5.11 a ± 0.17 | 3.58 b ± 0.12 | 3.91 b ± 0.13 | 3.52 b ± 0.12 | 11.17 c ± 0.37 | 6.80 d ± 0.23 | 5.35 a ± 0.18 | 5.38 a ± 0.18 | 9.73 e ± 0.33 | 2.42 f ± 0.08 | |
| Ketones | 2018 | 4.48 i ± 0.15 | 4.62 hi ± 0.15 | 5.83 j ± 0.19 | 4.99 hk ± 0.16 | 4.98 hk ± 0.16 | 4.66 hi ± 0.15 | 7.38 l ± 0.24 | 5.18 k ± 0.17 | 2.36 e ± 0.08 | 4.03 a ± 0.13 | 3.40 bc ± 0.11 | 1.09 d ± 0.04 | 2.16 e ± 0.07 | 3.64 ab ± 0.12 | 3.24 c ± 0.11 | 1.46 df ± 0.05 | 2.13 eg ± 0.11 | 1.74 fg ± 0.06 | 1.14 d ± 0.04 |
| 2019 | 2.30 hj ± 0.08 | 5.38 g ± 0.18 | 1.77 c ± 0.06 | 2.14 dh ± 0.07 | 2.91 f ± 0.10 | 2.72 fi ± 0.06 | 2.52 ij ± 0.08 | 2.58 i ± 0.09 | 2.05 dh ± 0.07 | 4.84 a ± 0.16 | 1.51 b ± 0.05 | 1.95 cd ± 0.07 | 0.90 e ± 0.03 | 0.99 e ± 0.03 | 1.40 b ± 0.05 | 1.35 b ± 0.05 | 2.97 f ± 0.10 | 1.32 b ± 0.04 | 1.04 e ± 0.03 | |
| Furans | 2018 | 0.63 ef ± 0.02 | 1.84 h ± 0.06 | 0.59 e ± 0.02 | 1.75 hi ± 0.06 | 1.67 ci ± 0.05 | 0.76 af ± 0.02 | 0.80 a ± 0.03 | 3.36 j ± 0.11 | 0.60 e ± 0.02 | 0.81 a ± 0.03 | 1.60 bc ± 0.05 | 1.36 d ± 0.04 | 0.65 ef ± 0.02 | 1.53 b ± 0.05 | 0.32 g ± 0.01 | 0.64 ef ± 0.02 | 0.68 aef ± 0.01 | 0.43 g ± 0.01 | 0.44 g ± 0.01 |
