Abstract
Controlling soil-borne pathogens is a significant problem in agriculture. Arbuscular mycorrhizae have a potential role in controlling soil-borne pathogens by increasing plant phytohormone contents. However, the mechanism of resistance by mycorrhizae has not been fully elucidated, particularly against bacterial wilt disease in Solanaceae. This study examined the role of mycorrhizae in expressing genes involved in the signaling pathways mediated by jasmonic acid (JA) and salicylic acid (SA) in tropical chili pepper against the bacterium Ralstonia solanacearum. Seedlings from ten genotypes of chili pepper were inoculated with a consortium of five mycorrhizal species and/or inoculated with a mixture of nine isolates of R. solanacearum. The leaves of 10-week-old plants after the treatment were sampled for real-time polymerase chain reaction analysis. The results showed that the mycorrhizae strengthened the immune system of tropical chili pepper by increasing the relative gene expression levels of JA and SA in genotypes with high and low responsiveness to the mycorrhizae. The relative gene expression level of JA was related to the percentage colonization of mycorrhizae and the resistance of the tropical chili pepper genotypes to R. solanacearum. The relative gene expression level of SA was associated with the resistance of tropical chili pepper to R. solanacearum.
1. Introduction
Pepper and sweet pepper (Capsicum spp.) have many uses as spices, natural food coloring agents, and raw materials for making medicines and pesticide mixtures [1,2,3,4]. One of the obstacles to chili cultivation during the rainy season is the systemic vascular wilt disease caused by Ralstonia solanacearum. R. solanacearum is a most devastating soil-borne pathogen, particularly in the Solanaceae family [5,6,7,8,9,10,11,12]. The bacterial wilt pathogen R. solanacearum remains difficult to control because of its persistence. This pathogen can survive on plant debris or in the absence of a host. It has a broad host range, high genetic diversity, complex sub-species, a broad geographic distribution, and lives and multiplies in the xylem, thereby blocking the xylem tissue [5,7,13,14,15,16,17,18].
Current management of pathogens is based on chemical pesticides. However, using chemical pesticides produces residues in crops that are harmful to health, impact the environment, and result in an unsustainable agricultural system [5,19,20]. In recent years, the specific microbiome has been recognized as a potential tool for achieving environmentally friendly agriculture. The importance of the microbiome as a modulator of crop resistance has emerged [5,19,21,22,23,24]. The presence of certain microorganisms induces the reprogramming of plant metabolic pathways involved in the defense system and increases the ability of plants to survive under adverse conditions [5,19,23,24,25].
Endophytic fungi, such as arbuscular mycorrhizae, have potential roles in controlling plant diseases, particularly soil-borne pathogens [26,27,28]. Local and systemic mycorrhizal-induced resistance (MIR) increases phytohormone contents, such as salicylic acid (SA) and jasmonic acid (JA) [23,24,29,30,31]. The response of plants to biotrophic pathogens is generally controlled by activating a salicylic acid (SA) dependent response [32]. Beneficial microorganisms modulate the SA pathway [19]. Activating the SA pathway may also influence the favorable performance of the symbiont.
JA is another essential hormone in plant defense that regulates various processes related to plant development, symbiosis, and plant responses to insects and pathogens [32,33,34]. JA stimulates induced systemic resistance (ISR) activated by the interactions between plants and certain beneficial microorganisms. Antagonism of the JA and SA biosynthetic pathways occurs [35]; however, some researchers have reported synergistic interactions between them [36,37]. Modulation of the JA signaling pathway may be involved in the symbiosis by keeping the endophytic fungi in the asymptomatic stage [31,38].
Managing beneficial microbes for plants has been proposed as a new platform to revolutionize plant protection against pathogens. However, the mechanisms underlying the symbiotic relationship between plants and beneficial microorganisms are still being investigated. Several studies have shown that biocontrol agents efficiently reduce the incidence of bacterial wilt disease in greenhouses, but field trials have not yielded results [5].
The present study examined the symbiotic relationship between tropical chili pepper genotypes and mycorrhizae. Genotypes of peppers with different responsiveness to mycorrhizae and different resistance to bacterial wilt disease were studied. This study aimed to investigate the potential of mycorrhizae to increase the resistance of the tropical chili pepper genotypes against R. solanacearum through hormone signaling pathways. The potential of mycorrhizae was studied in relation to the relative expression of the genes associated with JA and SA signaling pathways. The effect of mycorrhizae on phenotypic traits of the tropical chili pepper was also investigated.
2. Materials and Methods
2.1. Chili Pepper Genotypes, Mycorrhizae, and the Ralstonia solanacearum Isolates
This study was carried out at the Agrotechnology Innovation Center of the Universitas Gadjah Mada at Kalitirto Yogyakarta, Indonesia (7°79′58.59″ N, 110°46′52.85″ E). The experiment examined the relative gene expression of JA and SA at the generative growth phase when the plant started flowering, of ten tropical chili pepper genotypes with different responses to mycorrhizae and resistance to R. solanacearum.
Ten chili pepper genotypes were used in this study. The genotypes consisted of eight accessions from the Genebank of the Universitas Gadjah Mada and East-West Seed Indonesia (Ewindo) and two commercial cultivars. Based on the published descriptions of the cultivars, the two commercial cultivars were used as the resistant check cultivar (C-50, Known You Seed) and the susceptible check cultivar (C-54, Oriental Seed) against R. solanacearum. The mycorrhizal inoculum was a mixture of Glomus sp., Funneliformis sp., Acaulospora sp., Gigaspora sp., and Scutellospora sp. with a zeolite carrier obtained from the Agricultural Microbiology Laboratory, Faculty of Agriculture, Universitas Gadjah Mada. Mycorrhizal content was 500 inoculums per gram of zeolite carrier. The R. solanacearum isolate used was a mixture of nine bacterial isolates.
2.2. Procedure of Experiments
The study consisted of three experiments in parallel using separate sets of experimental plants.
Experiment 1a.
Evaluation of genotypes responsiveness to mycorrhizae
The seeds of the ten chili pepper genotypes were germinated in a germination box for 5 days. Twenty seedlings of each genotype were transplanted to nursery pot trays filled with sterilized growing media. The medium was a mixture of soil, coco peat, and husk charcoal at a ratio of 2:1:1 (v:v:v). Before transplanting the seedlings, the medium was mixed with the mycorrhizal inoculum at a ratio of 10:1 (w:w). The seedlings were maintained in the greenhouse until they were 21 days old.
The 21-day-old seedlings were transplanted into a 10-L planter bag filled with 7 kg of planting media per planter bag. The planting media was a mixture of soil, compost, coco peat, and husk charcoal at a ratio of 2:1:1:1 (v:v:v:v). The plants were maintained in the screen house until 10 weeks old.
The growth response to mycorrhizal symbiosis was defined as an increase in plant biomass inoculated with mycorrhiza compared to the biomass of plants that were not inoculated [39]. The plants were sampled 10 weeks after transplant to examine the dry biomass. The plant biomass was represented by shoot and root dry weight. Dry shoots and roots were weighed after the biomass was sun-dried in a greenhouse for seven days and oven-baked at 68 °C for 48 h. The percentage of mycorrhizal colonization was determined by staining the roots using trypan blue 0.05% (w:v) as described in the previous study [40] with modifications. The percentage of mycorrhizal colonization was calculated from the proportion of the mycorrhizal root area to the total root area. The area was measured using the Fiji program [41].
Experiment 1b.
