Phytochemical Profiling, Mineral Elements, and Biological Activities of Artemisia campestris L. Grown in Algeria
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction of Secondary Metabolites
2.3. Inductively Coupled Plasma ICP-OES
2.4. Liquid Chromatography-Mass Spectrometry (LC-MS-MS)
2.5. Assessment of Total Phenolics, Flavonoids Compounds
2.5.1. Total Phenolics Content
2.5.2. Total Flavonoids Content
2.6. Antioxidant Activity
2.6.1. DPPH Scavenging Activity
2.6.2. ABTS Scavenging Activity
2.6.3. β-Carotene-Linoleic Acid Bleaching Activity
- I (%): percentage of inhibition
- AH0: Absorbance of β-carotene in extract at t = 0.
- AC0: Absorbance of β-carotene in negative control at t = 0.
- AHt: Absorbance of β-carotene in extract at 120 min.
- ACt: Absorbance of β-carotene in negative control at 120 min.
2.6.4. Galvinoxyl (GOR) Scavenging Activity
2.6.5. Reducing Power Activity
2.6.6. Cupric Reducing Antioxidant Capacity (CUPRAC) Activity
2.6.7. O-Phenanthroline Chelating Activity
2.7. Enzymes Inhibitory Activity
2.7.1. Cholinesterase Inhibitory Activity
2.7.2. α-Amylase Inhibitory Activity
2.7.3. Pancreatic Lipase Inhibitory Activity
- AbsA: the activity in the absence of an inhibitor;
- Absa: the negative control in the absence of an inhibitor;
- AbsB: the activity in the presence of an inhibitor;
- Absb: the negative control in the presence of an inhibitor.
2.8. Photoprotective Activity
2.9. Statistical Data Analysis
3. Results
3.1. Mineral Analysis
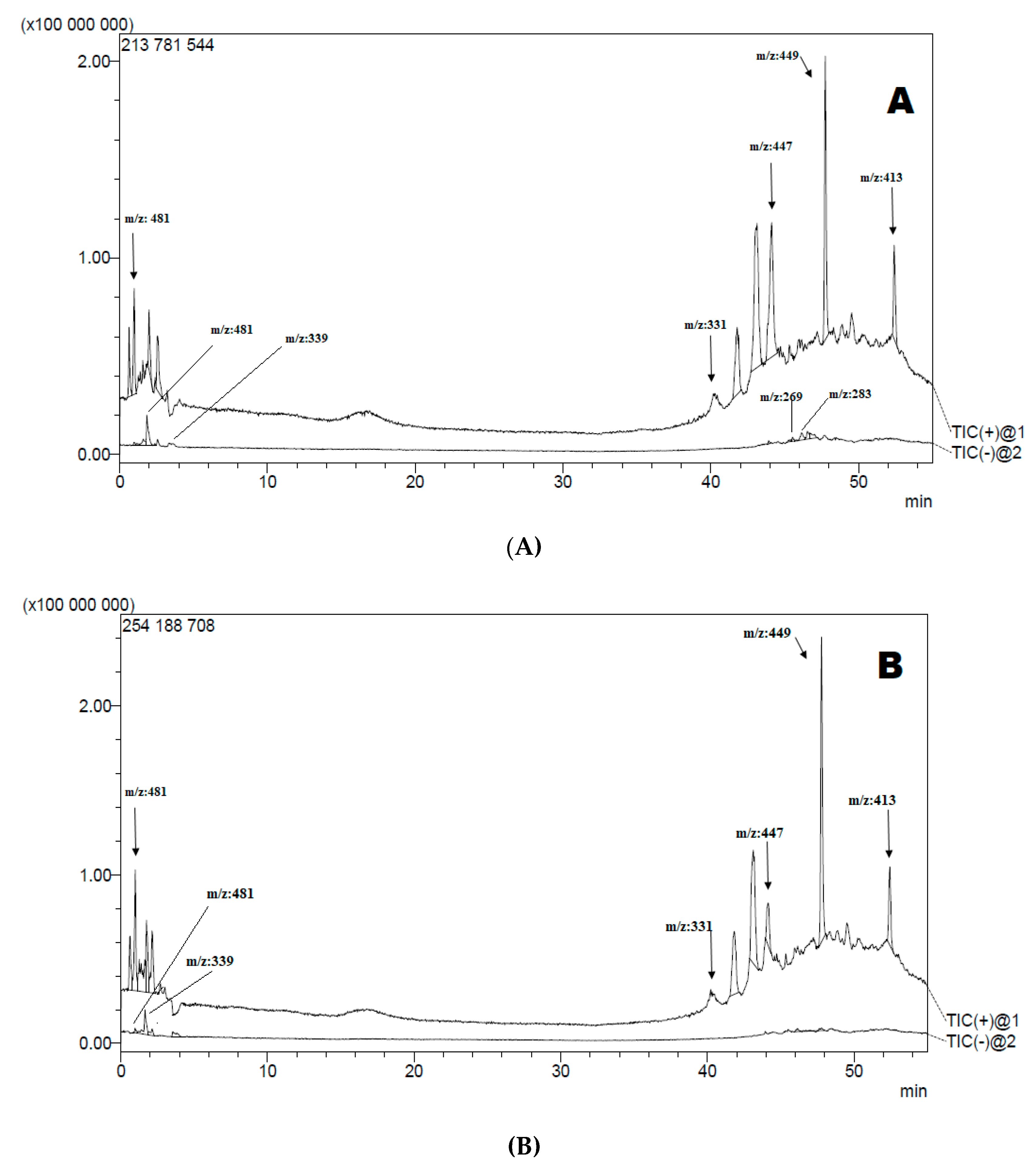
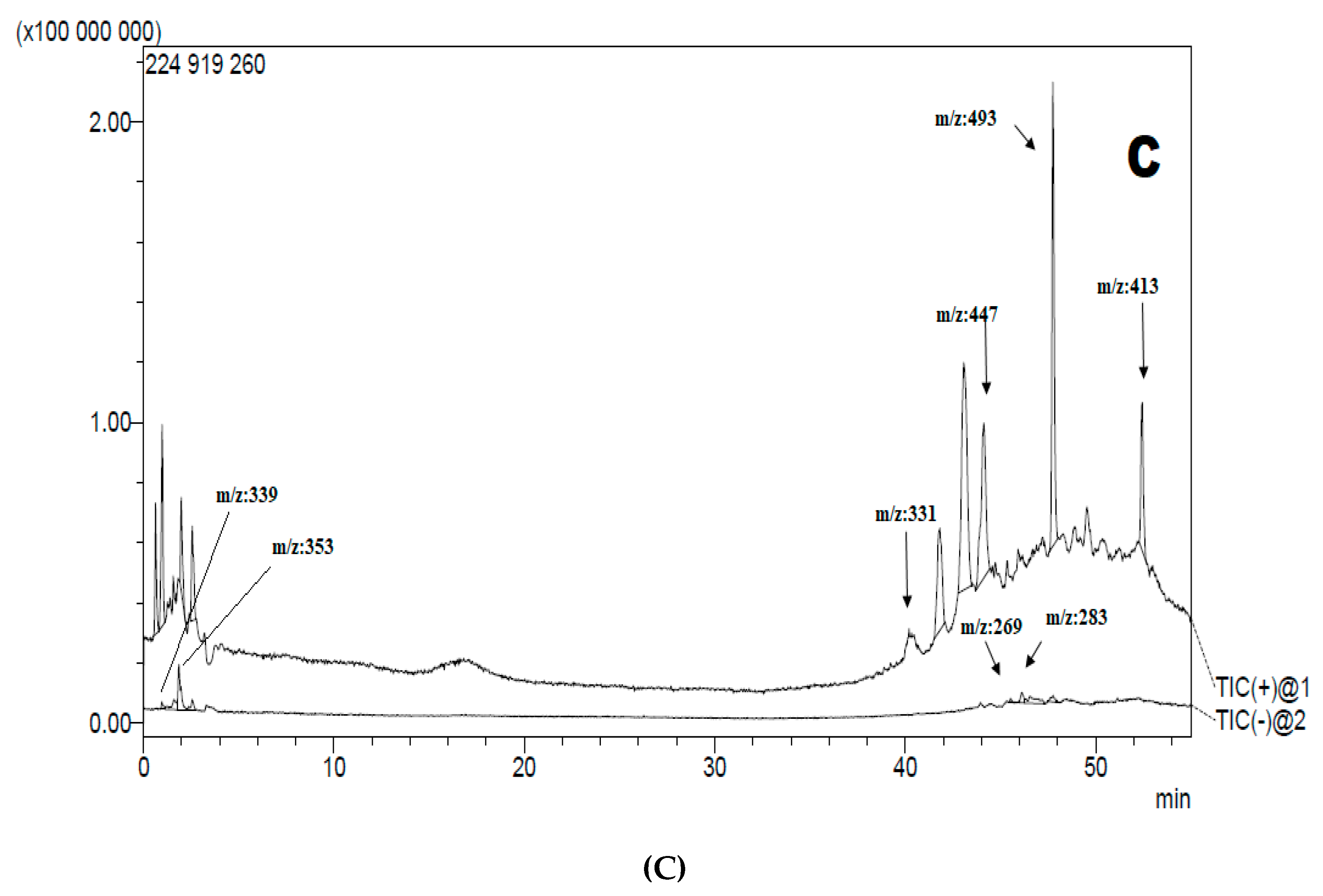
3.2. Liquid Chromatography-Mass Spectrometry Analysis LC-MS-MS
3.3. Assessment of Total Bioactive Compounds
3.4. Antioxidant Activity
3.4.1. DPPH Scavenging Activity
3.4.2. ABTS Scavenging Activity
3.4.3. β-Carotene-Linoleic Acid Bleaching Activity
3.4.4. Galvinoxyl (GOR) Scavenging Activity
3.4.5. Reducing Power Activity
3.4.6. Cupric Reducing Antioxidant Capacity Activity
3.4.7. O-Phenanthroline Activity
3.5. Enzymes Inhibition Activity
3.5.1. Cholinesterase Inhibitory Activity
3.5.2. α-Amylase Inhibitory Activity
3.5.3. Pancreatic Lipase Inhibitory Activity
3.6. Photoprotective Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AcME | Artemisa campestris Methanol Extract |
| AcPEE | Artemisa campestris Petroleum ether Extract |
| AcDE | Artemisa campestris Dichloromethane Extract |
| AcEAE | Artemisa campestris Ethyl Acetate Extract |
| AcBE | Artemisa campestris Butanol Extract |
| AcAE | Artemisa campestris Aqueous Extract |
| ACHE | acetylcholinesterase |
| BChE | butyrylcholinesterase |
| CUPRAC | cupric reducing antioxidant capacity |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| SPF | Sun Protective Factor |
| GOR | galvinoxyl radical |
| ICP-OES | Inductively coupled plasma-optical emission spectrometry |
| TIC+ | Total ion current in positive mode |
| TIC− | Total ion current in negative mode |
References
- Chan, K. Some aspects of toxic contaminants in herbal medicines. Chemosphere 2003, 52, 1361–1371. [Google Scholar] [CrossRef]
- Ghribia, L.; Ghouilaa, H.; Omrib, A.; Besbesb, M.; Janneta, H.B. Antioxidant and anti–acetylcholinesterase activities of extracts and secondary metabolites from Acacia cyanophylla. Asian Pac. J. Trop. Biomed. 2014, 4, S417–S423. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.W.; Yang, F.J.; Chen, C.L.; Lee, W.T.; Chen, R.S. Free radical scavenging activity and antiproliferative potential of Polygonum cuspidatum root extracts. J. Nat. Med. 2010, 64, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Tani, C.K.; Le Bourgeois, T.; Munoz, F. Aspects floristiques des adventices du domaine phytogéographique oranais (Nord-Ouest Algérien) et persistance d’espèces rares et endémiques. Fl. Medit. 2010, 20, 29–46. [Google Scholar]
- Quezel, P.; Santa, S. New flora of Algeria and southern desert regions. In New Flora of Algeria and Southern Desert Regions; CNRS: Paris, France, 1962. [Google Scholar]
- Gouveia, S.C.; Castilho, P.C. Artemisia annua L.: Essential oil and acetone extract composition and antioxidant capacity. Ind. Crops Prod. 2013, 45, 170–181. [Google Scholar] [CrossRef]