| 2019 | 0.66 j ± 0.02 | 0.48 g ± 0.02 | 0.15 bcf ± 0.01 | 0.17 f ± 0.01 | 0.17 f ± 0.01 | 0.24 d ± 0.01 | 0.14 bcf ± 0.00 | 0.38 i ± 0.01 | 0.37 hi ± 0.01 | 0.72 a ± 0.02 | 0.12 b ± 0.00 | 0.13 bc ± 0.00 | 0.25 de ± 0.01 | 0.12 b ± 0.00 | 0.24 d ± 0.01 | 0.16 cf ± 0.01 | 0.51 g ± 0.02 | 0.28 e ± 0.01 | 0.34 h ± 0.01 | |
| Nitrogen compounds | 2018 | 1.09 cef ± 0.04 | 1.13 bce ± 0.04 | 0.71 a ± 0.02 | 0.77 ag ± 0.02 | 0.81 ag ± 0.03 | 0.58 i ± 0.02 | 1.00 f ± 0.03 | 1.17 bcd ± 0.04 | 0.87 g ± 0.03 | 0.71 a ± 0.02 | 1.17 bcd ± 0.04 | 1.12 bce ± 0.04 | 1.05 ef ± 0.03 | 0.87 g ± 0.03 | 1.10 bce ± 0.04 | 1.25 dh ± 0.04 | 1.34 h ± 0.04 | 1.19 bd ± 0.04 | 1.33 h ± 0.04 |
| 2019 | 0.61 di ± 0.02 | 0.48 e ± 0.02 | 1.43 h ± 0.05 | 0.79 cf ± 0.03 | 0.64 i ± 0.02 | 0.65 gi ± 0.05 | 0.59 di ± 0.02 | 0.57 dei ± 0.02 | 1.17 bj ± 0.04 | 0.97 a ± 0.03 | 1.01 a ± 0.03 | 1.24 b ± 0.04 | 0.86 c ± 0.03 | 0.53 de ± 0.02 | 0.74 fg ± 0.02 | 1.41 h ± 0.05 | 0.61 di ± 0.02 | 0.87 c ± 0.03 | 1.11 j ± 0.04 | |
