1. Introduction
Nowadays, the demand for efficient farming processes in the horticulture and food industries increases rapidly. The state of horticulture depends on the products’ quality and quantity. Fungal diseases damage various parts of plants (for example, stems, leaves, inflorescences, and fruits), resulting in lower yields and final crop production, which leads to economic loss. Therefore, early detection, prevention, and management of plant diseases is crucial [
1].
The grey mould caused by
Botrytis cinerea damages many crop hosts (over 200 species) worldwide [
2,
3]. This fungus mainly enters the plant via direct penetration or natural openings or wounds [
4,
5].
B. cinerea is also a significant threat to greenhouse-grown plants, where disease control is usually challenging and expensive [
6]. The soil substrate used for plants might be the primary source of fungi in the greenhouse. In addition, the pathogens can be brought into the greenhouse in water. However, the enormous numbers of
Botrytis spores can be carried from outdoor plants and spread rapidly by air, infecting greenhouse crops. The chemical fungicides are used in almost all cases for disease prevention in the greenhouse. However, these are often used only after symptoms are visible, at which point a substantial epidemic may already be well underway, requiring multiple subsequent foliar sprays at regular intervals [
7]. Therefore, the ability to detect consistently and quantitatively the first signs of
Botrytis and monitor disease progression in a leaf can help to prevent still-intact parts of the crop production and reduce the usage of chemicals.
Several studies have addressed
B. cinerea diagnosis and detection in crops. Traditionally, fungal disease-causing pathogens have been detected by visual examination of symptoms, such as blight, rotting, spots, and wilting by using available guidelines and standards for assessment [
8]. Immunoassay-based diagnostic and molecular quantification based on a quantitative polymerase chain reaction (PCR) of quantifying fungal conidiospores offers increased throughput. However, these methods are destructive, require chemical and analytical steps during the analysis, and cannot be done rapidly. Therefore, the same individual plant cannot be monitored across time [
9]. Additional detection methods include plating and culturing plant pathogens for identification using microscopy techniques, but again, these approaches are challenging to scale for high-throughput, high-resolution temporal analysis [
10].
Plant disease symptoms are evident in various parts of plants. However, plant leaves are mainly used to detect infection. Plant leaves initiate the process of photosynthesis through which plants get their food. Diseases affect the leaves of plants so they fail to provide proper nourishment of the plant, leading to bad health or death [
1]. Therefore, early diagnosis and detection of the disease would allow more effective crop management practices to prevent outbreaks in field or greenhouse settings. Furthermore, fungal infections can alter leaf morphology and light reflectance, distinguished using leaf spectral indices [
11]. Again, having a rapid, simple, non-invasive way to detect the extent of
Botrytis disease is essential for plant pathologists interested in quantifying infection rates [
3].
Lettuce (
Lactuca sativa L.) is one of the most important leafy vegetables worldwide, as it is considered a rich source of vitamins (A, C, E, K), polyphenols, and antioxidants compounds [
12]. Grey mould caused by the fungus
B. cinerea has been considered as a major disease in greenhouse-grown lettuce [
13].
This study aimed to find out the specific parameters which could be used for the early detection of B. cinerea in lettuce. We hypothesised that non-destructive measurements could be used instead of biochemical analysis for the early detection of B. cinerea in lettuce. First, the two inculcation methods were used to evaluate the plant response to inner and outer infection with B. cinerea at different times after the inoculation. Then we measured the leaf spectral reflectance indices by non-destructive meters and determined phytochemicals (phenols, proteins, chlorophylls, carotenoids, and antiradical capacity) in lettuce. In the end, we compared the results of non-destructive and biochemical measurements to find out which of those parameters can potentially be used for early detection of B. cinerea in lettuce (1), and if the non-destructive measurements can be conditionally equated to biochemical analysis (2).