- Quezel, P.; Santa, S. Nouvelle flore de l’Algérie et des régions désertiques méridionales; Editions du Centre National de la recherche scientifique: Paris, France, 1963. [Google Scholar]
- Watson, L.E.; Bates, P.L.; Evans, T.M.; Unwin, M.M.; Estes, J.R. Molecular phylogeny of subtribe Artemisiinae (Asteraceae), including Artemisia and its allied and segregate genera. BMC Evol. Biol. 2002, 2, 17. [Google Scholar] [CrossRef]
- Toumi, M.; Messirene, A.; Benkhalifa, A. Les plantes à usage thérapeutique dans le Hoggar: Cas de la ville de Tamanrasset et son environnement rural. In 3ème Exposition d’Ethnobotanique Et 4ème Atelier d’Initiation à La Phytothérapie; Jardin d’Essai du Hamma: Mohamed Belouizdad, Algeria, 2016; pp. 63–65. [Google Scholar]
- Hamza, N.; Berke, B.; Umar, A.; Cheze, C.; Gin, H.; Moore, N. A review of Algerian medicinal plants used in the treatment of diabetes. J. Ethnopharmacol. 2019, 238, 111841. [Google Scholar] [CrossRef]
- Boudjelal, A.; Henchiri, C.; Sari, M.; Sarri, D.; Hendel, N.; Benkhaled, A.; Ruberto, G. Herbalists and wild medicinal plants in M’Sila (North Algeria): An ethnopharmacology survey. J. Ethnopharmacol. 2013, 148, 395–402. [Google Scholar] [CrossRef]
- Tardío, J.; Pardo-de-Santayana, M.; Morales, R. Ethnobotanical review of wild edible plants in Spain. Bot. J. Linn. Soc. 2006, 152, 27–71. [Google Scholar] [CrossRef]
- Webster, D.; Taschereau, P.; Belland, R.J.; Sand, C.; Rennie, R.P. Antifungal activity of medicinal plant extracts; preliminary screening studies. J. Ethnopharmacol. 2008, 115, 140–146. [Google Scholar] [CrossRef]
- Dib, I.; Tits, M.; Angenot, L.; Wauters, J.N.; Assaidi, A.; Mekhfi, H.; Ziyyat, A. Antihypertensive and vasorelaxant effects of aqueous extract of Artemisia campestris L. from Eastern Morocco. J. Ethnopharmacol. 2017, 206, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Sefi, M.; Fetoui, H.; Makni, M.; Zeghal, N. Mitigating effects of antioxidant properties of Artemisia campestris leaf extract on hyperlipidemia, advanced glycation end products and oxidative stress in alloxan-induced diabetic rats. Food Chem. Toxicol. 2010, 48, 1986–1993. [Google Scholar] [CrossRef] [PubMed]
- Ghlissi, Z.; Sayari, N.; Kallel, R.; Bougatef, A.; Sahnoun, Z. Antioxidant, antibacterial, anti-inflammatory and wound healing effects of Artemisia campestris aqueous extract in rat. Biomed. Biomed. Pharmacother. 2016, 84, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Younsi, F.; Mehdi, S.; Aissi, O.; Rahali, N.; Jaouadi, R.; Boussaid, M.; Messaoud, C. Essential oil variability in natural populations of Artemisia campestris (L.) and Artemisia herba-alba (Asso) and incidence on antiacetylcholinesterase and antioxidant activities. Chem. Biodivers. 2017, 14, e1700017. [Google Scholar] [CrossRef] [PubMed]
- Golubkina, N.; Logvinenko, L.; Konovalov, D.; Garsiya, E.; Fedotov, M.; Alpatov, A.; Caruso, G. Foliar Application of Selenium under Nano Silicon on Artemisia annua: Effects on Yield, Antioxidant Status, Essential Oil, Artemisinin Content and Mineral Composition. Horticulturae 2022, 8, 597. [Google Scholar] [CrossRef]
- Ferchichi, L.; Merza, J.; Landreau, A.; Le Ray, A.M.; Legseir, B.; Seraphin, D.; Richomme, P. Occurrence of isocoumarinic and phenolic derivatives in Artemisia campestris L. subsp. campestris. Biochem. Syst. Ecol. 2006, 34, 829–832. [Google Scholar] [CrossRef]
- Larkem, I.; Tarai, N.; Benchikha, N.; Messaoudi, M.; Begaa, S.; Martins, M.; Silva, A.M.S.; Pinto, D.C.G.A. Chemical profile and antioxidant activity of Sesbania bispinosa (Jacq.) W. Wight aerial parts and seeds extracts. J. Food Process. Preserv. 2021, 45, e15468. [Google Scholar] [CrossRef]
- Messaoudi, M.; Rebiai, A.; Sawicka, B.; Atanassova, M.; Ouakouak, H.; Larkem, I.; Egbuna, C.; Awuchi, C.G.; Boubekeur, S.; Ferhat, M.A. Effect of Extraction Methods on Polyphenols, Flavonoids, Mineral Elements, and Biological Activities of Essential Oil and Extracts of Mentha pulegium L. Molecules 2022, 27, 11. [Google Scholar] [CrossRef]