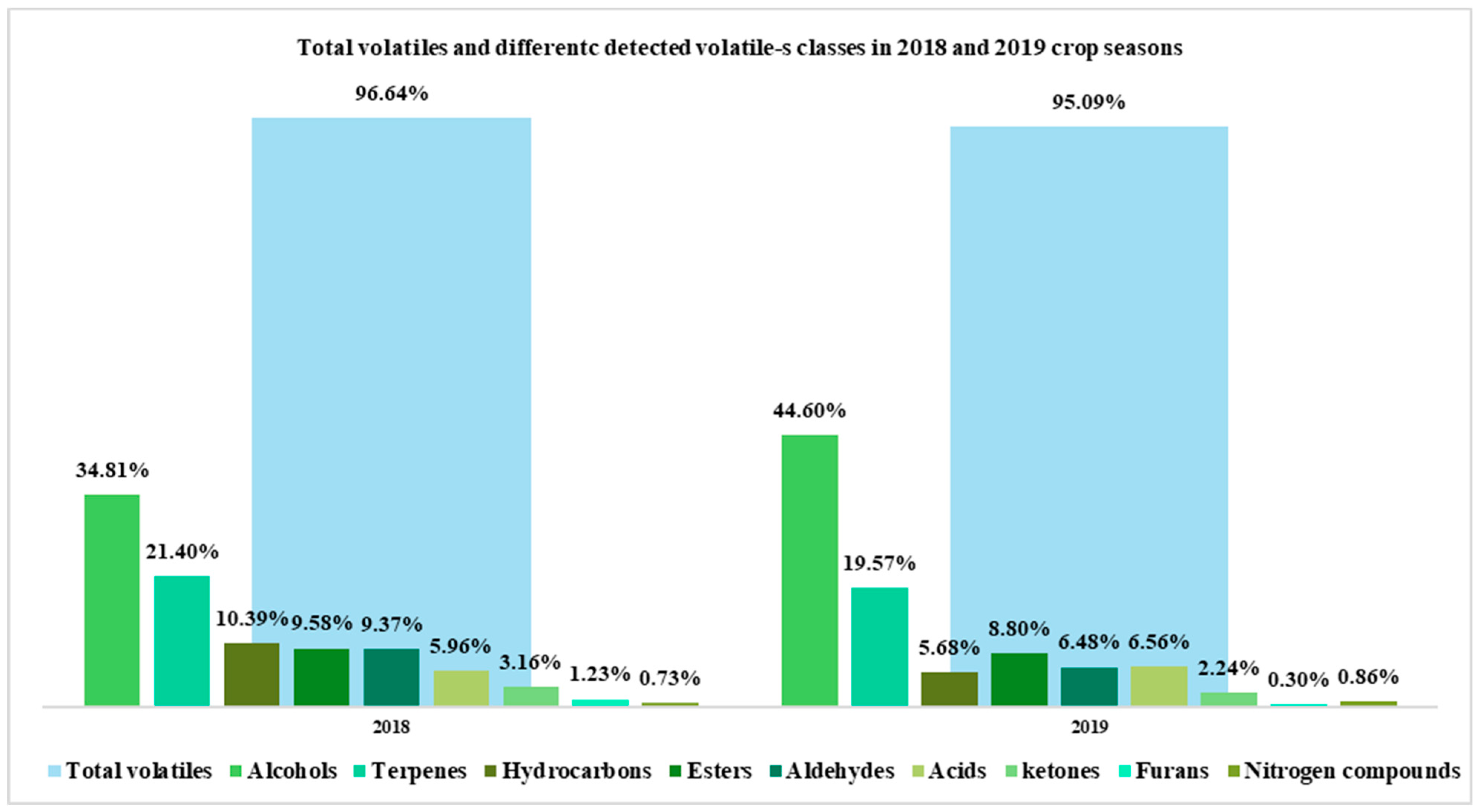
| Total volatiles | 2018 | 95.11 | 98.67 | 96.65 | 94.12 | 94.71 | 96.10 | 97.02 | 96.98 | 97.56 | 96.46 | 95.35 | 95.96 | 97.09 | 96.42 | 97.44 | 97.52 | 97.86 | 97.80 | 97.31 |
| 2019 | 96.81 | 94.56 | 96.87 | 96.83 | 97.09 | 94.98 | 90.79 | 89.34 | 96.27 | 95.74 | 94.75 | 97.63 | 98.04 | 95.60 | 96.26 | 95.12 | 87.36 | 96.55 | 96.13 | |
3.2. Season Impact
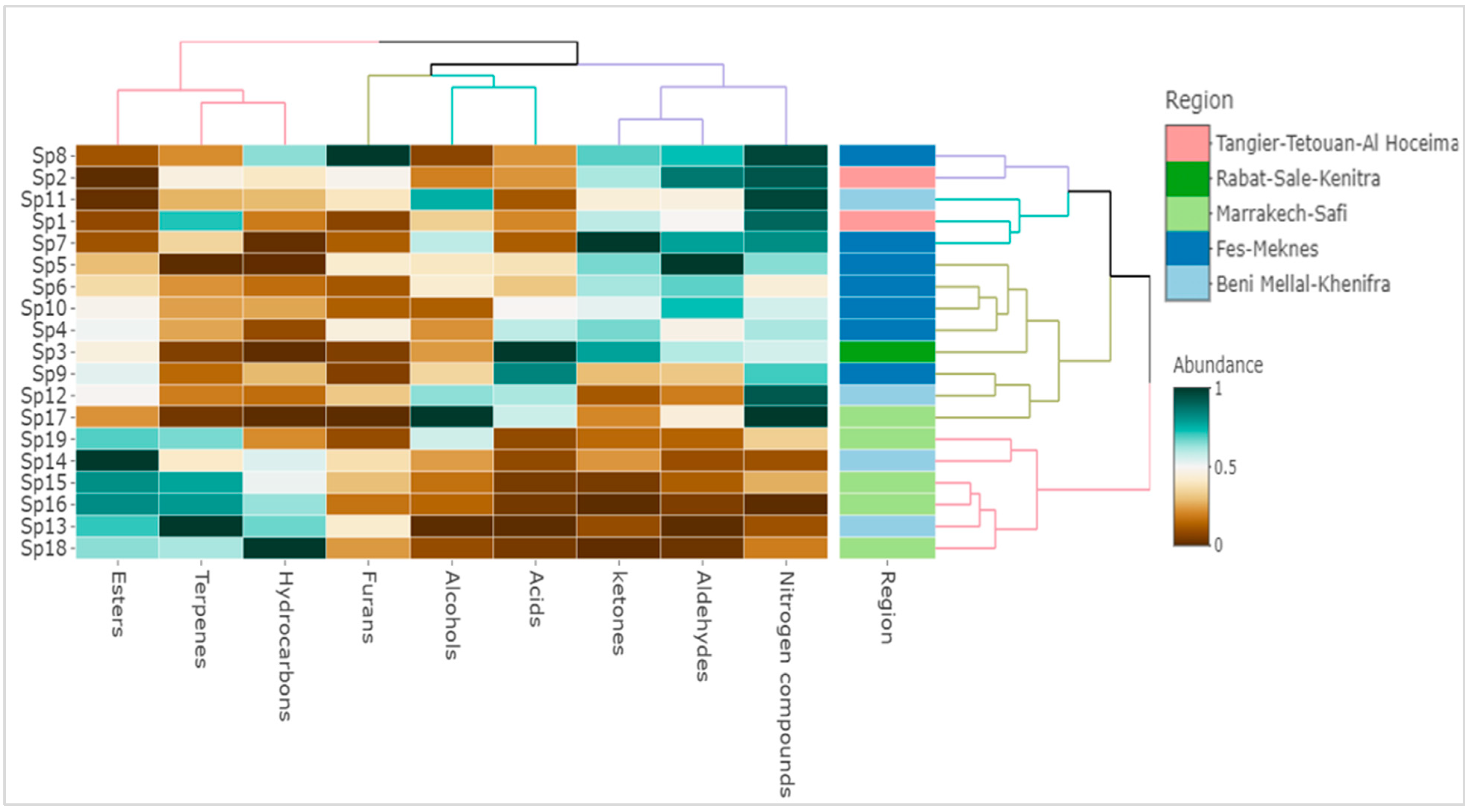
3.3. Geographical Origin Influence
3.4. Pedo-Climatic Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive oil volatile compounds, flavour development and quality: A critical review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Ait Hmida, A. Stratégie de Valorisation de L’huile D’olive par L’origine et la Qualité: Évaluation du Projet Tyout-Chiadma, Première AOP au Maroc. 2016, pp. 161–167. Available online: https://om.ciheam.org/om/pdf/a118/00007175.pdf (accessed on 2 April 2019).