2. Materials and Methods
2.1. Plant Material and Growth Conditions
The experiments were performed at Lithuanian Research Centre for Agriculture and Forestry, Institute of Horticulture, in the greenhouse in July–September 2020. The lettuce “Little Gem” (Green vegetable seeds, United Kingdom) seeds were sown in the plastic trays (50 holes, 54 × 28 cm) with peat substrate (Terraerden, Latvia) with NPK (100–160; 110–180; 120–200 mg L−1) with microelements Mn, Cu, Mo, B, Zn and Fe (pH H2O 5.5–6.5; electrical conductivity (EC) ms cm−1 < 1.10). During vegetation experiments, the average day and night temperatures were 24 ± 5 °C/21 ± 5 °C, and the relative air humidity was 75 ± 5%. On the 10th day after sowing, the seedlings were transferred to plastic pots (9 × 9 × 10 cm) and grown for an additional 17 days. Plants were watered daily with tap water. The experiments were conducted twice.
2.2. Artificial Infection In Vivo
The artificial inoculation of lettuce leaves with B. cinerea isolate was done in two ways: (1) inoculation with a pathogen disc (Infected-1), depicting the entry of the pathogen from the outside; and (2) inoculation with a spore suspension (Infected-2), depicting the entry of the pathogen from the inside.
The single spore isolates of
B. cinerea from infected strawberry fruit (LT11B_BRA_189) was obtained from LAMMC IH Laboratory of plant protection isolate collection. The isolate was morphologically identified as
B. cinerea and verified by species-specific PCR, according to Rasiukevičiūtė et al. (2018) [
14]. The isolate was maintained in Petri dishes with potato dextrose agar (PDA) at 22 ± 2 °C for seven days.
Infected-1. The centre of lettuce leaves artificially inoculated with 7 mm B. cinerea mycelial discs cut from the periphery of 7-day-old cultures.
Infected-2. The spore suspension of 7 day-old B. cinerea was prepared by adding 1 mL of sterile sdeionised water to each plate. The spore’s concentration was counted using a Neubauer hemocytometer counting chamber. The spore concentration adjusted to 1 × 104 spores mL−1. 20 µL of the suspension was applied on the surface of lettuce near the lettuce leaf’s central vein.
Non-infected. The control plants were not inoculated with B. cinerea.
The changes in non-destructive measurements of leaf spectral reflectance indices and biochemical compounds of non-infected and infected lettuces were determined after 12, 18, 36, 60, and 84 h. The same plants used for non-destructive measurements were analysed by biochemical analysis. Therefore, the different plants were evaluated at each time after the inoculation.
2.3. Non-Destructive Measurements of Leaf Spectral Indexes
The non-destructive measurements were done by putting the meter where it could avoid the main vein of the leaf. Ten different plants from each group (Non-infected, Infected-1 and Infected-2) were measured at each time of assessment after inoculation with B. cinerea.
The leaf spectral reflectance indexes were measured using a leaf spectrometer (CI-710, CID Bioscience, USA) from 9:00 to 12:00 a.m. The reflection spectra obtained were used to calculate the photochemical reflectance index (PRI), which shows changes in the xanthophyll cycle, using the following formula:
The snormalised difference vegetation index (NDVI), which shows changes in biomass content, was calculated by:
The plant senescence reflectance index (PSRI), which shows changes in dry or senescent carbon, was calculated by:
The carotenoid reflectance index (CRI), which shows changes in the carotenoids-to-chlorophyll ratio, was calculated by:
where R800, R750, R700, R680, R570, R531, R510 represent the leaf reflectance integrated over 10 nm wavelenght centered on 800, 750, 680, 570, 531, and 510 nm, respectively.
The relative chlorophyll (CHL), flavonols (FLA) contents, and nitrogen balance index (NBI) was measured using the non-destructive meter Dualex (Force-A, France).
2.4. Biochemical Analysis and Water Content Measurements
The conjugated biological samples of the infected leaves from three randomly selected control and inoculated with B. cinerea lettuce were used for the biochemical analysis. Fresh plant tissues were immediately frozen with 10 mL of liquid nitrogen (N2) and stored in an ultra-low freezer until the analysis.
Each plant shoot FW (g) and DW (g) was measured using an electronic analytical balance (Mettler Toledo, Columbus, OH, USA). Shoots were dried in an oven (Venticell-BMT, Czech Republic) at 70 °C for 48 h before DW measurements. Water content was calculated as the fraction of the difference between shoot FW and DW in FW and used to re-calculate biochemical compound contents in the DW of plants.