- Fassel, V.A.; Kniseley, R.N. Inductively coupled plasma. Optical emission spectroscopy. Anal. Chem. 1974, 46, 1110A–1120A. [Google Scholar] [CrossRef]
- Butler, C.C.; Kniseley, R.N.; Fassel, V.A. Inductively coupled plasma-optical emission spectrometry. Application to the determination of alloying and impurity elements in low and high alloy steels. Anal. Chem. 1975, 47, 825–829. [Google Scholar] [CrossRef]
- Müller, L.; Gnoyke, S.; Popken, A.M.; Böhm, V. Antioxidant capacity and related parameters of different fruit formulations. Lwt-Food Sci. Technol. 2010, 43, 992–999. [Google Scholar] [CrossRef]
- Topçu, G.; Ay, M.; Bilici, A.; Sarıkürkcü, C.; Öztürk, M.; Ulubelen, A. A new flavone from antioxidant extracts of Pistacia Terebinthus. Food Chem. 2007, 103, 816–822. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Marco, G.J. A rapid method for evaluation of antioxidants. J. Am. Oil Chem. Soc. 1968, 45, 594–598. [Google Scholar] [CrossRef]
- Shi, H.; Noguchi, N.; Niki, E. Galvinoxyl method for standardizing electron and proton donation activity. Methods Enzymol. 2001, 335, 157–166. [Google Scholar]
- Oyaizu, M. Antioxidative activities of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Dianoczki, C.; Recseg, K.; Karlovits, G.; Szłyk, E. Determination of antioxidant capacities of vegetable oils by ferric-ion spectrophotometric methods. Talanta 2008, 76, 899–905. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Zengin, G.; Sarikurkcu, C.; Aktumsek, A.; Ceylan, R.; Ceylan, O. A comprehensive study on phytochemical characterization of Haplophyllum myrtifolium Boiss. endemic to Turkey and its inhibitory potential against key enzymes involved in Alzheimer, skin diseases and type II diabetes. Ind. Crops Prod. 2014, 53, 244–251. [Google Scholar] [CrossRef]
- Souza, S.P.D.; Pereira, L.L.S.; Souza, A.A.; Santos, C.D.D. Inhibition of pancreatic lipase by extracts of Baccharis trimera (Less.) DC., Asteraceae: Evaluation of antinutrients and effect on glycosidases. Rev. Bras. Farmacogn. 2011, 21, 450–455. [Google Scholar] [CrossRef]
- Santos, B.C.S.; Pires, A.S.; Yamamoto, C.H.; Couri, M.R.C.; Taranto, A.G.; Alves, M.S.; Araújo, A.L.D.S.D.M.; de Sousa, O.V. Methyl Chavicol and Its Synthetic Analogue as Possible Antioxidant and Antilipase Agents Based on the In Vitro and In Silico Assays. Oxid. Med. Cell. Longev. 2018, 2018. Available online: https://www.semanticscholar.org/paper/Methyl-Chavicol-and-Its-Synthetic-Analogue-as-and-Santos-Pires/56fd111a214c845f2828b32bbbc046ed0f2554a3 (accessed on 1 September 2022). [CrossRef] [PubMed]
- Mansur, J.D.S.; Breder, M.N.R.; Mansur, M.C.D.A.; Azulay, R.D. Determinaçäo do fator de proteçäo solar por espectrofotometria. An. Bras. Dermatol. 1986, 40, 121–124. [Google Scholar]
- Sayre, R.M.; Agin, P.P.; LeVee, G.J.; Marlowe, E. A comparison of in vivo and in vitro testing of sunscreening formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef]
- Aissani, F.; Grara, N.; Bensouici, C.; Bousbia, A.; Ayed, H.; Idris, M.H.M.; Teh, L.K. Algerian Sonchus oleraceus L.: A comparison of different extraction solvent on phytochemical composition, antioxidant properties and anti-cholinesterase activity. Adv. Trad. Med. 2022, 22, 383–394. [Google Scholar] [CrossRef]
- Bakchiche, B.; Gherib, A.; Bronze, M.R.; Ghareeb, M.A. Identification, quantification, and antioxidant activity of hydroalcoholic extract of Artemisia campestris from Algeria. Turk. J. Pharm. Sci. 2019, 16, 234. [Google Scholar] [CrossRef]
- Bourgou, S.; Rebey, I.B.; Mkadmini, K.; Isoda, H.; Ksouri, R.; Ksouri, W.M. LC-ESI-TOF-MS and GC-MS profiling of Artemisia herba-alba and evaluation of its bioactive properties. Int. Food Res. J. 2017, 99, 702–712. [Google Scholar] [CrossRef]
- Carazzone, C.; Mascherpa, D.; Gazzani, G.; Papetti, A. Identification of phenolic constituents in red chicory salads (Cichorium intybus) by high-performance liquid chromatography with diode array detection and electrospray ionisation tandem mass spectrometry. Food Chem. 2013, 138, 1062–1071. [Google Scholar] [CrossRef]