- IOC. International Olive Council. Available online: https://www.internationaloliveoil.org/ (accessed on 24 July 2022).
- Morales, M.T.; Tsimidou, M. The Role of Volatile Compounds and Polyphenols in Olive Oil Sensory Quality. In Handbook of Olive Oil: Analysis and Properties; Harwood, J., Aparicio, R., Eds.; Springer: Boston, MA, USA, 2000; pp. 393–458. [Google Scholar] [CrossRef]
- Guth, H.; Grosch, W. A Comparative Study of the Potent Odorants of Different Virgin Olive Oils. Lipid/Fett 1991, 93, 335–339. [Google Scholar] [CrossRef]
- Žanetić, M.; Špika, M.J.; Ožić, M.M.; Bubola, K.B. Comparative Study of Volatile Compounds and Sensory Characteristics of Dalmatian Monovarietal Virgin Olive Oils. Plants 2021, 10, 1995. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using gas chromatography–ion mobility spectrometry (GC–IMS)’. Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef]
- Reiners, J.; Grosch, W. Odorants of Virgin Olive Oils with Different Flavor Profiles. J. Agric. Food Chem. 1998, 46, 2754–2763. [Google Scholar] [CrossRef]
- Genovese, A.; Caporaso, N.; Sacchi, R. Flavor Chemistry of Virgin Olive Oil: An Overview. Appl. Sci. 2021, 11, 1639. [Google Scholar] [CrossRef]
- Aparicio, R.; Harwood, J. Handbook of Olive Oil: Analysis and Properties; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Angerosa, F.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G. Volatile compounds in virgin olive oil: Occurrence and their relationship with the quality. J. Chromatogr. A 2004, 1054, 17–31. [Google Scholar] [CrossRef]
- Kiritsakis, A.K. Flavor components of olive oil-A review. J. Amer. Oil Chem. Soc. 1998, 75, 673–681. [Google Scholar] [CrossRef]
- Kosma, I.S.; Kontominas, M.G.; Badeka, A.V. The Application of Chemometrics to Volatile Compound Analysis for the Recognition of Specific Markers for Cultivar Differentiation of Greek Virgin Olive Oil Samples. Foods 2020, 9, 1672. [Google Scholar] [CrossRef]
- Cecchi, L.; Migliorini, M.; Mulinacci, N. Virgin Olive Oil Volatile Compounds: Composition, Sensory Characteristics, Analytical Approaches, Quality Control, and Authentication. J. Agric. Food Chem. 2021, 69, 2013–2040. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Liu, S.; Liu, Y.; Song, H. Comparison of flavour fingerprint, electronic nose and multivariate analysis for discrimination of extra virgin olive oils. R. Soc. Open Sci. 2019, 6, 190002. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.D.G.; Freitas, A.M.C.; Cabrita, M.J.; Garcia, R. Olive oil composition: Volatile compounds. In Olive Oil-Constituents, Quality, Health Properties and Bioconversions; InTech: London, UK, 2012. [Google Scholar]
- Boskou, D. Olive Oil: Chemistry and Technology; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Flath, R.A.; Forrey, R.R.; Guadagni, D.G. Aroma components of olive oil. J. Agric. Food Chem. 1973, 21, 948–952. [Google Scholar] [CrossRef]
- Kosma, I.; Vavoura, M.; Kontakos, S.; Karabagias, I.; Kontominas, M.; Apostolos, K.; Badeka, A. Characterization and Classification of Extra Virgin Olive Oil from Five Less Well-Known Greek Olive Cultivars. J. Am. Oil Chem. Soc. 2016, 93, 837–848. [Google Scholar] [CrossRef]
- Haddada, F.M.; Manai, H.; Daoud, D.; Fernandez, X.; Lizzani-Cuvelier, L.; Zarrouk, M. Profiles of volatile compounds from some monovarietal Tunisian virgin olive oils. Comparison with French PDO. Food Chem. 2007, 103, 467–476. [Google Scholar] [CrossRef]