Evaluation of genotypes resistance to R. solanacearum
The seeding procedure was conducted similarly to Experiment 1a. Shortly after the seedlings were transplanted into the nursery pot trays, the R. solanacearum was inoculated using the drenching method. A mixture of nine strains of the R. solanacearum bacterial suspension at a concentration of 1 × 108 CFU mL−1 (OD600 = 0.1) was poured (20 mL of each) into the planting medium around the root area.
The resistance of the ten tropical chili pepper genotypes to R. solanacearum was evaluated based on the wilt symptoms of seedlings The wilt symptoms in the seedlings were observed in the morning on days 3, 7, 14, 21, 28, and 35 after the bacterial inoculation based on the scoring method as described in the previous study [7,17] with modifications. Score 0: all leaves or plants remained healthy with no symptoms; score 1: ≤20% withered leaves or plants; score 2: 21–40% withered leaves or plants; score 3: 41–60% withered leaves or plants; score 4: 61–80% of withered leaves or plants; and score 5: >80% withered leaves or plants/dead plant. Disease intensity (DI) and disease incidence (DInc.), and the area under the disease progress curve (AUDPC) were calculated using the formula as referred to from the previous study [17,42,43], respectively. The DI was used to determine the resistance of each genotype, including the check cultivar. Genotypes were categorized as resistant if the DI was <30%, moderately resistant if the DI was 31–40%, moderately susceptible if the DI was 41–50%, and susceptible if the DI was >50% [17].
Experiment 2.
Evaluation of relative gene expression JA and SA
The second experiment combined the responsiveness of the genotypes to mycorrhizae with their resistance to R. solanacearum to evaluate the relative gene expression of JA and SA. The treatments applied in this study consisted of seedlings that were not inoculated with mycorrhizae and not inoculated with R. solanacearum (M0R0), seedlings that were inoculated with mycorrhiza but not inoculated with R. solanacearum (M1R0), seedlings that were not inoculated with mycorrhiza but inoculated with R. solanacearum (M0R1), and seedlings that were inoculated with mycorrhiza and inoculated with R. solanacearum (M1R1).
The seeds of the ten chili pepper genotypes were germinated in a germination box for 5 days. Twenty seedlings of each genotype were transplanted to nursery pot trays filled with sterilized growing media. The medium was a mixture of soil, coco peat, and husk charcoal at a ratio of 2:1:1 (v:v:v). Before transplanting the seedlings, the medium was mixed with the mycorrhizal inoculum at a ratio of 10:1 (w:w). The seedlings were maintained in the greenhouse until they were 21 days old.
The 21-day-old seedlings were transplanted into a 10-L planter bag filled with 7 kg of planting media per planter bag. The planting media was a mixture of soil, compost, coco peat, and husk charcoal at a ratio of 2:1:1:1 (v:v:v:v). The bacterium was inoculated on 21-day-old seedlings using the soil drenching method. A mixture of nine strains of the R. solanacearum suspension at a concentration of 1 × 108 CFU mL−1 (OD600 = 0.1) was poured into the seedling media around the root area. Then, the seedlings were watered in the morning and afternoon, as required. A total of 20 planter bags were prepared for each genotype and repeated three times for each treatment. The bags were arranged in a completely randomized block design. The seedlings inoculated with R. solanacearum were placed in separate screen houses from those that were not inoculated. The plants were raised until they entered the generative growth phase, and leaf samples were taken (approximately 10 weeks after transplanting).
2.3. Isolation of Leaf RNA
The expression of JA and SA pathway genes was detected by quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR) (BIO-RAD CFX96tm Real-Time System; Bio-Rad Laboratories, Hercules, CA, USA) using RNA samples extracted from leaf tissue. RNA was isolated from the youngest leaf tissue for qRT-PCR using the Geneaid RNA mini kit (Plant) [44]. A sample of 100 mg of leaf tissue was crushed in liquid nitrogen. The crushed tissue was placed in a 1.5 mL microtube and 500 µL of PRB Buffer and 5 µL of β-mercaptoethanol was added and mixed until homogeneous. The mixture was incubated at 60 °C for 5 min, transferred to a filter column with a 2 mL collection tube, and centrifuged at 3000 rpm (1000× g) for 1 min. Then, the filter column was discarded. The supernatant was taken from the collection tube and transferred to a 1.5 mL microtube. Absolute ethanol was added to the supernatant at half the volume of the total supernatant taken and homogenized with a vortex mixer. The supernatant was transferred to an RB column with a 2 mL collection tube and centrifuged at 12,000 rpm (16,000× g) for 1 min. The 2 mL of solution in the collection tube was discarded, the supernatant was returned to the RB column, and 400 µL of W1 buffer was added and centrifuged at 12,000 rpm (16,000× g) for 30 s. The solution in the collection tube was discarded and the supernatant was returned to the RB column. Then, 600 µL of wash buffer was added to the RB column, the eluate was centrifuged at 12,000 rpm (16,000× g) for 1 min, and the solution in the collection tube was discarded. The washing step with the wash buffer was repeated. The column tube was dried by centrifugation at 12,000 rpm (16,000× g) for 3 min. Then, the RB column was transferred to a 1.5 mL microtube, and 50 µL of RNAse-free water was added to the center of the RB column and allowed to stand for 2 min. In the last stage, the eluate was centrifuged at 12,000 rpm (16,000× g) for 1 min, and the RNA was eluted into a 1.5 mL microtube.
2.4. qRT-PCR for GAPDH-cp and Genes of Interest
The qRT-PCR analysis was performed using the Bio-Rad CFX-96 real-time PCR system. The target genes were oxophytodienoic acid reductase-3 (OPR3) (jasmonic acid), and ICL (salicylic acid), and the reference gene was GAPDH-cp (Table 1). The total reaction volume was 20 µL, including 10 µL of 2 SensiFAST SYBR No-ROX One-Step Mix, 0.2 µL of reverse transcriptase, 0.4 µL of riboSafe RNAse inhibitor, 1.6 µL of the primers (0.8 µL of the10 µM forward primer and 0.8 µL of the 10 µM reverse primer), 4 µL of the RNA template, and 3.8 µL of RNAse-free water. The qRT-PCR was implemented in three stages. Stage I was reverse transcription at 45 °C for 10 min, stage II was polymerase activation at 95 °C for 2 min, and stage III was 35 cycles of denaturation at 95 °C for 1 min, annealing for 30 s at the optimum temperature for each primer, elongation at 72 °C for 1 min, and a melting curve analysis at 65–95 °C for 5 s. The analysis was repeated in triplicate for each gene. The relative quantification of the genes of interest was assessed using the 2−ΔΔCT method [45].

Table 1.
Primer and reference genes used in the real-time RT-PCR analysis.
2.5. Phenotypic Response of the Genotypes to Mycorrhizae and R. solanacearum
The phenotypic response was analyzed from the data of plant height and stem diameter of ten tropical chili pepper genotypes from all the treatments, i.e., M0R0, M1R0, M0R1 and M1R1. Plant height and stem diameter were measured at 10 weeks after transplanting. Plant height was measured from the ground surface to the shoot tip. The stem diameter was measured at approximately 5 cm above the ground. The data was collected from 15 plants for each genotype.