- He, Z.Z.; Yan, J.F.; Song, Z.J.; Ye, F.; Liao, X.; Peng, S.L.; Ding, L.S. Chemical constituents from the aerial parts of Artemisia minor. J. Nat. Prod. 2009, 72, 1198–1201. [Google Scholar] [CrossRef]
- Rechek, H.; Haouat, A.; Hamaidia, K.; Allal, H.; Boudiar, T.; Pinto, D.C.G.A.; Cardoso, S.M.; Bensouici, C.; Soltani, N.; Silva, A.M.S. Chemical composition and antioxidant, anti-inflammatory, and enzyme inhibitory activities of an endemic species from southern algeria: Warionia saharae. Molecules 2021, 26, 5257. [Google Scholar] [CrossRef] [PubMed]
- Ivanescu, B.; Vlase, L.; Corciova, A.; Lazar, M.I. HPLC-DAD-MS study of polyphenols from Artemisia absinthium, A. annua, and A. vulgaris. Chem. Nat. Compd. 2010, 46, 468–470. [Google Scholar] [CrossRef]
- Ivanescu, B.; Lungu, C.; Vlase, L.; Gheldiu, A.M.; Grigorescu, C.; Corciova, A. Bioactive compounds from Artemisia campestris L. subsp. campestris. Dementia 2018, 2, 3. [Google Scholar] [CrossRef]
- Sasikumar, V.; Kalaisezhiyen, P. Evaluation of Free Radical Scavenging Activity of Various Leaf Extracts from Kedrostis Foetidissima (Jacq.) Cogn. Biochem. Anal. Biochem. 2014, 3, 1. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. SPJ 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Polinsky, R.J. Clinical pharmacology of rivastigmine: A new-generation acetylcholinesterase inhibitor for the treatment of Alzheimer’s disease. Clin. Ther. 1998, 20, 634–647. [Google Scholar] [CrossRef]
- Jamshidi-Kia, F.; Lorigooini, Z.; Amini-Khoei, H. Medicinal plants: Past history and future perspective. J. HerbMed Pharmacol. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Messaoudi, M.; Begaa, S. Dietary Intake and Content of Some Micronutrients and Toxic Elements in Two Algerian Spices (Coriandrum sativum L. and Cuminum cyminum L.). Biol. Trace Elem. Res. 2019, 188, 508–513. [Google Scholar] [CrossRef]
- Benarfa, A.; Begaa, S.; Messaoudi, M.; Hamlat, N.; Sawicka, B. Elemental composition analysis of Pistacia lentiscus L.; leaves collected from Mitidja plain in Algeria using instrumental neutron activation analysis (INAA) technique. Radiochim. Acta 2020, 108, 821–828. [Google Scholar] [CrossRef]
- Zazzo, J.F. Oligo-éléments, vitamines et immunité. Nutr. Clin. Metab. 1993, 7, 121–129. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, M.A. Concentrations and health risk assessment of trace elements in cereals, fruits, and vegetables of Bangladesh. Biol. Trace Elem. Res. 2019, 191, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Islam, M.A.; Zaved, M.M. Assessment of Essential and Potentially Toxic Elements and Possible Health Risks in Hylocereus undatus and Punica granatum. Biol. Trace Elem. Res. 2020, 198, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Codex, F.A.O. Alimentarius, General Requirements (Food Hygiene); FAO: Rome, Italy, 1995. [Google Scholar]
- World Health Organization. WHO Global Report on Traditional and Complementary Medicine 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Lasisi, A.A.; Ejelonu, B.C.; Nwosu, F.O.; Olayiwola, M.A.; Yusuff, A.A. Heavy metals and macronutrients content in selected herbal plants of South-Western Nigeria. Hamdard Med. 2006, 49, 71–76. [Google Scholar]
- Sánchez-Moreno, C. Methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci. Technol. Int. 2002, 8, 121–137. [Google Scholar] [CrossRef]
- Megdiche-Ksouri, W.; Trabelsi, N.; Mkadmini, K.; Bourgou, S.; Noumi, A.; Snoussi, M.; Barbria, R.; Tebourbi, O.; Ksouri, R. Artemisia campestris phenolic compounds have antioxidant and antimicrobial activity. Ind. Crops Prod. 2015, 63, 104–113. [Google Scholar] [CrossRef]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Inchuen, S.; Narkrugsa, W.; Pornchaloempong, P. Effect of drying methods on chemical composition, color and antioxidant properties of Thai red curry powder. Agric. Nat. Resour. 2010, 44, 142–151. [Google Scholar]
- Fidrianny, I. Evaluation of antioxidant activities from various extracts of Dragon fruit peels using DPPH, ABTS assays and correlation with phenolic, flavonoid, carotenoid content. Int. J. Pharm. Sci. 2014, 5, 104–111. [Google Scholar]