- Guinda, A.; Lanzón, A.; Albi, T. Differences in Hydrocarbons of Virgin Olive Oils Obtained from Several Olive Varieties. J. Agric. Food Chem. 1996, 44, 1723–1726. [Google Scholar] [CrossRef]
- Cajka, T.; Riddellova, K.; Klimankova, E.; Cerna, M.; Pudil, F.; Hajslova, J. Traceability of olive oil based on volatiles pattern and multivariate analysis. Food Chem. 2010, 121, 282–289. [Google Scholar] [CrossRef]
- Bajoub, A.; Sánchez-Ortiz, A.; Ouazzani, N.; Fernández-Gutiérrez, A.; Beltrán, G.; Carrasco-Pancorbo, A. First comprehensive characterization of volatile profile of north Moroccan olive oils: A geographic discriminant approach. Food Res. Int. 2015, 76, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Lechhab, T.; Lechhab, W.; Cacciola, F.; Salmoun, F. Sets of internal and external factors influencing olive oil (Olea europaea L.) composition: A review. Eur. Food Res. Technol. 2022, 248, 1069–1088. [Google Scholar] [CrossRef]
- Inglese, P.; Famiani, F.; Galvano, F.; Servili, M.; Esposto, S.; Urbani, S. Factors Affecting Extra-Virgin Olive Oil Composition. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 83–147. [Google Scholar] [CrossRef]
- Kiralan, M.; Ozkan, G.; Koyluoglu, F.; Ugurlu, H.A.; Bayrak, A.; Kiritsakis, A. Effect of cultivation area and climatic conditions on volatiles of virgin olive oil. Eur. J. Lip. Sci. Technol. 2012, 114, 552–557. [Google Scholar] [CrossRef]
- Issaoui, M.; Flamini, G.; Brahmi, F.; Dabbou, S.; Ben Hassine, K.; Taamali, A.; Chehab, H.; Ellouz, M.; Zarrouk, M.; Hammami, M. Effect of the growing area conditions on differentiation between Chemlali and Chétoui olive oils. Food Chem. 2010, 119, 220–225. [Google Scholar] [CrossRef]
- Romero, N.; Saavedra, J.; Tapia, F.; Sepúlveda, B.; Aparicio, R. Influence of agroclimatic parameters on phenolic and volatile compounds of Chilean virgin olive oils and characterization based on geographical origin, cultivar and ripening stage. J. Sci. Food Agric. 2016, 96, 583–592. [Google Scholar] [CrossRef]
- Rached, M.B.; Galaverna, G.; Cirlini, M.; Boujneh, D.; Zarrouk, M.; Guerfel, M. Pedologic factors affecting virgin olive oil quality of “Chemlali” olive trees (Olea europaea L.). J. Oleo Sci. 2017, 66, 907–915. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chtourou, F.; Valli, E.; Bendini, A.; Lazzez, A.; Toschi, T.G.; Bouaziz, M. Effects of Olive Trees Age on the Minor Components of Oueslati Virgin Olive Oils Produced from Olives Harvested at Different Ripening Degrees. J. Am. Oil Chem. Soc. 2017, 94, 435–447. [Google Scholar] [CrossRef]
- Tura, D.; Prenzler, P.D.; Bedgood, D.R.; Antolovich, M.; Robards, K. Varietal and processing effects on the volatile profile of Australian olive oils. Food Chem. 2004, 84, 341–349. [Google Scholar] [CrossRef]
- Servili, M.; Esposto, S.; Lodolini, E.; Selvaggini, R.; Taticchi, A.; Urbani, S.; Montedoro, G.; Serravalle, M.; Gucci, R. Irrigation Effects on Quality, Phenolic Composition, and Selected Volatiles of Virgin Olive Oils Cv. Leccino. J. Agric. Food Chem. 2007, 55, 6609–6618. [Google Scholar] [CrossRef] [PubMed]
- Stefanoudaki, E.; Williams, M.; Chartzoulakis, K.; Harwood, J. Effect of Irrigation on Quality Attributes of Olive Oil. J. Agric. Food Chem. 2009, 57, 7048–7055. [Google Scholar] [CrossRef] [PubMed]
- Polari, J.J.; Mori, M.; Wang, S.C. Olive Oil from “Sikitita” under Super-High-Density Planting System in California: Impact of Harvest Time and Crop Season. J. Am. Oil Chem. Soc. 2020, 97, 1179–1190. [Google Scholar] [CrossRef]