2.6. Data Analysis
The statistical analysis was performed using R Studio software version 3.6.2 [48]. The means of all observed traits were calculated using the least square means and analysis of variance. The Scott-Knott post hoc analysis was used to detect differences between the mean values of the observed characteristics. A p-value < 0.05 was considered significant. Structural equation modeling-partial least square (SEM-PLS) was used to construct the path coefficient and assess the cumulative effect of genotype responsiveness to mycorrhizae and the genotype resistance to R. solanacearum on the relative expression of the JA and SA genes [49,50]. A standardized stepwise regression was used to investigate the parameters of each genotype responsiveness variable to the mycorrhizae and the resistance of the chili genotypes to R. solanacearum, which affected the relative expression of the JA and SA genes [51]. SEM-PLS analysis and standardized stepwise regression were performed using PROC GLM and PROC REG in SAS 9.4 [52]. A heatmap was created using the Euclidean distance and the average heatmap method in R [53]. The heatmap was used to classify the genotypes based on their responsiveness to the mycorrhizae, resistance to R. solanacearum, and the relative gene expression of JA and SA. The heatmap was also used to determine the responsiveness to the mycorrhizae and/or the resistance to R. solanacearum related to the relative gene expression of JA and SA.
3. Results
3.1. Evaluation of the Growth Response to Arbuscular Mycorrhizae and Resistance to R. solanacearum
The growth response by the ten genotypes to the mycorrhizae was divided into three groups. Genotype C-38 had the highest growth response to the mycorrhizae, reaching 388.00% ± 37.40%, while genotype C-37 had the lowest growth response of 27.00% ± 2.60%. The growth response to mycorrhizae of four genotypes (C-08, C-30, C-09, C-38) was high; the responses of the other four genotypes (C-34, C-49, C-02, C-37) and the two commercial cultivars (C-50, C-54) were low (Figure 1).
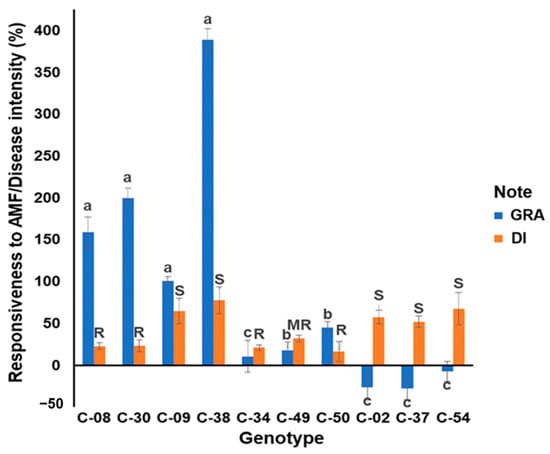
Figure 1.
The growth responsiveness of the tropical chili pepper genotypes to mycorrhizae (GRA) and the disease intensity of the tropical chili pepper genotypes to R. solanacearum (DI) at 35 days after inoculation (DAI). Note: The same lowercase letters in the GRA histogram indicate no significant differences between the genotypes at a significance level of 0.95 according to the Scott-Knott test. The uppercase letters in the DI histogram indicate the resistance of the genotypes based on the disease intensity at 35 DAI. Bars represent the mean ± standard deviation. AMF: arbuscular mycorrhizal fungi. GRA: the growth response of genotype to mycorrhiza; DI: disease intensity at 35 DAI. R: resistant; MR: moderately resistant; and S: susceptible; C-50: resistant check cultivar and C-54: susceptible check cultivar to R. solanacearum according to their published cultivar’s description.
All tropical chili pepper genotypes were infected with R. solanacearum 35 days after inoculation (DAI) but with different DI values. Among the eight accessions tested, three were classified as resistant, one was moderately resistant, and four were susceptible genotypes (Figure 1). The susceptible check had a DI value of 67.19% ± 0.19%. The four accessions susceptible to R. solanacearum had DIs of 51.97–77.61%. The susceptible check in this evaluation resulted in DI, which was the same as the cultivar description. The resistant check cultivar had a DI of 16.22% ± 0.07% at 35 DAI, which was the same as the cultivar description. Three accessions classified as resistant had higher DIs than the resistant check, ranging from 44.52 to 59.21% and 20.96 to 23.22%, respectively. One accession classified as moderately resistant had a DI of 31.91% ± 0.04% (Figure 1).
3.2. Effect of Arbuscular Mycorrhizae on the Relative Gene Expression of JA
The relative gene expression of JA in genotypes with high responsiveness to mycorrhizae and resistance to R. solanacearum (C-08 and C-30) was highest in the M1R1 treatment. In genotypes with high responsiveness to mycorrhizae and susceptibility to R. solanacearum (C-09 and C-38), the relative gene expression of JA was highest in M0R1. In genotypes with low responsiveness to mycorrhizae and susceptibility to R. solanacearum (C-02 and C-37), the relative gene expression of JA was highest in the M0R1 treatment. In genotypes with low responsiveness to mycorrhizae and resistance or moderate resistance to R. solanacearum (C-34 and C-49), the relative gene expression of JA was highest in M1R1 (Figure 2).
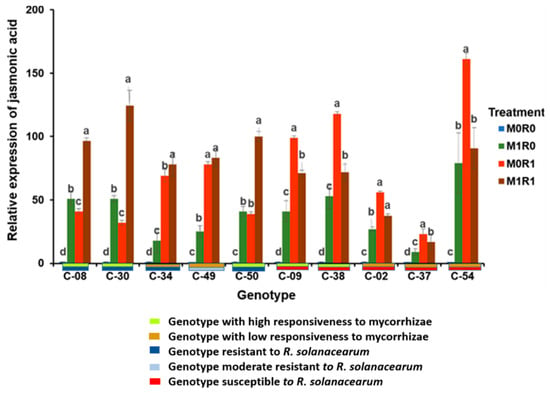
Figure 2.
Relative gene expression of jasmonic acid (JA) in the tropical chili pepper genotypes. Note: Plants without mycorrhizae (M0); plants inoculated with mycorrhizae (M1); plants without R. solanacearum (R0); plants inoculated with R. solanacearum (R1). Bars represent the mean ± standard deviation. The same lowercase letters indicate no significant difference in the mean values between treatments in each genotype, according to the Scott-Knott test, at a significance level of 0.95.
Inoculating mycorrhizae but not R. solanacearum (M1R0) showed that mycorrhizae caused the relative gene expression of JA to increase in the chili pepper genotypes. The relative gene expression of JA was higher in genotypes with high responsiveness to mycorrhizae than in those with low responsiveness. The relative gene expression of JA in the genotypes with high responsiveness to mycorrhizae ranged from 40.65 to 53.31 times, while the genotypes with low responsiveness only ranged from 9.33 to 27.20 times.
Inoculating with R. solanacearum but not the mycorrhizae (M0R1) showed that the relative gene expression of JA from the genotypes resistant to R. solanacearum was lower than that of the susceptible genotypes. However, the opposite result occurred in the genotypes resistant to R. solanacearum. The relative gene expression of JA from genotypes resistant to R. solanacearum ranged from 31.64 to 78.14. The relative gene expression of JA in the genotypes susceptible to R. solanacearum went from 38.38 to 118.04 times.
The genotypes inoculated with R. solanacearum and mycorrhizae (M1R1) triggered the relative gene expression of JA. The relative magnitude of JA gene expression depended on the resistance of the genotype to R. solanacearum and the responsiveness to the mycorrhizae. The relative gene expression of JA in the genotypes resistant to R. solanacearum increased in the range of 77.88–124.44 times Genotypes susceptible to R. solanacearum in the presence of mycorrhizal symbiosis increased their relative gene expression of JA in the range of 16.88–71.71 times.