- Akrout, A.; El Jani, H.; Amouri, S.; Neffati, M. Screening of antiradical and antibacterial activities of essential oils of Artemisia campestris L.; Artemisia herba alba asso, & thymus capitatus hoff. Et link. Growing wild in the southern of Tunisia. Res. Sci. Technol. Educ. 2009, 2, 29–39. [Google Scholar]
- Akrout, A.; Gonzalez, L.A.; El Jani, H.; Madrid, P.C. Antioxidant and antitumor activities of Artemisia campestris and Thymelaea hirsuta from southern Tunisia. Food Chem. Toxicol. 2011, 49, 342–347. [Google Scholar] [CrossRef]
- Hsu, B.; Coupar, I.M.; Ng, K. Antioxidant activity of hot water extract from the fruit of the Doum palm. Hyphaene Thebaica. Food Chem. 2006, 98, 317–328. [Google Scholar] [CrossRef]
- Boulanouar, B.; Abdelaziz, G.; Aazza, S.; Gago, C.; Miguel, M.G. Antioxidant activities of eight Algerian plant extracts and two essential oils. Ind. Crops Prod. 2013, 46, 85–96. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, Y.; Jin, S.; Hu, Y.; Wang, T.; Tian, R.; Han, Z.; Xu, D.; Jiang, Q. Circulating vitamin E levels and Alzheimer’s disease: A Mendelian randomization study. Neurobiol. Aging 2018, 72, 189-e1. [Google Scholar] [CrossRef] [PubMed]
- Mesulam, M.; Guillozet, A.; Shaw, P.; Quinn, B. Widely spread butyrylcholinesterase can hydrolyze acetylcholine in the normal and Alzheimer brain. Neurobiol. Dis. 2002, 9, 88–93. [Google Scholar] [CrossRef]
- Cheraif, K.; Bakchiche, B.; Gherib, A.; Bardaweel, S.K.; Çol Ayvaz, M.; Flamini, G.; Ascrizzi, R.; Ghareeb, M.A. Chemical composition, antioxidant, anti-tyrosinase, anti-cholinesterase and cytotoxic activities of essential oils of six Algerian plants. Molecules 2020, 25, 1710. [Google Scholar] [CrossRef]
- Szwajgier, D. Anticholinesterase Activity of Selected Phenolic Acids and Flavonoids-Interaction Testing in Model Solutions. Ann. Agric. Environ. Med. 2015, 22. Available online: https://agro.icm.edu.pl/agro/element/bwmeta1.element.agro-0469e05d-0b2c-4ff9-aedc-ce3ad01bf0ba (accessed on 1 September 2022). [CrossRef]
- Murray, A.P.; Faraoni, M.B.; Castro, M.J.; Alza, N.P.; Cavallaro, V. Natural AChE inhibitors from plants and their contribution to Alzheimer’s disease therapy. Curr. Neuropharmacol. 2013, 11, 388–413. [Google Scholar] [CrossRef]
- Boukhalkhal, S.; Gourine, N.; Pinto, D.C.G.A.; Silva, A.M.S.; Yousfi, M. UHPLC-DAD-ESI-MSn profiling variability of the phenolic constituents of Artemisia campestris L. populations growing in Algeria. Biocatal. Agric. Biotechnol. 2020, 23, 101483. [Google Scholar] [CrossRef]
- Wresdiyati, T.; SA’DIAH, S.; Winarto, A.D.I.; Febriyani, V. Alpha-glucosidase inhibition and hypoglycemic activities of Sweitenia mahagoni seed extract. HAYATI J. Biosci. 2015, 22, 73–78. [Google Scholar] [CrossRef]
- Rasouli, H.; Hosseini-Ghazvini, S.M.-B.; Adibi, H.; Khodarahmi, R. Differential α-amylase/α-glucosidase inhibitory activities of plant-derived phenolic compounds: A virtual screening perspective for the treatment of obesity and diabetes. Food Funct. 2017, 8, 1942–1954. [Google Scholar] [CrossRef]
- Bljajić, K.; Brajković, A.; Čačić, A.; Vujić, L.; Jablan, J.; Saraiva de Carvalho, I.; Zovko Končić, M. Chemical composition, antioxidant, and α-glucosidase-inhibiting activity of aqueous and hydroethanolic extracts of traditional antidiabetics from Croatian ethnomedicine. Horticulturae 2021, 7, 15. [Google Scholar] [CrossRef]
- De la Garza, A.L.; Milagro, F.I.; Boque, N.; Campión, J.; Martínez, J.A. Natural inhibitors of pancreatic lipase as new players in obesity treatment. Planta Med. 2011, 77, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Dutra, E.A.; Kedor-Hackmann, E.R.M.; Santoro, M.I.R.M. Determination of sun protection factor (SPF) of sunscreens by ultraviolet spectrophotometry. Rev. De Cienc. Farm. Basica E Apl. 2004, 40, 381–385. [Google Scholar] [CrossRef]
- Saewan, N.; Jimtaisong, A. Photoprotection of natural flavonoids. J. Appl. Pharm. Sci. 2013, 3, 129–141. [Google Scholar]
- Commission of European Communities. Recommendation of 22 September. On Sun Screen Products and Manufacturers Claims. Off. J. Eur. Union 2006. Available online: https://eur-lex.europa.eu (accessed on 1 September 2022).