- Prenzler, Z.; Bedgood, D., Jr.; Bishop, A.; Robards, K. Volatile profile of olive oils. Adv. Hortic. Sci. 2002, 16, 246–252. [Google Scholar]
- Ben-Hassine, K.; Taamalli, A.; Ferchichi, S.; Mlaouah, A.; Benincasa, C.; Romano, E.; Flamini, G.; Lazzez, A.; Grati-Kamoun, N.; Perri, E.; et al. Physicochemical and sensory characteristics of virgin olive oils in relation to cultivar, extraction system and storage conditions. Food Res. Int. 2013, 54, 1915–1925. [Google Scholar] [CrossRef]
- Lechhab, T.; Salmoun, F.; Lechhab, W.; El Majdoub, Y.O.; Russo, M.; Camillo, M.R.T.; Trovato, E.; Dugo, P.; Mondello, L.; Cacciola, F. Determination of bioactive compounds in extra virgin olive oils from 19 Moroccan areas using liquid chromatography coupled to mass spectrometry: A study over two successive years. Eur. Food Res. Technol. 2021, 247, 2993–3012. [Google Scholar] [CrossRef]
- Lechhab, T.; Lechhab, W.; Trovato, E.; Salmoun, F.; Mondello, L.; Cacciola, F. Impact of edaphoclimatic conditions and crop season on olive oils fatty acids. Agron. J. 2022. [Google Scholar] [CrossRef]
- Mansouri, F.; Ben Moumen, A.; Richard, G.; Fauconnier, M.-L.; Sindic, M.; Caid, H.S.; Elamrani, A. Flavor profiles of monovarietal virgin olive oils produced in the Oriental region of Morocco. Oilseeds Fats Crops Lipids 2017, 24, 2017. [Google Scholar] [CrossRef]
- Tanouti, K.; Serghini-Caid, H.; Sindic, M.; Wathelet, J.-P.; Elamrani, A. Volatile Compounds, Profiles of Virgin Olive Oils Produced In the Eastern Morocco: Oxidative Stability and Sensory Defects. J. Food Res. 2012, 1, 194–206. [Google Scholar] [CrossRef][Green Version]
- Nigri, S.; Oumeddour, R.; Fernandez, X. Analysis of some Algerian virgin olive oils by headspace solid phase micro-extraction coupled to gas chromatography/mass spectrometry. Riv. Ital. Sostanze Grasse 2012, 84, 54–61. [Google Scholar]
- Cherfaoui, M.; Cecchi, T.; Keciri, S.; Boudriche, L. Volatile and Sensory Profiles of Algerian Extra-Virgin Olive Oil from Souidi and Zeletni Cultivars. Chem. Biodivers. 2019, 16, e1900297. [Google Scholar] [CrossRef]
- Aparicio, R.; Morales, M.T.; Alonso, M.V. Relationship between volatile compounds and sensory attributes of olive oils by the sensory wheel. J. Am. Oil Chem. Soc. 1996, 73, 1253–1264. [Google Scholar] [CrossRef]
- Aparicio, R.; Morales, M.T.; Alonso, V. Authentication of European Virgin Olive Oils by Their Chemical Compounds, Sensory Attributes, and Consumers’ Attitudes. J. Agric. Food Chem. 1997, 45, 1076–1083. [Google Scholar] [CrossRef]
- Eriotou, E.; Karabagias, I.K.; Maina, S.; Koulougliotis, D.; Kopsahelis, N. Geographical origin discrimination of “Ntopia” olive oil cultivar from Ionian islands using volatile compounds analysis and computational statistics. Eur. Food Res. Technol. 2021, 247, 3083–3098. [Google Scholar] [CrossRef] [PubMed]
- Vichi, S.; Guadayol, J.M.; Caixach, J.; López-Tamames, E.; Buxaderas, S. Monoterpene and sesquiterpene hydrocarbons of virgin olive oil by headspace solid-phase microextraction coupled to gas chromatography/mass spectrometry. J. Chromatogr. A 2006, 1125, 117–123. [Google Scholar] [CrossRef]
- Morales, M.T.; Alonso, M.V.; Rios, J.J.; Aparicio, R. Virgin olive oil aroma: Relationship between volatile compounds and sensory attributes by chemometrics. J. Agric. Food Chem. 1995, 43, 2925–2931. [Google Scholar] [CrossRef]