3.3. Effect of Arbuscular Mycorrhizae on the Relative Gene Expression of SA
The relative gene expression of SA in the genotypes with high responsiveness to mycorrhizae and resistance to R. solanacearum (M0R1) (C-08 and C-30) was the highest. The relative SA gene expression in the M1R1 and M1R0 treatments was higher than in the M0R1 in the resistant check cultivar (C-50). In the genotypes with high responsiveness to mycorrhizae and susceptibility to R. solanacearum (C-09 and C-38), the relative gene expression of SA in the M1R1 treatment was highest. The relative gene expression of SA in the resistant check cultivar was highest in the M1R1 treatment. In the genotypes with low responsiveness to mycorrhizae and susceptibility to R. solanacearum (C-02 and C-37), the relative gene expression of SA was highest in the M1R1 treatment. In the genotypes with low responsiveness to mycorrhizae and resistance or moderate resistance to R. solanacearum (C-34 and C-49), the relative gene expression of SA was highest in the M1R1 treatment, followed by M1R0 and M0R1 (Figure 3).
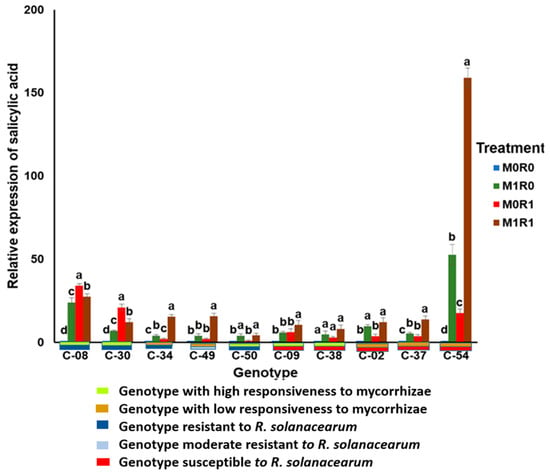
Figure 3.
Relative gene expression of salicylic acid (SA) in the tropical chili pepper genotypes. Note: Plants without mycorrhizae (M0); plants inoculated with mycorrhizae (M1); plants not inoculated with R. solanacearum (R0); plants inoculated with R. solanacearum (R1). Bars represent mean ± standard deviation. The same lowercase letters indicate no significant difference in mean values between treatments in each genotype according to the Scott-Knott test at a significance level of 0.95.
Genotypes inoculated with mycorrhizae but not with R. solanacearum (M1R0) showed the relative gene expression of SA depended on the responsiveness of the genotype to the arbuscular mycorrhizal. Genotypes with high responsiveness to the mycorrhizae revealed higher relative gene expression of SA than genotypes with low responsiveness. The range of relative gene expression of SA in the responsive genotypes was 4.81–23.7 times, which was higher than those in genotypes with low responsiveness at 4.15–9.63 times.
The resistant genotypes tended to express more SA than the susceptible genotypes. This observation can be seen in the genotypes treated with bacterial inoculation without mycorrhizae (M0R1). The relative gene expression range of SA in the resistant genotypes was 2.93–34.21 times, which was higher than that in the susceptible genotypes at 2.02–17.68 times.
The combined inoculation treatment with R. solanacearum and mycorrhizae (M1R1) showed that the arbuscular mycorrhizal symbiosis increased the relative gene expression of SA. The increase in relative SA expression depended on the resistance of the genotype to R. solanacearum. In the presence of mycorrhizae, the relative gene expression of SA in the resistant genotypes ranged from 12.16 to 74.45 times. It tended to be higher than that in the susceptible genotypes which were only 8.13–13.81 times.
3.4. Structural Equation Modeling-PLS and Standardized Stepwise Regression
The results of the SEM-PLS analysis showed that the resistance to R. solanacearum had a significant path coefficient on the relative gene expression of JA (p < 0.048*) (Figure 4). However, the relative gene expression of SA was not directly affected by either the responsiveness to mycorrhizae or the resistance to R. solanacearum (Figure 5).
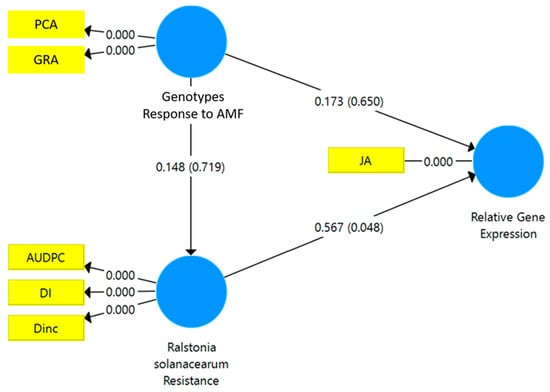
Figure 4.
Structural equation modeling-partial least square (SEM-PLS) of the relationship between the responsiveness to mycorrhizae and the resistance of the tropical chili pepper genotypes to the R. solanacearum variables and the relative gene expression of JA. AMF: arbuscular mycorrhizal fungi. PCA: percentage colonization of mycorrhiza, GRA: the growth response of the tropical chili pepper genotype to mycorrhizae, DI: disease intensity, DInc.: disease incidence, AUDPC: area under disease progress curve, JA: jasmonic acid.
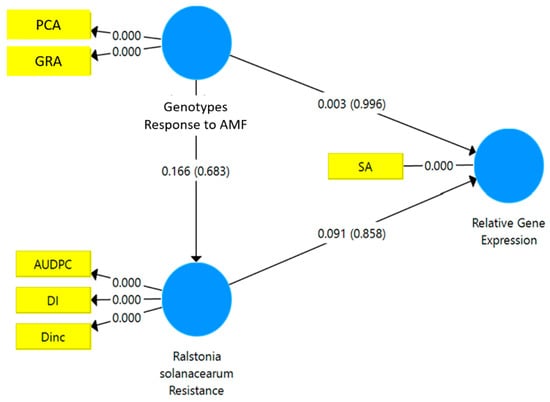
Figure 5.
Structural equation modeling-partial least square (SEM-PLS) of the relationship between the responsiveness to mycorrhizae and the resistance of the tropical chili pepper genotypes to R. solanacearum variables and the relative gene expression of SA. AMF: arbuscular mycorrhizal fungi. PCA: percentage colonization of mycorrhizae, GRA: the growth response of the tropical chili pepper genotypes to mycorrhizae, DI: disease intensity, DInc.: disease incidence, AUDPC: area under disease progress curve, SA: salicylic acid.
The standardized stepwise regression showed that the relative gene expression of JA was significantly affected by the percentage colonization of mycorrhizae (PCA) (0.935***) (R2 = 0.874***) and the DInc (0.9480***) (R2 = 0.928***). Similarly, the relative expression of the SA gene was significantly affected only by DInc (0.648*) (R2 = 0.648*). Thus, the PCA is a responsiveness variable, and DInc is a variable of resistance. Therefore, the relative gene expression of JA was strongly affected by the responsiveness of the tropical chili pepper genotypes to mycorrhizae and the resistance to R. solanacearum. The relative gene expression of SA was related to the resistance of the tropical chili pepper genotypes to R. solanacearum.
3.5. Clustering of the Genotypes Based on the Responsiveness to Mycorrhizae, Resistance to R. solanacearum, and the Relative Gene Expression of JA and SA
The genotypes were grouped into four clusters based on the heatmap (Figure 6). The heatmap shows high diversity in several traits, i.e., PCA, genotype responsiveness to mycorrhizae (GRA), DI, DInc, the AUDPC, and the relative gene expression of JA and SA. The relative variation in JA and SA gene expression in the tropical chili pepper genotypes was similar (Figure 2 and Figure 3). The heatmap shows that the relative gene expression of SA was related to variable resistance of the genotypes to R. solanacearum. In contrast, the relative gene expression of JA was related to variable resistance of the genotypes to R. solanacearum and the GRA. The results of this heatmap agree with the standardized stepwise regression results regarding the relative determinants of JA and SA gene expression.