| Longueur D’onde λ (nm) | EE (λ) × I(λ) (Norms) |
|---|---|
| 290 | 0.0150 |
| 295 | 0.0817 |
| 300 | 0.2874 |
| 305 | 0.3278 |
| 310 | 0.1864 |
| 315 | 0.0837 |
| 320 | 0.0180 |
| Total | 1.0000 |
| Elements | Mean ± SD * | V (%) ** |
|---|---|---|
| Ca | 10,538.1 ± 69.9 | 0.66 |
| Cd | 0.0988 ± 0.0098 | 9.92 |
| Co | 1.28 ± 0.148 | 11.56 |
| Cr | 1.083 ± 0.055 | 5.08 |
| Fe | 1181.8 ± 8.23 | 6.97 |
| Li | 46.6 ± 4.45 | 9.55 |
| Mg | 990.7 ± 3.145 | 0.32 |
| Mn | 41.06 ± 6.51 | 15.85 |
| Mo | 0.29 ± 0.068 | 23.45 |
| Ni | 2.75 ± 0.43 | 15.64 |
| Pb | 0.68 ± 0.305 | 44.85 |
| Sr | 23.93 ± 0.768 | 3.21 |
| Ti | 20.38 ± 1.61 | 7.90 |
| Zn | 47.07 ± 0.797 | 1.69 |
| Na | 298.41 ± 1.319 | 0.44 |
| Cu | 70.22 ± 0.94 | 1.34 |
| Extract | tR (min) | Ionisation Mode (m/z) | m/z | Tentatively Identified Compound | Molecular Formula | Ref |
|---|---|---|---|---|---|---|
| AcDE AcEAE AcBE | 0.645 0.627 0.646 | [M + H]+ | 172 | NI | NI | - |
| AcDE AcEAE | 0.983 0.986 | [M + H]+ | 481 | 15-O-β-D-glucopyranosyl-11β, 13-dihydro urospermal A | C21H30O10 | [39] |
| AcBE | 0.987 | [M + H]+ | 437 | NI | NI | - |
| AcEAE | 1.629 | [M + H]+ | 365 | NI | NI | - |
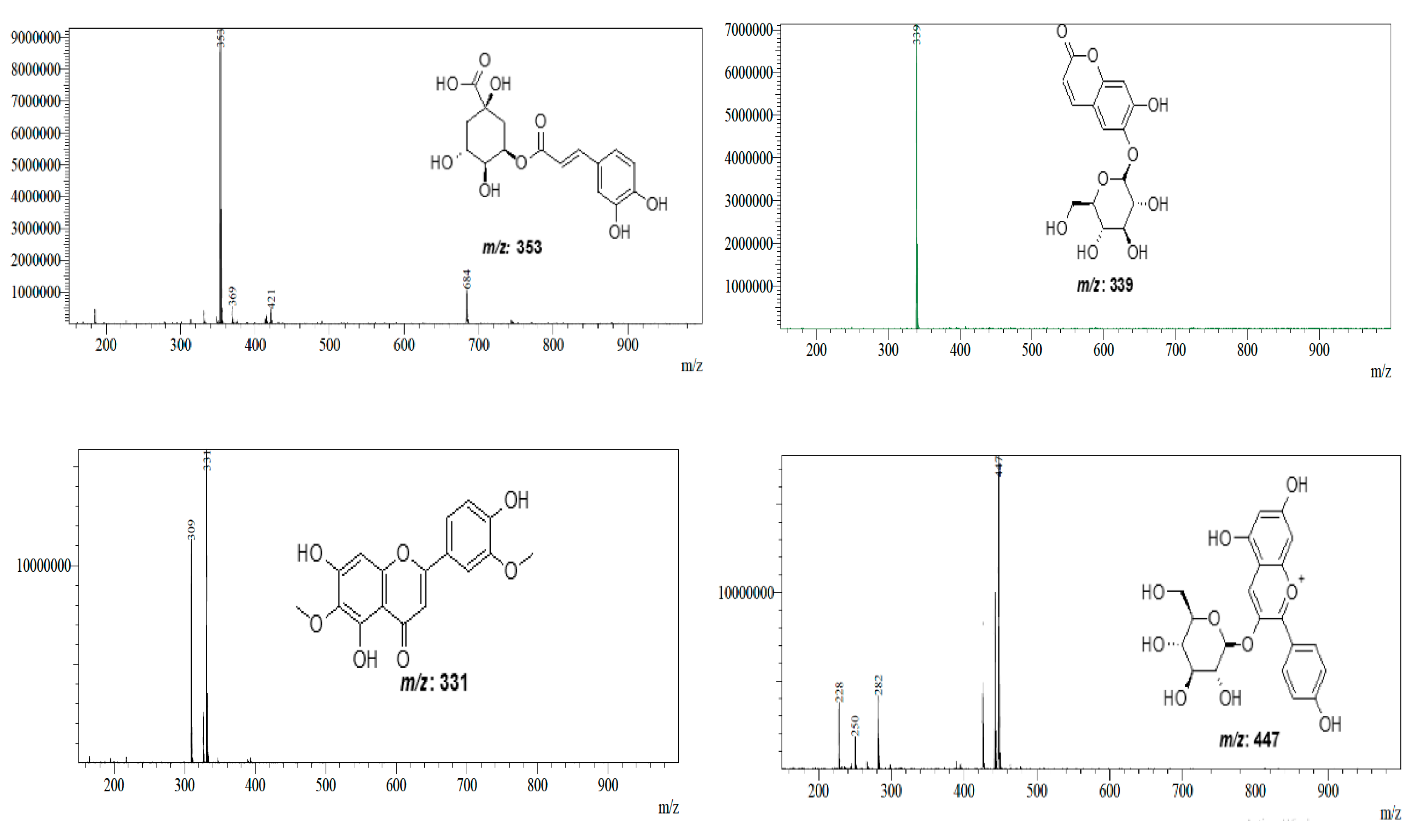
| AcDE AcEAE AcBE | 1.998 1.764 1.995 | [M + H]− | 353 | 5-O-caffeoylquinic acid | C16H18O9 | [40] |
| AcDE AcEAE AcBE | 2.564 2.144 2.568 | [M + H]+ | 381 | NI | NI | - |
| AcDE AcEAE AcBE | 41.787 41.831 41.805 | [M + H]+ | 331 | Jaceosidin | C17H14O7 | [41] |
| AcDE AcEAE AcBE | 43.128 43.130 43.083 | [M + H]+ | 367 | NI | NI | - |
| AcDE AcEAE AcBE | 44.107 44.133 44.115 | [M + H]+ | 447 | Pelargonidin-3-O-glucuronide | C21H19O11 | [42] |
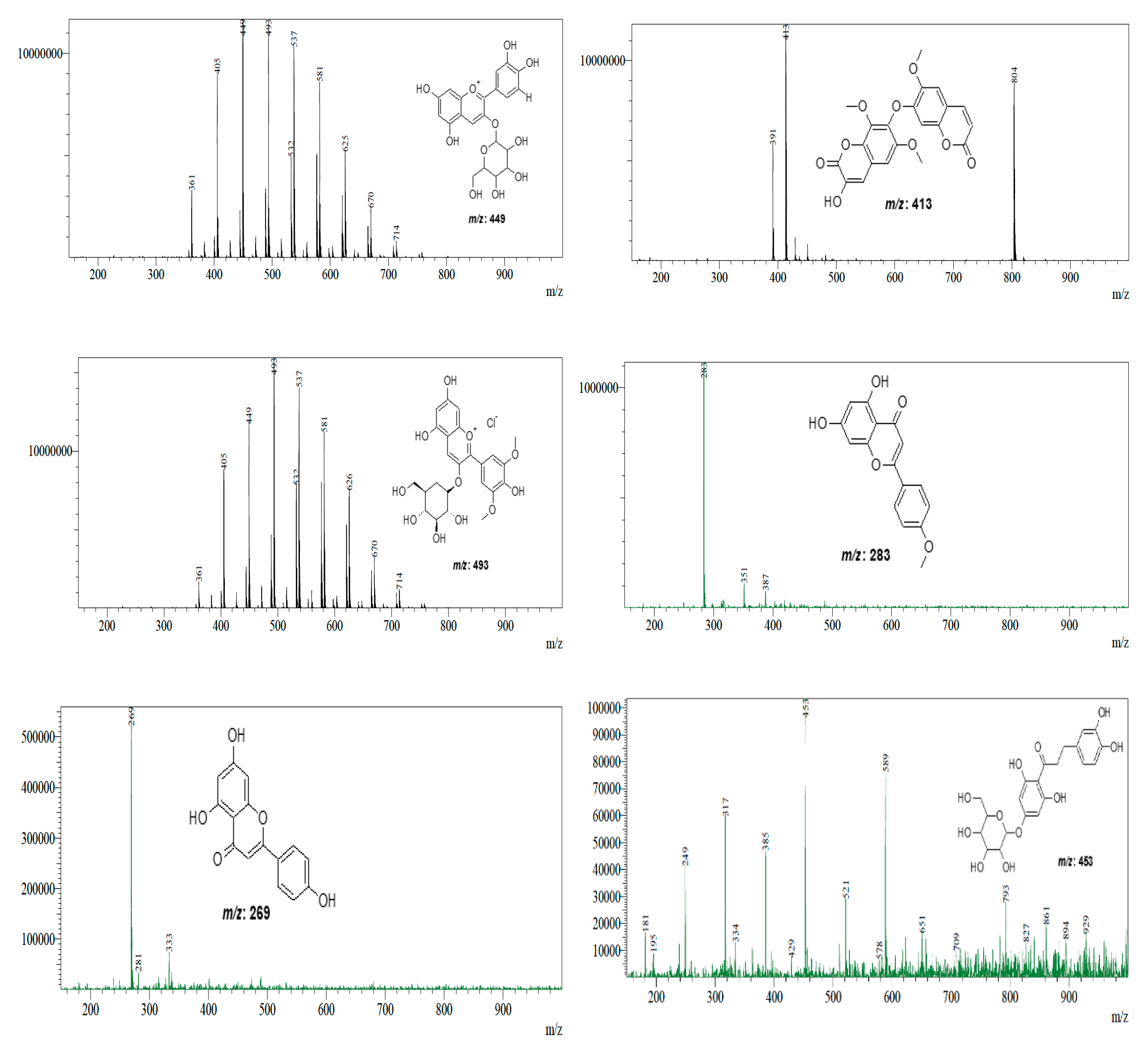
| AcDE AcEAE | 47.738 47.771 | [M + H]+ | 449 | Cyanidin-3-O-glucoside | C21H20O11 | [42] |
| AcDE AcEAE AcBE | 52.406 52.432 52.427 | [M + H]+ | 413 | Arteminorin B | C21H16O9 | [43] |
| AcBE | 47.747 | [M + H]+ | 493 | Malvidin 3-O-glucoside | C23H25ClO12 | [42] |
| AcDE AcEAE AcBE | 0.978 0.974 0.970 | [M − H]− | 239 | NI | NI | - |
| AcDE AcBE | 1.600 1.597 | [M − H]− | 369 | NI | NI | - |
| AcDE AcEAE AcBE | 1.854 1.646 1.871 | [M − H]− | 339 | Esculetin-6-O-glucoside | C15H16O9 | [44] |
| AcDE AcEAE AcBE | 2.387 2.134 2.565 | [M − H]− | 403 | NI | NI | - |
| AcEAE | 0.218 | [M − H]− | 453 | 3-hydroxyphloretin 6′-O-hexoside | C21H24O11 | [41] |