2.4.1. Total Phenolic Content
The total phenolic content of lettuce was determined spectrophotometrically [
15] with modifications. 0.5 g of frozen lettuce tissue was homogenised in a ceramic mortar with 5 mL of 80% ice-cold methanol and transferred to a 15 mL polypropylene conical centrifuge tube (Labbox Labware S.L., Barcelona, Spain). The extract was incubated at 4 °C for 24 h. Samples were centrifuged (Hermle Z 300 K, Hermle Labortechnik, Wehingen, Germany) at a relative centrifugal force of 4000 rpm min
−1 for 10 min at room temperature. The supernatant was filtered through a 70 mm qualitative filter paper (Frisenette ApS, Knebel, Denmark). First, 100 µL of the filtrate was diluted with 200 µL of 10% (
v/
v) Folin & Ciocalteu′s phenol reagent and vortexed thoroughly. Then, 800 µL of 700 mM of sodium carbonate was added. After 20 min, the absorbance of the samples was measured at 765 nm using a spectrophotometer CamSpec M501 (Spectronic CamSpec Ltd., Garforth, UK). The total phenolic content was calculated using a standard curve of gallic acid (R
2 > 0.95). Data are presented as the mean of three analytical measurements of total phenolic content (in mg g
−1) on a dry basis of the lettuce.
2.4.2. Total Protein Content
Total protein content was determined according to the spectrophotometric method [
16]. Frozen fresh plant material was ground with N
2 and extracted with 50 mM phosphate buffer containing 1 mM ethylenediaminetetraacetic acid (EDTA), 10 mM 2-mercaptoethanol, 100 μM phenylmethylsulfonyl fluoride (PMSF). The extract was centrifuged for 10 min at 4000 rpm min
−1 (Hermle Z 300 K, Hermle Labortechnik, Wehingen, Germany), the supernatant was mixed with diluted (1:5) Bradford reagent. Absorbance was read at 595 nm on the CamSpec M501 spectrophotometer (Spectronic CamSpec Ltd., Garforth, UK). The bovine serum albumin calibration curve determined total protein contents. Data are presented as the mean of three analytical measurements (in mg g
−1) on a dry basis of the lettuce.
2.4.3. Measurements of Antioxidant Capacity
For the ferric-reducing antioxidant power (FRAP) assay, the working reagent was prepared by mixing acetate buffer (300 mM, pH 3.6), a solution of 10 mM 2,4,6-tripyridyl-s-triazine (TPTZ) in 40 mM hydrochloric acid, and 20 mM iron (III) chloride hexahydrate (FeCl
3 × 6H
2O) at 10:1:1 (
v/
v/
v) [
17]. A total of 20 µL of the sample were mixed with 3 mL of working solution and incubated in the dark for 30 min. Then, the absorbance at 593 nm was read. The antioxidant power was expressed as Fe
2+ antioxidant capacity (Fe
2+ μmol g
−1 of dry plant weight).
The antiradical activity was evaluated according to the spectrophotometric 2,2-Diphenyl-1-picrylhydrazyl (DPPH) scavenging activity method [
18,
19] with modifications. A total of 100 µL of 80% methanol extracts used for the total phenolic content assay were diluted with 1 mL of 60 µM DPPH solution. The absorbance was measured after 16 min using the CamSpec M501 spectrophotometer at 515 nm. The ability of plant extract to scavenge DPPH free radicals was calculated using the DPPH solution as a blank. Data are presented as the mean of three analytical samples to scavenge DPPH free radicals (in µmol g
−1) on a dry basis of the plant.