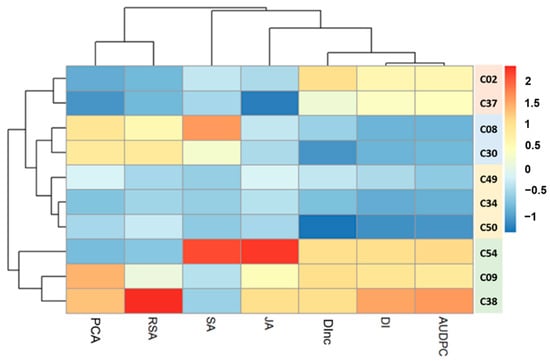
Figure 6.
Heatmap describing the diversity of responsiveness to the mycorrhizae, the resistance to R. solanacearum, and the relative gene expression of JA and SA. Blue and red indicate an increase and decrease in the value of each trait, respectively. The black lines on the top and at the left of the heatmap indicate dendogram of variables and genotypes, respectively. Color-bar on the right side of the dendogram indicates the genotypes in the same cluster. PCA: percentage colonization of mycorrhiza, GRA: genotype responsiveness to mycorrhiza, SA: salicylic acid, JA: jasmonic acid, DInc: disease incidence, DI: disease intensity, AUDPC: area under the disease progress curve.
3.6. Phenotypic Response Based on the Responsiveness to Mycorrhizae and Resistance to R. solanacearum
Figure 7 shows the response of ten genotypes of tropical chili pepper based on responsiveness to mycorrhizae, resistance to R. solanacearum, and relative gene expression of JA and SA on plant height. In general, the genotypes inoculated with R. solanacearum and arbuscular mycorrhizae (M1R1) were able to suppress the growth of R. solanacearum, the plants were taller than plants infected R. solanacearum but not inoculation mycorrhizae (M0R1). The same phenomenon occurs in the variable stem diameters of ten genotypes with various mycorrhizal responsiveness and R. solanacearum resistance, mycorrhizae were able to suppress the growth of R. solanacearum (Figure 8). The relative gene expression of JA and SA induced by mycorrhizae in the presence of R. solanacearum (M0R1) could suppress the growth of R. solanacearum. This was indicated by the higher plants and larger stem diameters of the tropical chili pepper genotypes than the plants infected R. solanacearum but not inoculation mycorrhizae (M0R1) (Figure 7 and Figure 8).
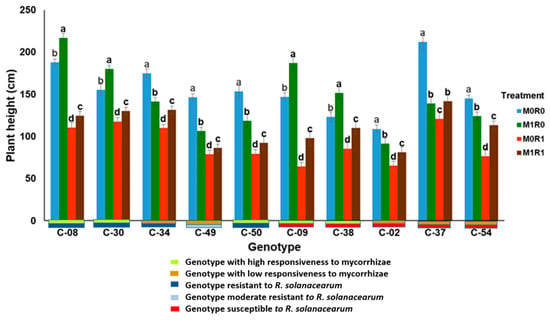
Figure 7.
Plant height of ten genotypes tropical chili pepper as a phenotypic response to the treatments. Bars represent mean ± standard deviation. The same lowercase letters indicate no significant difference in mean values between treatments in each genotype according to the Scott-Knott test at a significance level of 0.95.
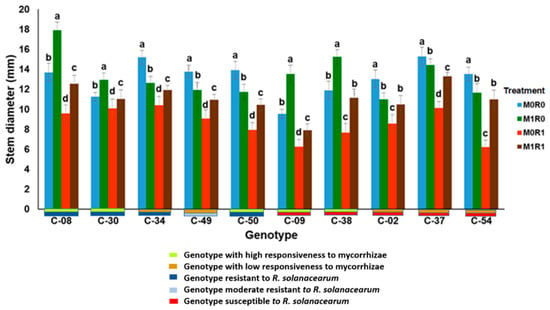
Figure 8.
Stem diameter of ten genotypes tropical chili pepper as a phenotypic response to the treatments. Bars represent mean ± standard deviation. The same lowercase letters indicate no significant difference in mean values between treatments in each genotype according to the Scott-Knott test at a significance level of 0.95.
4. Discussion
4.1. Responsiveness to Arbuscular Mycorrhizae and the Resistance to R. solanacearum
Figure 1 shows the diverse growth responses of the tropical chili pepper genotypes to the mycorrhizae. The genotypes differed in their response to arbuscular mycorrhizal colonization. The effectiveness of the symbiosis with mycorrhizae is highly dependent on the species and the genotype of the host plant [54,55]. The genotype is an essential factor determining the response to mycorrhizae [31,56,57]. Differences in the response to mycorrhizae emphasize a genetic basis for the interactions between plants and mycorrhizae [31]. Many studies have reported significant differences in the responsiveness to mycorrhizae between genotypes within the same host plant species [27,54,57]. The symbiosis with arbuscular mycorrhizae changed the growth of the tropical chili peppers. Janos [58] stated that the responses of plant genotypes to mycorrhizal colonization can be classified into positive, neutral, and negative. The positive reactions of four of the tropical chili pepper genotypes indicated that mycorrhizal colonies and the host plant got benefit from the symbiosis. A small positive symbiotic response indicated that the tropical chili pepper as the host plant did not respond to the presence of mycorrhizae, and the mycorrhizae did not interfere with the host plant. This observation indicates that there was no effect on the host plant in the presence of arbuscular mycorrhizal symbioses.
In contrast, a negative response was found in two of the tropical chili pepper genotypes, as the mycorrhizal symbioses inhibited plant growth or induced symbiotic parasitism. The reaction to the mycorrhizae is neutral or negative in cases where mycorrhizal colonization does not increase plant growth [27]; the biomass produced by plants does not increase even though the plants are well colonized with mycorrhizae [57].
Screening for resistance to R. solanacearum in chilies has been carried out but has focused on red chilies and sweet pepper (Capsicum annuum). Several sources of resistance to bacterial wilt disease have been identified in Capsicum pepper [12,42,59,60,61,62,63,64]. Three chili pepper genotypes from the germplasm were resistant, one was moderately resistant, and four were susceptible (Figure 1). These results demonstrate that resistant genotypes can be obtained from Indonesia, as reported by several researchers [62,64,65]. This also explains why Southeast Asia is an essential source of chili germplasm resistant to R. solanacearum bacterial wilt disease. The germplasms were more adapted to the various environment. They collected represented centers of chili pepper production areas in the three provinces in Jawa Island. Resistant genotypes are used as a source of resistance genes in breeding programs. As R. solanacearum easily adapts to the environment, including infecting resistant host plants, the screening results in this study could be used to breed new resistant cultivars.
4.2. Interaction between the Genotypes of Tropical Chili Pepper, the Arbuscular Mycorrhizae, and R. solanacearum on the Relative Gene Expression of JA and SA, and Phenotypic Response
Arbuscular mycorrhizal fungi change the community and the biotic interactions in the rhizosphere. This mycorhizospheric effect alters the communities of beneficial microorganisms and suppresses pathogens in the soil [66,67,68]. Associations with mycorrhizae directly benefit plants by producing secondary metabolites or modulating plant defenses. A recent study showed that different endophytes induce plant resistance to biotic or abiotic stressors [32,69]. However, the plant microbiome is strongly influenced by its host. Plants must recognize endophytes during colonization, as signaling molecules, and identifying microbes as beneficial partners will create a symbiotic mutualism.