- Baccouri, B.; Rajhi, I. Terpene Compounds of New Tunisian Extra-Virgin Olive Oil: Effect of Ripening Stage; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Tomé-Rodríguez, S.; Ledesma-Escobar, C.A.; Penco-Valenzuela, J.M.; Priego-Capote, F. Influence of the fatty acid profile on the volatile components of virgin olive oil subjected to thermal stress. J. Sci. Food Agric. 2021, 101, 4829–4837. [Google Scholar] [CrossRef] [PubMed]
- Tura, D.; Failla, O.; Pedò, S.; Gigliotti, C.; Bassi, D.; Serraiocco, A. Effects of seasonal weather variability on olive oil composition in Northern Italy. Acta Hortic. 2008, 791, 769–776. [Google Scholar] [CrossRef]
- Fernandes-Silva, A.A.; Falco, V.; Correia, C.M.; Villalobos, F.J. Sensory analysis and volatile compounds of olive oil (cv. Cobrancosa) from different irrigation regimes. Grasas Aceites 2013, 64, 59–67. [Google Scholar] [CrossRef]
- Klisović, D.; Novoselić, A.; Lukić, I.; Bubola, K.B. Extra virgin olive oil under simulated consumption conditions: Evaluation of quality, health, and flavour properties. J. Food Comp. Anal. 2022, 110, 104570. [Google Scholar] [CrossRef]
- Vichi, S.; Pizzale, L.; Conte, L.S.; Buxaderas, S.; López-Tamames, E. Solid-Phase Microextraction in the Analysis of Virgin Olive Oil Volatile Fraction: Characterization of Virgin Olive Oils from Two Distinct Geographical Areas of Northern Italy. J. Agric. Food Chem. 2003, 51, 6572–6577. [Google Scholar] [CrossRef] [PubMed]
- Baccouri, B.; Rajhi, I.; Mohamed, S.N.; Flamini, G. Monitoring the fatty acid and volatile compositions of Tunisian virgin olive oils using HS-SPME–GC–MS with regard to growing area. Eur. Food Res. Technol. 2022. [Google Scholar] [CrossRef]
- Youssef, O.; Guido, F.; Manel, I.; Youssef, N.B.; Luigi, C.P.; Mohamed, H.; Daoud, D.; Mokhtar, Z. Volatile compounds and compositional quality of virgin olive oil from Oueslati variety: Influence of geographical origin. Food Chem. 2011, 124, 1770–1776. [Google Scholar] [CrossRef]
- Conte, L.; Bendini, A.; Valli, E.; Lucci, P.; Moret, S.; Maquet, A.; Lacoste, F.; Brereton, P.; Garcia-Gonzalez, D.L.; Moreda, W.; et al. Olive oil quality and authenticity: A review of current EU legislation, standards, relevant methods of analyses, their drawbacks and recommendations for the future. Trend Food Sci. Technol. 2020, 105, 483–493. [Google Scholar] [CrossRef]
- Pannelli, G.; Servili, M.; Servaggini, R.; Baldioli, M.; Montedoro, G.F. Effect of agronomic and seasonal factors on olive (Olea europaea L.) Production and on the qualitative characteristics of the oil. Acta Hortic. 1994, 356, 239–244. [Google Scholar] [CrossRef]
- Ranalli, A.; De Mattia, G.; Patumi, M.; Proietti, P. Quality of virgin olive oil as influenced by origin area. Grasas Aceites 1999, 50, 249–259. [Google Scholar] [CrossRef]
- Cresti, M.; Ciampolini, F.; Tattini, M.; Cimato, A. Effect of salinity on productivity and oil quality of olive (Olea europaea L.) plants. Adv. Hort. Sci. 1994, 8, 211–214. [Google Scholar]
- Campestre, C.; Angelini, G.; Gasbarri, C.; Angerosa, F. The Compounds Responsible for the Sensory Profile in Monovarietal Virgin Olive Oils. Molecules 2017, 22, 1833. [Google Scholar] [CrossRef]
- Theodosi, S.; Kosma, I.S.; Badeka, A.V. Quality characteristics of Koroneiki olive oil from Zakynthos island (Greece) and differentiation depending on the altitude level. Eur. Food Res. Technol. 2021, 247, 1235–1248. [Google Scholar] [CrossRef]