2.4.4. Determination of Chlorophylls and Carotenoids by Chromatographic Method
Contents of chlorophylls (
a,
b) and carotenoids (neoxanthin, violaxanthin, lutein and zeaxanthin, α- and β-carotenes) were evaluated according to the methods of Edelenbos (2001) using high-performance liquid chromatography (HPLC) on a Chromegabond C30 column (3 µm particle size, 15 × 2.1 mm) (ES Industries, West Berlin, NJ, USA) [
20]. Carotenoids were extracted using 80% acetone (500 mg of sample grounded with 10 mL liquid N
2), centrifuged (10 min, 4000 rpm min
−1), and filtrated through a 13 mm and 0.22 µm nylon syringe filter (BGB Analytik AG, Böckten, Switzerland). The HPLC 10A system (Shimadzu, Kyoto, Japan) equipped with a diode array (SPD-M 10A VP) detector was used for analysis. Peaks were detected at 440 nm. The mobile phase consisted of A (80% methanol, 20% water) and B (100% ethyl acetate). Gradient: 0 min; 20% B, 2.5 min; 22.5% B, 20–22.5 min; 50% B, 24–26 min; 80% B, 31–34 min; 100% B, 42–47 min; and 20% B, flow rate 1 mL min
−1. The contents of chlorophylls and carotenoids in each group of lettuce were evaluated after 18, 36, 60, and 84 h after inoculation. The results are expressed as mg g
−1 in the dry weight of plants.
2.5. Statistical Analysis
For linear regression analysis, data were processed using Microsoft 365 Excel software. For statistical analysis, data were processed using the XLSTAT software (Addinsoft, France), using one-way analysis of variance, ANOVA, and Tukey’s HSD test at confidence level p ≤ 0.05.
4. Discussion
Plants have developed complex sensory mechanisms to identify biotic invasion and overcome the detriment of growth, pre- and postharvest yield, and survival [
21,
22,
23]. The plant responds to this fungal pathogen with transcriptional reprogramming of genes, which encode proteins involved in pathogen perception, signaling, transcription, hormonal signaling, secondary metabolic pathways and proteins with a diverse role in defence against biotic stress [
23]. Therefore, the discovery of specific parameters indicating such mechanisms at an early stage when no visible disease symptoms appear would allow helping protect plants against further spread of the pathogen and reduce the usage of chemical fungicides.
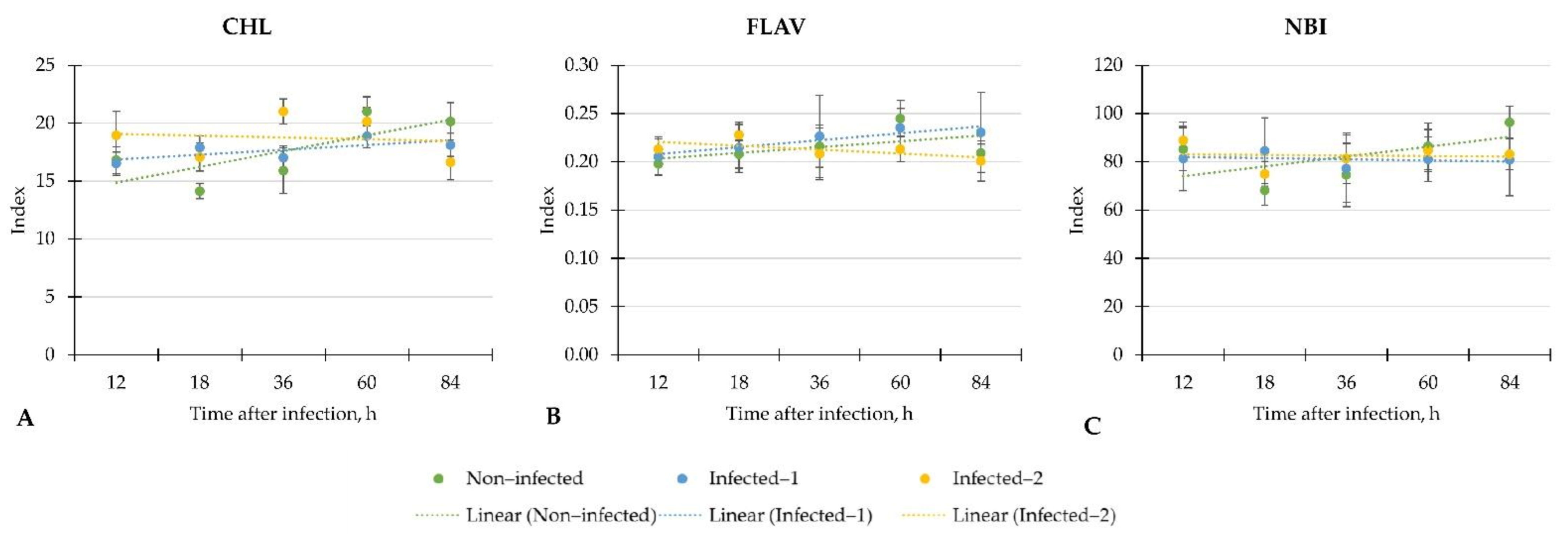
Our study evaluated the early physiological response according to leaf spectral indices to two different inoculation methods of B. cinerea on a commercially important greenhouse crop—lettuce. Moreover, we determined main plant metabolites to know if the response according to the leaf spectral indices is the same as the biochemical level. The summarised results showed weak, moderate, or strong linear regression of leaf spectral indices and phytochemicals throughout the investigation period from 12 to 84 h after inoculation with B. cinerea.