All of these factors affect different plant species in different microbiomes [19]. This study investigated the function of the symbiosis of chili pepper and mycorrhizae and the resistance to R. solanacearum. Ten tropical chili pepper genotypes with different responsiveness to mycorrhizae and resistance to R. solanacearum were tested. The function of the mycorrhizae was evaluated and the relative gene expression of JA and SA in each tropical chili pepper genotype was determined.
JA is an essential hormone involved in the plant resistance system. JA regulates various processes related to plant development, symbiotic interactions, and plant responses to pathogens, particularly soil-borne pathogens. JA triggers ISR, which is activated by the relationship between the plant and certain beneficial microorganisms. ISR is marked by the accumulation of JA [19,32,33]. The relative gene expression of JA in the ten tropical chili pepper genotypes is shown in Figure 2 and Figure 6. In all ten tropical chili pepper genotypes, treatment with mycorrhizae and R. solanacearum infection (M1R1) increased the relative gene expression of JA. The increase in the relative gene expression of JA varied (Figure 2). For the resistant and moderately resistant genotypes (with blue bar under the X-axis), the relative gene expression of JA was higher in the M1R1 treatments. In this genotype group, the difference between M1R1 and M0R1 was larger in the genotypes which are responsive to mycorrhizae (with green and blue bar under the X-axis). It means that the effect of mycorrhizae was higher in the genotypes with high responsiveness to mycorrhizae. In contrast, the relative gene expression of JA in the five susceptible genotypes was higher in the M0R1 treatment (orange histograms with red bar under the X-axis). This finding indicates that JA is always expressed in the infected plants, but with different expressions between resistant and susceptible plants. For resistant plants, the relative expression of JA was depended on responsiveness to mycorrhiza. Genotypes C-08, C-30, and C-50 were resistant to R. solanacearum and had a high responsiveness to mycorrhizae. It showed a high expression of JA even in the treatment without infection. Meanwhile, for the susceptible plants, the expression of JA depended mainly on the presence R. solanacearum infection.
R. solanacearum infection increased the expression of the OPR3 gene, and the relative gene expression of JA was higher (Figure 2). The SEM-PLS in Figure 4 shows a relationship between JA gene expression and the resistance of the genotypes to R. solanacearum. The heatmap revealed similar results (Figure 6). As the accumulation JA was altered by inoculating the endophytic fungi, it can be argued that the expression of several marker genes for different steps in the JA biosynthetic pathway and other genes is associated with plant resistance [19].
The M1R0 treatment increased the relative gene expression of JA in all ten tropical chili pepper genotypes. However, differences were observed in the relative gene expression of JA. The relative gene expression of JA was higher in genotypes with a high mycorrhizal response than in those inoculated with R. solanacearum only (M0R1). A similar result was found for the resistant check cultivar. However, this result differed from genotypes with high responsiveness to the mycorrhizae but susceptibility to R. solanacearum in which the relative gene expression of JA was the lowest. This result is the same as in genotypes classified as poor responders to arbuscular mycorrhizae. A similar result was found in the susceptible check cultivar. The JA signaling involved in symbiosis must be modulated to maintain arbuscular mycorrhizal fungal endophytes in the asymptomatic stage [19,38].
The relative gene expression of JA in the ten genotypes inoculated with arbuscular mycorrhizae and R. solanacearum (M1R1) increased, even in the genotypes with a low or negative response to the mycorrhizae. The mycorrhizae increased the relative gene expression of JA in all ten tropical chili pepper genotypes that were inoculated with R. solanacearum. Based on the standardized stepwise regression, the relative gene expression of JA was significantly affected by the DInc and the PCA. The heatmap analysis provided the same results (Figure 6).
In general, antagonism occurs between the JA and SA biosynthetic pathways. Thus, if the relative gene expression of SA is high, the relative gene expression of JA is low. These hormonal signaling pathways do not act independently but affect each other through a complex network of regulatory interactions [35]. Several researchers have reported synergistic interactions between the JA and SA biosynthetic pathways [36,37]. Arbuscular mycorrhizal symbioses increased the production of SA. The increase was not dependent on the genotype’s responsiveness to mycorrhizae, but it depended on their resistance to R. solanacearum. The results of the standardized stepwise regression reinforced this result. The relative gene expression of SA was significantly affected by DInc., either the mycorrhizal inoculation only (M1R0) or both arbuscular mycorrhizal and R. solanacearum (M1R1) inoculation.
It is known that the presence of endophytic mycorrhiza can mediate plant resistance by changing endogenous hormones. Symbiosis with endophytic fungi is usually associated with inhibiting the SA pathway and increasing JA production [19,38]. The results showed a significant increase in the relative gene expression of the JA and SA pathways, indicating that mycorrhizal colonization of the tropical chili pepper genotypes increased plant defense. Similarly, the tropical chili pepper genotypes inoculated with mycorrhizae increased their relative gene expression of JA, and the increase was higher than that of SA (Figure 2 and Figure 3). The expression of JA and SA were in the opposite way. When JA was expressed higher, the SA would be low, and vice versa. Mycorrhizal colonization induced gene expression, activated the JA pathway, and increased plant resistance to R. solanacearum. JA levels increased significantly, particularly in the genotypes inoculated with mycorrhizae and infected with R. solanacearum (M1R1). Figure 7 and Figure 8 showed a comparison of plant height and stem diameter of chili pepper plants with and without mycorrhiza inoculation. It may represent an increase in plant resistance to R. solanacearum. The chili pepper plant inoculated with mycorrhiza and R. solanacearum (M1R1) showed a higher plant height and thicker stem diameter compared to the chili pepper plant inoculated with R. solanacearum but without mycorrhiza (M0R1). Mycorrhizal colonization protected the tropical chili pepper from R. solanacearum and enhanced the immune priming system by producing JA and SA in genotypes with high and low responsiveness or hostile responses to mycorrhizae. However, the increase in the relative gene expression of the JA and SA signaling pathways was affected by the resistance properties of the tropical chili pepper genotypes to R. solanacearum.
As biotrophic organisms, mycorrhiza can trigger plant defense responses during the early stages of colonization, similar to biotrophic pathogen infection [67,70]. Plant responses occur in defense of colonization. Modulating plant defense responses leads to tissue preconditioning to activate plant defenses systemically during a pathogen attack by activating JA signaling, which is referred to as priming. Plant responses are faster and more robust in dealing with a pathogen infection when primed so they are more efficient at increasing plant resistance and plant health [66,67,71,72]. This priming phenomenon was found in the mycorrhizae and R. solanacearum (M1R1) inoculated treatment, especially in the genotypes resistant to R. solanacearum. The relative gene expression of JA increased in the five genotypes of tropical chili peppers in the M1R1 treatment, regardless of the responsiveness of the chili pepper to the mycorrhizae (Figure 2).
Resistance to R. solanacearum is closely related to and controlled by JA and SA-mediated signaling [73]. Mycorrhizae potentially control plant diseases, particularly soil-borne pathogens [26,27,28]. The presence of mycorrhizae induces the reprogramming of plant metabolic pathways involved in defense, which may increase the ability of the plants to survive adverse conditions [5,19,23,24,25]. Local and systemic MIR increases the content of plant hormones, such as SA and JA [23,24,29,30,31].