| AcDE AcBE | 45.530 45.522 | [M − H]− | 269 | Apigenin | C15H10O5 | [45] |
| AcDE AcEAE AcBE | 46.137 46.135 46.112 | [M − H]− | 283 | Acacetin | C16H12O5 | [46] |
| AcEAE | 3.552 | [M − H]− | 198 | NI | NI | - |
| Extracts | Total Phenolic Compounds Content (µg GAE/mg) * | Flavonoids Content (µg QE/mg) ** |
|---|---|---|
| AcME | 135.37 ± 1.35 a | 61.59 ± 0.58 a |
| AcPEE | 30.27 ± 0.33 b | 65.69 ± 0.29 b |
| AcDE | 203.60 ± 0.67 c | 69.44 ± 1.47 c |
| AcEAE | 527.33 ± 0.61 d | 203.19 ± 0.14 d |
| AcBE | 130.27 ± 0.33 e | 66.87 ± 0.29 e |
| AcAE | 141.64 ± 1.52 f | 63.4 ± 0.14 f |
| Extracts | DPPH IC50 (μg/mL) | ABTS IC50 (μg/mL) | β-Carotene Linoleic Acid IC50 (μg/mL) | GOR IC50 (μg/mL) | Phenanthroline A0.5 (μg/mL) | Reducing Power A0.5 (μg/mL) | CUPRAC A0.5 (μg/mL) |
|---|---|---|---|---|---|---|---|
| AcME | 141.47 ± 0.65 a | 26.04 ± 0.39 a | ≥200 | 68.21 ± 0.13 a | ≥50 | 54.00 ± 0.33 a | 449.57 ± 4.87 a |
| AcPEE | ≥200 | ≥200 | ≥200 | ≥100 | ≥50 | ≥50 | ≥200 |
| AcDE | 73.82 ± 1.98 b | 23.26 ± 0.42 b | >200 | 16.11 ± 0.02 b | 31.95 ± 0.22 b | 96.58 ± 1.51 b | 56.44 ± 1.11 b |
| AcEAE | 10.45 ± 0.19 c | 9.52 ± 0.12 c | >200 | 2.45 ± 0.03 c | 7.12 ± 0.15 c | 16.05 ± 0.16 c | 9.94 ± 0.21 c |
| AcBE | 147.09 ± 0.17 d | 66.52 ± 0.94 d | 183.87 ± 1.30 d | 62.37 ± 0.16 d | 35.56 ± 1.51 d | ≥200 | 91.58 ± 2.67 d |
| AcAE | 126.09 ± 1.63 e | 58.67 ± 0.58 e | ≥50 | 152.18 ± 0.47 e | 136.67 ± 1.53 e | 103.25 ± 1.09 e | 233.33 ± 0.58 e |
| BHT * | 22.32 ± 1.19 f | 1.29 ± 0.30 f | 1.05 ± 0.01 f | 3.32 ± 0.18 f | 2.24 ± 0.17 f | ≥200 | 9.62 ± 0.87 f |
| BHA * | 5.73 ± 0.41 g | 1.81 ± 0.10 g | 0.90 ± 0.02 g | 5.38 ± 0.06 g | 0.93 ± 0.07 g | 8.41 ± 0.67 g | 3.64 ± 0.19 g |
| Ascorbic acid * | NT | NT | NT | NT | NT | 9.01 ± 1.46 h | NT |
| Extracts | Anti-Cholinesterase | Anti-α-Amylase | Anti-Lipase | |||||
|---|---|---|---|---|---|---|---|---|
| AChE IC50 (μg/mL) | Inhibition (%) (200 μg/mL) | BChE IC50 (μg/mL) | Inhibition (%) (200 μg/mL) | α-Amylase IC50 (μg/mL) | Inhibition (%) (4000 μg/mL) | Lipase IC50 (μg/mL) | Inhibition (%) (1000 μg/mL) | |
| AcME | NA | NA | ≥200 | 30.51 ± 0.29 | NT | NT | NT | NT |
| AcPEE | 59.03 ± 0.58 a | 88.01 ± 0.68 | 93.50 ± 1.60 a | 56.13 ± 1.49 | 11.79 ± 0.14 a | 97.91 ± 0.63 | 40.15 ± 1.36 a | 75.72 ± 1.34 |