| Geographical Origins Variables | Volatile Classes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Alcohols | Terpenes | Hydrocarbons | Esters | Aldehydes | Acids | Ketones | Furans | Nitrogen Compounds | Total Volatiles | |
| Rainfall | −0.14 | −0.47 * | −0.50 * | −0.52 * | 0.79 **** | 0.46 * | 0.79 **** | 0.11 | 0.45 | −0.43 |
| Temperature | −0.11 | 0.02 | −0.01 | 0.11 | 0.09 | −0.05 | 0.06 | −0.01 | −0.17 | 0.15 |
| Wind speed | 0.11 | 0.11 | 0.08 | 0.2 | −0.17 | −0.14 | −0.18 | −0.24 | −0.22 | 0.33 |
| Relative humidity | −0.02 | −0.15 | −0.3 | −0.42 | 0.44 | 0.19 | 0.51 * | −0.06 | 0.32 | −0.18 |
| Clay | −0.01 | −0.06 | −0.09 | −0.31 | 0.25 | 0.04 | 0.28 | 0.11 | 0.27 | −0.06 |
| Silt | −0.31 | 0.14 | 0.25 | 0.53 * | −0.35 | 0.03 | −0.31 | 0.13 | −0.38 | 0.18 |
| Sand | 0.46 * | −0.03 | −0.25 | −0.27 | 0.01 | −0.07 | 0.03 | −0.26 | 0.18 | −0.27 |
| pH | −0.13 | −0.12 | 0.28 | 0.39 | −0.11 | −0.06 | −0.28 | 0.17 | −0.36 | 0.4 |
| Conductivity | 0.01 | 0.06 | 0.44 | 0.11 | −0.26 | −0.19 | −0.33 | −0.1 | −0.18 | 0.38 |
| Organic matter | −0.22 | 0.15 | −0.14 | 0.12 | 0.12 | −0.15 | 0.3 | −0.1 | −0.23 | −0.36 |
| Limestone | −0.29 | −0.18 | 0.14 | −0.15 | 0.41 | 0.03 | 0.22 | 0.12 | 0.05 | 0.28 |
| N | −0.2 | 0.22 | −0.04 | −0.07 | 0.17 | −0.32 | 0.32 | 0.34 | −0.04 | −0.2 |
| P | 0.19 | 0.04 | −0.23 | −0.3 | 0.17 | −0.09 | 0.41 | −0.15 | 0.19 | 0.03 |
| K | −0.13 | 0.08 | 0.15 | 0.23 | −0.14 | −0.22 | 0.02 | 0.3 | −0.28 | −0.15 |
| Ca2+ | −0.35 | −0.17 | 0.11 | 0.09 | 0.3 | −0.05 | 0.25 | 0.41 | −0.14 | 0.06 |
| Mg2+ | 0.05 | 0.12 | 0.23 | 0.18 | −0.32 | −0.12 | −0.38 | 0.24 | 0.01 | 0.15 |
| Na+ | −0.17 | 0.2 | 0.58 ** | 0.23 | −0.38 | −0.25 | −0.41 | −0.09 | −0.33 | 0.31 |
| K+ | −0.17 | 0.09 | 0.14 | 0.12 | −0.05 | −0.23 | 0.12 | 0.32 | −0.23 | −0.15 |
| C/N | −0.23 | −0.1 | −0.08 | 0.2 | 0.09 | 0.25 | 0.09 | −0.4 | −0.2 | −0.14 |
| Latitude | −0.15 | −0.37 | −0.44 | −0.64 ** | 0.79 **** | 0.38 | 0.85 **** | 0.14 | 0.53 * | −0.35 |
| Longitude | −0.11 | −0.41 | −0.38 | −0.50 * | 0.67 ** | 0.34 | 0.74 *** | 0.2 | 0.46 * | −0.39 |
| Altitude | 0.08 | 0.14 | 0.13 | 0.03 | −0.26 | −0.03 | −0.34 | −0.19 | 0.09 | −0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lechhab, T.; Lechhab, W.; Trovato, E.; Salmoun, F.; Mondello, L.; Cacciola, F. Screening of the Volatile Composition of Moroccan Olive Oils by Using SPME/GC-MS-FID over a Two-Year Period: A Pedoclimatic Discrimination. Horticulturae 2022, 8, 925. https://doi.org/10.3390/horticulturae8100925
Lechhab T, Lechhab W, Trovato E, Salmoun F, Mondello L, Cacciola F. Screening of the Volatile Composition of Moroccan Olive Oils by Using SPME/GC-MS-FID over a Two-Year Period: A Pedoclimatic Discrimination. Horticulturae. 2022; 8(10):925. https://doi.org/10.3390/horticulturae8100925
Chicago/Turabian StyleLechhab, Touria, Wafae Lechhab, Emanuela Trovato, Farida Salmoun, Luigi Mondello, and Francesco Cacciola. 2022. "Screening of the Volatile Composition of Moroccan Olive Oils by Using SPME/GC-MS-FID over a Two-Year Period: A Pedoclimatic Discrimination" Horticulturae 8, no. 10: 925. https://doi.org/10.3390/horticulturae8100925
APA StyleLechhab, T., Lechhab, W., Trovato, E., Salmoun, F., Mondello, L., & Cacciola, F. (2022). Screening of the Volatile Composition of Moroccan Olive Oils by Using SPME/GC-MS-FID over a Two-Year Period: A Pedoclimatic Discrimination. Horticulturae, 8(10), 925. https://doi.org/10.3390/horticulturae8100925