Symbiosis with mycorrhizae increases the root surface area more than 100 times compared to non-symbiotic roots [74]. Colonization with arbuscular mycorrhizae changes the root morphology, and these changes make it difficult for soil-borne pathogens to enter the root system. The symbiosis directly causes an increase in mycorrhizal exudation from root branches, which changes the root microbiome and stimulates root exudates that inhibit pathogens. This also increases the acquisition of mineral nutrients and modulates plant hormonal balance. Arbuscular mycelia protect the roots from the damaging effects of various pathogenic hydrolytic enzymes that disrupt the epidermal cell walls. Arbuscular mycelia block the molecular crosstalk required for the host plant/pathogen interaction with the rhizosphere [23,24,75].
5. Conclusions
Among the eight accessions, three groups were resistant to R. solanacearum. Three genotypes were resistant, one genotype was moderately resistant, and four genotypes were susceptible to R. solanacearum. Based on the responsiveness to the arbuscular mycorrhizae, the ten genotypes were classified as four tropical chili pepper genotypes with high responsiveness and six with low responsiveness to the arbuscular mycorrhizae, three of which exhibited negative responsiveness. Inoculating the tropical chili peppers with arbuscular mycorrhizae induced resistance to R. solanacearum through a priming action by increasing the relative expression of the genes associated with JA and SA. However, the relationship was different between the JA and SA pathways. Mycorrhizae induced the relative expression of the gene associated with JA in the tropical chili peppers, which depended on the responsiveness to mycorrhizae and resistance to R. solanacearum. The relative expression of the gene associated with SA was depended on the resistance of the genotypes to R. solanacearum. These results suggest that mycorrhizal colonization modulates the signaling pathways mediated by JA and SA to activate plant defense genes against bacterial wilt caused by R. solanacearum and increase plant resistance.
Author Contributions
Conceptualization, E.A., T., T.A. (Triwidodo Arwiyanto) and J.W.; methodology, E.A., T., T.A. (Triwidodo Arwiyanto) and J.W.; software, E.A. and T.A. (Taufan Alam); validation, E.A., T., T.A. (Triwidodo Arwiyanto) and J.W.; formal analysis, E.A., T.A. (Taufan Alam) and I.P.A.; investigation, E.A.; resources, E.A.; data curation, E.A.; writing of the original draft, E.A.; writing, reviewing, and editing, E.A., T., T.A. (Triwidodo Arwiyanto) and J.W.; visualization, E.A., T.A. (Taufan Alam) and I.P.A.; supervision, T., T.A. (Triwidodo Arwiyanto) and J.W.; project administration, E.A. and T.A. (Taufan Alam); funding acquisition, E.A. and T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Universitas Gadjah Mada, grant number 3550/UN1.P.III/Dit-Lit/PT.01.05/2022.
Institutional Review Board Statement
Not applicable for studies not involving humans or animals.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Our profound gratitude is addressed to Woro Darmini, who assisted in culturing the bacterial suspension of R. solanacearum, and Yeni Fatmawati and Putri Eka Amdella, who assisted in the preparation and operation of the qRT-PCR equipment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bosland, P.W.; Votava, E.J. Peppers: Vegetable and Spice Capsicums, 2nd ed.; CABI Publishing: Willingford, UK; Volume 2, pp. 1–230. [CrossRef]
- López, P.; Gorzalczany, S.; Acevedo, C.; Alonso, R.; Ferraro, G. Chemical Study and Anti-Inflammatory Activity of Capsicum chacoense and C. baccatum. Rev. Bras. Farmacogn. 2012, 22, 455–458. [Google Scholar] [CrossRef]
- Pereira, J.A.P.; Vieira, I.J.C.; Freitas, M.S.M.; Prins, C.L.; Martins, M.A.; Rodrigues, R. Effects of Arbuscular Mycorrhizal Fungi on Capsicum Spp. J. Agric. Sci. 2016, 154, 828–849. [Google Scholar] [CrossRef]
- Sanati, S.; Razavi, B.M.; Hosseinzadeh, H. A Review of the Effects of Capsicum annuum L. and Its Constituent, Capsaicin, in Metabolic Syndrome. Iran. J. Basic Med. Sci. 2018, 21, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Yang, J.; Tan, Y.; Munir, S.; Liu, Q.; Zhang, J.; Ji, G.; Zhao, Z. Ralstonia solanacearum, a Deadly Pathogen: Revisiting the Bacterial Wilt Biocontrol Practices in Tobacco and Other Solanaceae. Rhizosphere 2022, 21, 100479. [Google Scholar] [CrossRef]
- Aguk, J.A.; Karanja, N.; Schulte-Geldermann, E.; Bruns, C.; Kinyua, Z.; Parker, M. Control of Bacterial Wilt (Ralstonia solanacearum) in Potato (Solanum tuberosum) Using Rhizobacteria and Arbuscular Mycorrhiza Fungi. Afr. J. Food Agric. Nutr. Dev. 2018, 18, 13371–13387. [Google Scholar] [CrossRef]
- Du, H.; Chen, B.; Zhang, X.; Zhang, F.; Miller, S.A.; Rajashekara, G.; Xu, X.; Geng, S. Evaluation of Ralstonia solanacearum Infection Dynamics in Resistant and Susceptible Pepper Lines Using Bioluminescence Imaging. Plant Dis. 2017, 101, 272–278. [Google Scholar] [CrossRef]
- Kang, Y.J.; Ahn, Y.K.; Kim, K.T.; Jun, T.H. Resequencing of Capsicum annuum Parental Lines (YCM334 and Taean) for the Genetic Analysis of Bacterial Wilt Resistance. BMC Plant Biol. 2016, 16, 235. [Google Scholar] [CrossRef]
- Mamphogoro, T.P.; Babalola, O.O.; Aiyegoro, O.A. Sustainable Management Strategies for Bacterial Wilt of Sweet Peppers (Capsicum annuum) and Other Solanaceous Crops. J. Appl. Microbiol. 2020, 129, 496–508. [Google Scholar] [CrossRef]
- Thakur, P.P.; Mathew, D.; Nazeem, P.A.; Abida, P.S.; Indira, P.; Girija, D.; Shylaja, M.R.; Valsala, P.A. Identification of Allele-Specific AFLP Markers Linked with Bacterial Wilt [Ralstonia solanacearum (Smith) Yabuuchi et al.] Resistance in Hot Peppers (Capsicum annuum L.). Physiol. Mol. Plant Pathol. 2014, 87, 19–24. [Google Scholar] [CrossRef]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 Plant Pathogenic Bacteria in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef]
- Lebeau, A.; Daunay, M.C.; Frary, A.; Palloix, A.; Wang, J.F.; Dintinger, J.; Chiroleu, F.; Wicker, E.; Prior, P. Bacterial Wilt Resistance in Tomato, Pepper, and Eggplant: Genetic Resources Respond to Diverse Strains in the Ralstonia solanacearum Species Complex. Phytopathology 2011, 101, 154–165. [Google Scholar] [CrossRef]
- Álvarez, B.; Biosca, E.G.; López, M.M. On the Life of Ralstonia solanacearum, a Destructive Bacterial Plant Pathogen. In Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Formatex Research Center: Badajoz, Spain, 2010; pp. 267–279. [Google Scholar]