| AcDE | ≥200 | 4.94 ± 0.50 | 185.11 ± 2.5 b | 52.49 ± 1.52 | 28.33 ± 1.35 b | 92.10 ± 0.36 | 86.29 ± 2.60 b | 62.49 ± 0.38 |
| AcEAE | 23.16 ± 0.19 c | 83.25 ± 0.39 | ≥ 200 | 26.49 ± 1.55 | 284.33 ± 3.9 c | 51.91 ± 0.62 | 155.47 ± 3.44 c | 55.04 ± 0.58 |
| AcBE | NA | NA | NA | NA | 21.55 ± 0.66 d | 88.98 ± 0.07 | NA | NA |
| AcAE | NA | NA | NA | NA | NA | NA | NA | NA |
| Galantamine * | 6.27 ± 1.15 e | 94.77 ± 0.34 | 34.75 ± 1.99 e | 78.95 ± 0.58 | NA | NT | NT | NT |
| Acarbose * | NT | NT | NT | NT | 3650.93 ± 10.7 f | 53.05 ± 1.59 | NT | NT |
| Orlistat * | NT | NT | NT | NT | NT | NT | 0.06 ± 0.001 g | 79.84 ± 1.07 |
| Nivea * | Vichy * | AcME | AcPEE | AcDE | AcEAE | AcBE | AcAE | |
|---|---|---|---|---|---|---|---|---|
| SPF | 50.11 ± 0.53 | 44.22 ± 0.35 | 42.07 ± 0,17 | 24.79 ± 0.07 | 40.76 ± 0.11 | 39.51 ± 0.09 | 38.00 ± 0.05 | 26.07 ± 0.22 |
| V ** | 1.00 | 0.79 | 0.22 | 0.28 | 0.27 | 0.23 | 0.13 | 0.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahnit, W.; Smara, O.; Bechki, L.; Bensouici, C.; Messaoudi, M.; Benchikha, N.; Larkem, I.; Awuchi, C.G.; Sawicka, B.; Simal-Gandara, J. Phytochemical Profiling, Mineral Elements, and Biological Activities of Artemisia campestris L. Grown in Algeria. Horticulturae 2022, 8, 914. https://doi.org/10.3390/horticulturae8100914
Zahnit W, Smara O, Bechki L, Bensouici C, Messaoudi M, Benchikha N, Larkem I, Awuchi CG, Sawicka B, Simal-Gandara J. Phytochemical Profiling, Mineral Elements, and Biological Activities of Artemisia campestris L. Grown in Algeria. Horticulturae. 2022; 8(10):914. https://doi.org/10.3390/horticulturae8100914
Chicago/Turabian StyleZahnit, Wafa, Ouanissa Smara, Lazhar Bechki, Chawki Bensouici, Mohammed Messaoudi, Naima Benchikha, Imane Larkem, Chinaza Godswill Awuchi, Barbara Sawicka, and Jesus Simal-Gandara. 2022. "Phytochemical Profiling, Mineral Elements, and Biological Activities of Artemisia campestris L. Grown in Algeria" Horticulturae 8, no. 10: 914. https://doi.org/10.3390/horticulturae8100914
APA StyleZahnit, W., Smara, O., Bechki, L., Bensouici, C., Messaoudi, M., Benchikha, N., Larkem, I., Awuchi, C. G., Sawicka, B., & Simal-Gandara, J. (2022). Phytochemical Profiling, Mineral Elements, and Biological Activities of Artemisia campestris L. Grown in Algeria. Horticulturae, 8(10), 914. https://doi.org/10.3390/horticulturae8100914