In the study, most attention was paid to the linear regression of measured indices which changed in inoculated with B. cinerea lettuce compared to non-infected plants. We found that changes depended on the inoculation method. For example, the changes of CHL in plants that were infected with pathogen spore disc were similar to non-infected plants. On the contrary, the linear regression of CHL in lettuce infected with pathogens spore suspension sealed to the central vein of the plant was weak and led to the significantly lower CHL at the end of the investigation (84 h) compared to non-infected plants. Such changes in lettuce may indicate the inconsistent plant response to inoculation with B. cinerea at different analysis times. Also, the significant increment of CHL in lettuce infected with pathogen spore disc after 18 h may be considered a plant response to the pathogen.
The strong linear regression of FLA was found in the lettuce inoculated with the pathogen spore disc. In contrast, the linear regression of FLA was similar in control and infected with spore suspension plants. However, we did not find differences in FLA in infected lettuce compared to control plants.
In general, the inoculation with B. cinerea did not change NBI in lettuce. Moreover, the weak regression of NBI in all investigated groups of plants showed inconsistent changes throughout the evaluation period. Similar trends were found on NDVI, PSRI, CRI, and PRI in all lettuce. However, the significant increment of CRI, NDVI and PRI in lettuce infected with pathogen spore disc compared to non-infected plants after 18 h shows the potential of these indices for early disease detection in lettuce.
Generally, the early detection of the
B. cinerea infection in horticultural crops using non-destructive methods is an emerging topic. Such an evaluation method requires considering the relative position between the leaf and detector and the lighting conditions [
3]. The study of Wu et al. [
24] showed 85% accuracy before infection symptoms were visible in eggplant leaves when the hyperspectral visible near-infrared (VNIR) spectroradiometer to measure reflectance intensities of healthy and
B. cinerea inoculated plants was used. In another study, the 552 nm and 701 nm wavelengths for detecting discolouration in lettuce leaves with over 99% accuracy were approved, but not for
Botrytis fungal infection [
25]. Scarboro et al. demonstrated a process for selecting optimal spectral bands integrated into a two-band multispectral (540 and 670 nm) camera imaging system and evaluating its performance for detecting
B. cinerea infection on lettuce leaves to monitor disease progression producing a true positive rate of 95.25% with a false positive rate of 9.316% [
3]. Such findings, as mentioned earlier, can be considered for choosing leaf spectral reflectance indices according to the wavelengths used in the formulas of their calculation.
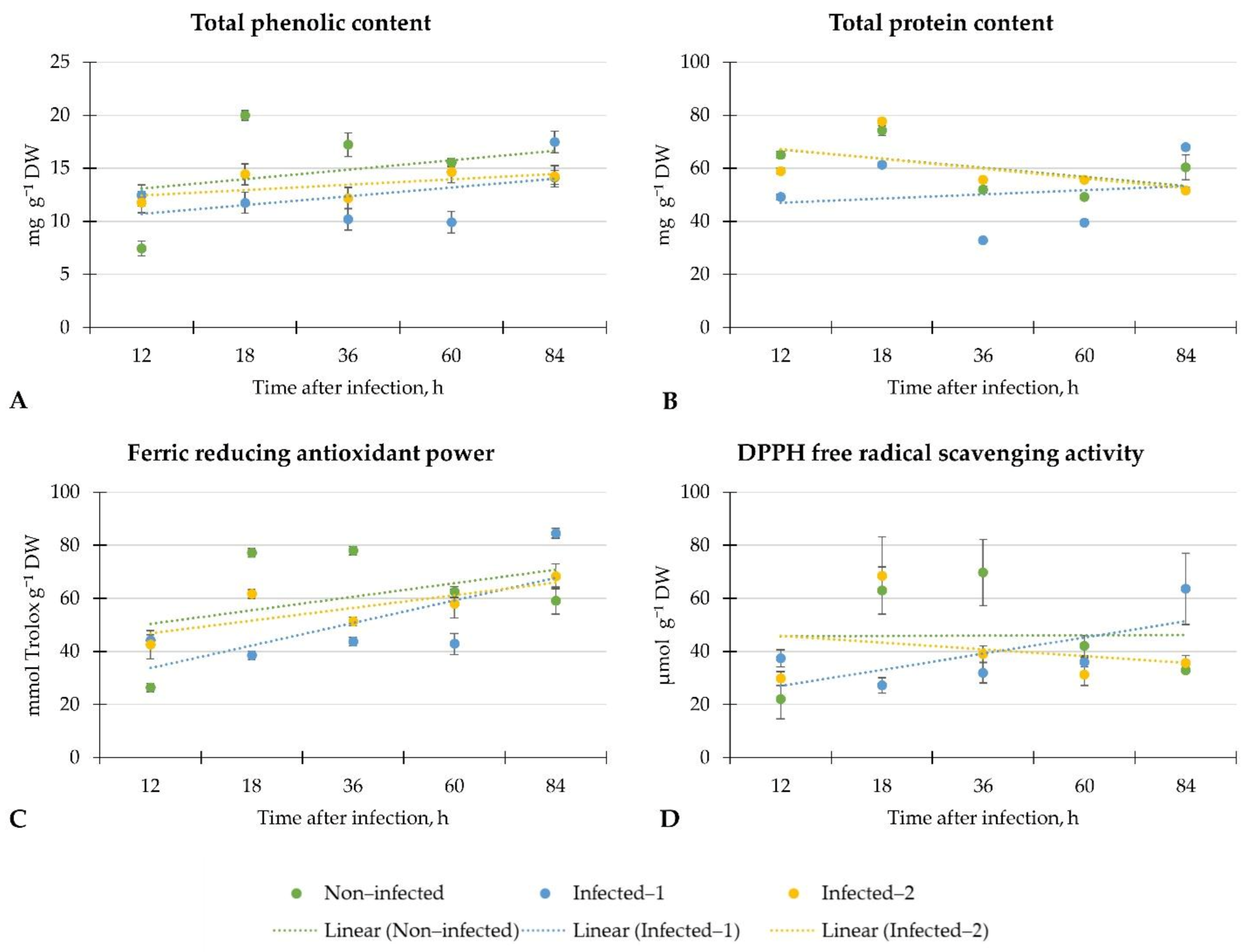
Our results demonstrated the weak linear regression of total phenolic and protein contents and DPPH free radical scavenging activity in the plants from each investigation group. Only ferric-reducing antioxidant power linear regression in infected lettuce was moderate, while in non-infected plants, it was weak. However, total phenolic content increased twice after 12 h regardless of the inoculation method, and similar results were found on ferric-reducing antioxidant power in lettuce. However, the decrement of these phytochemicals was later observed. Surprisingly, at the end of the investigation period, the total phenolic and protein content and antiradical activity according to the DPPH and ferric-reducing antioxidant power methods increased in lettuce infected with the pathogen spore disc.
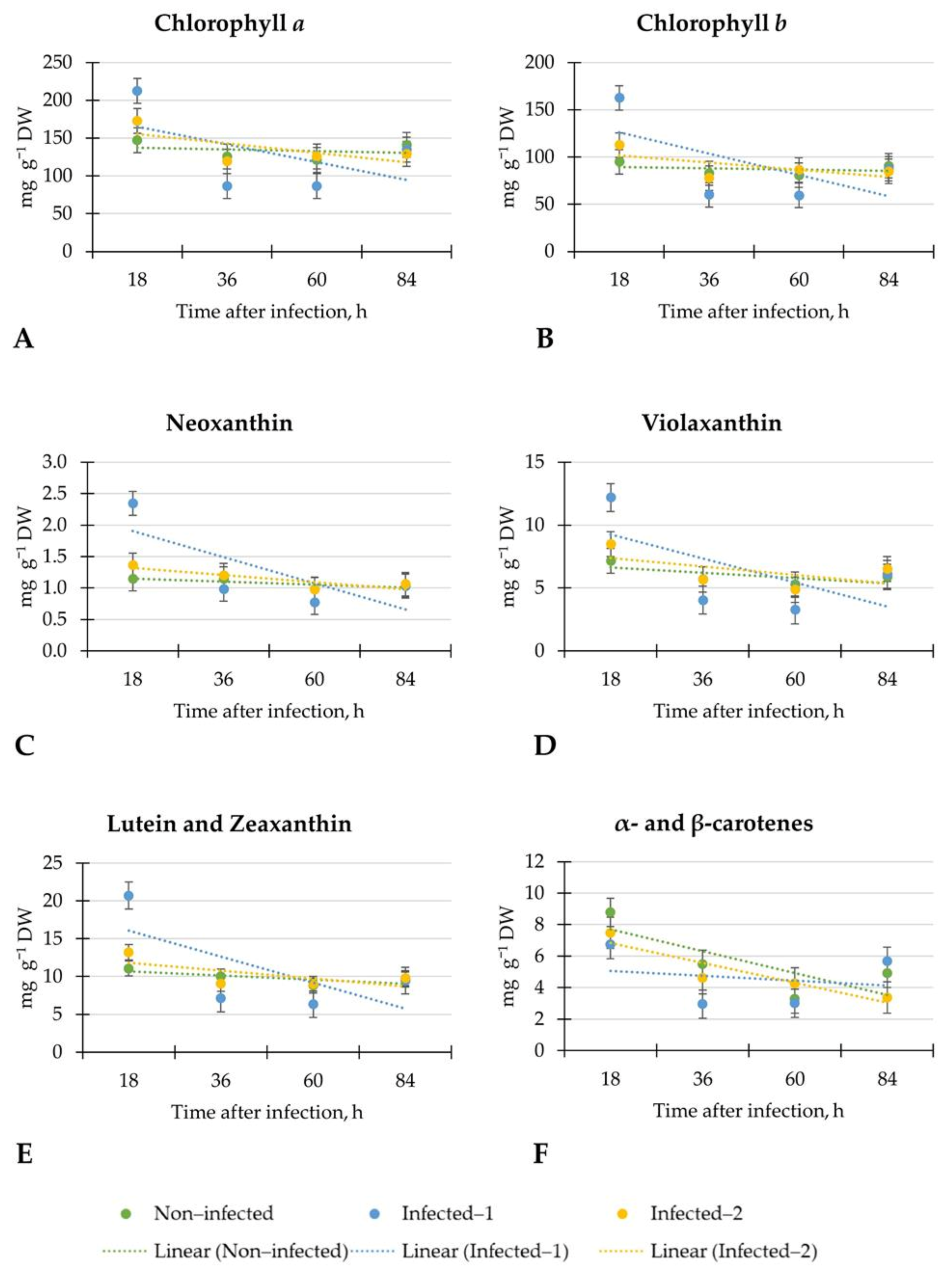
In all tested groups of lettuce, the linear regression of chlorophylls was weak, showing inconsistent plant response at each evaluation time. Similar changes were observed in contents of neoxanthin, violaxanthin, lutein, and zeaxanthin, α- and β-carotenes in all tested lettuce. However, in lettuce inoculated with pathogen spore disc, the linear regression of α- and β-carotenes was weak. It may show the inconsistent plant response because of the affected metabolism of these carotenoids. However, after 18 h, the significant increment of chlorophyll b (1.7-fold), neoxanthin (2.0-fold), lutein, and zeaxanthin (1.9-fold) were observed in inoculated with the pathogen spore disc compared to non-infected lettuce. In addition, the changes in contents of chlorophylls after 18 h were similar to non-destructive CHL measurements. There is a lack of results on early detection of B. cinerea on lettuce by non-destructive measurements of leaf spectral reflectance indices and biochemical compounds. For precise and optimal B. cinerea control, early detection is essential because when the disease can be seen visually, it is difficult to stop it.