- Yadeta, K.A.; Thomma, B.P.H.J. The Xylem as a Battleground for Plant Hosts and Vascular Wilt Pathogens. Front. Plant Sci. 2013, 4, 97. [Google Scholar] [CrossRef]
- Jiang, G.; Wei, Z.; Xu, J.; Chen, H.; Zhang, Y.; She, X.; Macho, A.P.; Ding, W.; Liao, B. Bacterial Wilt in China: History, Current Status, and Future Perspectives. Front. Plant Sci. 2017, 8, 1549. [Google Scholar] [CrossRef] [PubMed]
- Agoncillo, E.S. Control Bacterial Wilt Disease Caused by Ralstonia Solanacearum in Pepper Using Arbuscular Mycorrhizal Fungi (Mykovam). J. Nat. Sci. Res. 2018, 8, 62–66. [Google Scholar]
- Namisy, A.; Chen, J.R.; Prohens, J.; Metwally, E.; Elmahrouk, M.; Rakha, M. Screening Cultivated Eggplant and Wild Relatives for Resistance to Bacterial Wilt (Ralstonia Solanacearum). Agriculture 2019, 9, 157. [Google Scholar] [CrossRef]
- Kim, B.-S.; French, E.; Caldwell, D.; Harrington, E.J.; Iyer-Pascuzzi, A.S. Bacterial Wilt Disease: Host Resistance and Pathogen Virulence Mechanisms. Physiol. Mol. Plant Pathol. 2016, 95, 37–43. [Google Scholar] [CrossRef]
- Llorens, E.; Scalschi, L.; Sharon, O.; Vicedo, B.; Sharon, A.; García-Agustín, P. Jasmonic Acid Pathway Is Required in the Resistance Induced by Acremonium sclerotigenum in Tomato against Pseudomonas syringae. Plant Sci. 2022, 318, 111210. [Google Scholar] [CrossRef]
- Yuan, S.; Li, M.; Fang, Z.; Liu, Y.; Shi, W.; Pan, B.; Wu, K.; Shi, J.; Shen, B.; Shen, Q. Biological Control of Tobacco Bacterial Wilt Using Trichoderma harzianum Amended Bioorganic Fertilizer and the Arbuscular Mycorrhizal Fungi Glomus mosseae. Biol. Control 2016, 92, 164–171. [Google Scholar] [CrossRef]
- Latz, M.A.C.; Jensen, B.; Collinge, D.B.; Jørgensen, H.J.L. Endophytic Fungi as Biocontrol Agents: Elucidating Mechanisms in Disease Suppression. Plant Ecol. Divers. 2018, 11, 555–567. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. The Impact of Microbes in the Orchestration of Plants’ Resistance to Biotic Stress: A Disease Management Approach. Appl. Microbiol. Biotechnol. 2019, 103, 9–25. [Google Scholar] [CrossRef]
- Dowarah, B.; Gill, S.S.; Agarwala, N. Arbuscular Mycorrhizal Fungi in Conferring Tolerance to Biotic Stresses in Plants. J. Plant Growth Regul. 2021, 41, 1429–1444. [Google Scholar] [CrossRef]
- Sanmartín, N.; Pastor, V.; Pastor-Fernández, J.; Flors, V.; Pozo, M.J.; Sánchez-Bel, P. Role and Mechanisms of Callose Priming in Mycorrhiza-Induced Resistance. J. Exp. Bot. 2021, 71, 2769–2781. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Gupta, R.; Pandey, R. Rice Seed Priming with Picomolar Rutin Enhances Rhizospheric Bacillus subtilis CIM Colonization and Plant Growth. PLoS ONE 2016, 11, e01460132. [Google Scholar] [CrossRef] [PubMed]
- Whipps, J.M. Prospects and Limitations for Mycorrhizas in Biocontrol of Root Pathogens. Can. J. Bot. 2004, 82, 1198–1227. [Google Scholar] [CrossRef]
- Jacott, C.N.; Murray, J.D.; Ridout, C.J. Trade-Offs in Arbuscular Mycorrhizal Symbiosis: Disease Resistance, Growth Responses and Perspectives for Crop Breeding. Agronomy 2017, 7, 75. [Google Scholar] [CrossRef]
- Bonfante, P.; Genre, A. Mechanisms Underlying Beneficial Plant—Fungus Interactions in Mycorrhizal Symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garrido, J.M. Regulation of the Plant Defence Response in Arbuscular Mycorrhizal Symbiosis. J. Exp. Bot. 2002, 53, 1377–1386. [Google Scholar] [CrossRef]
- Hause, B.; Mrosk, C.; Isayenkov, S.; Strack, D. Jasmonates in Arbuscular Mycorrhizal Interactions. Phytochemistry 2007, 68, 101–110. [Google Scholar] [CrossRef]
- Hohmann, P.; Messmer, M.M. Breeding for Mycorrhizal Symbiosis: Focus on Disease Resistance. Euphytica 2017, 213, 113. [Google Scholar] [CrossRef]
- Llorens, E.; García-Agustín, P.; Lapeña, L. Advances in Induced Resistance by Natural Compounds: Towards New Options for Woody Crop Protection. Sci. Agric. 2017, 74, 90–100. [Google Scholar] [CrossRef]
- Koo, A.J.K.; Howe, G.A. The Wound Hormone Jasmonate. Phytochemistry 2009, 70, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- De Geyter, N.; Gholami, A.; Goormachtig, S.; Goossens, A. Transcriptional Machineries in Jasmonate-Elicited Plant Secondary Metabolism. Trends Plant Sci. 2012, 17, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; Van Der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C.M. Hormonal Modulation of Plant Immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed]
- Halim, V.A.; Altmann, S.; Ellinger, D.; Eschen-Lippold, L.; Miersch, O.; Scheel, D.; Rosahl, S. PAMP-Induced Defense Responses in Potato Require Both Salicylic Acid and Jasmonic Acid. Plant J. 2009, 57, 230–242. [Google Scholar] [CrossRef]
- Scalschi, L.; Vicedo, B.; Camañes, G.; Fernandez-Crespo, E.; Lapeña, L.; González-Bosch, C.; García-Agustín, P. Hexanoic Acid Is a Resistance Inducer That Protects Tomato Plants against Pseudomonas Syringae by Priming the Jasmonic Acid and Salicylic Acid Pathways. Mol. Plant Pathol. 2013, 14, 342–355. [Google Scholar] [CrossRef]
- Navarro-Meléndez, A.L.; Heil, M. Symptomless Endophytic Fungi Suppress Endogenous Levels of Salicylic Acid and Interact with the Jasmonate-Dependent Indirect Defense Traits of Their Host, Lima Bean (Phaseolus lunatus). J. Chem. Ecol. 2014, 40, 816–825. [Google Scholar] [CrossRef]
- Hetrick, B.A.D.; Wilson, G.W.T.; Cox, T.S. Mycorrhizal Dependence of Modern Wheat Cultivars and Ancestors: A Synthesis. Can. J. Bot. 1992, 71, 512–518. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved Procedures for Clearing Roots and Staining Parasitic and Vesicular-Arbuscular Mycorrhizal Fungi for Rapid Assessment of Infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Lafortune, D.; Béramis, M.; Daubèze, A.M.; Boissot, N.; Palloix, A. Partial Resistance of Pepper to Bacterial Wilt Is Oligogenic and Stable under Tropical Conditions. Plant Dis. 2005, 89, 501–506. [Google Scholar] [CrossRef]
- Lillemo, M.; Joshi, A.K.; Prasad, R.; Chand, R.; Singh, R.P. QTL for Spot Blotch Resistance in Bread Wheat Line Saar Co-Locate to the Biotrophic Disease Resistance Loci Lr34 and Lr46. Theor. Appl. Genet. 2013, 126, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Geneaid Biotech Ltd. Home Page. Available online: https://www.geneaid.com/ (accessed on 16 March 2022).
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Fukushige, H.; Hildebrand, D.F.; Gan, S. Evidence Supporting a Role of Jasmonic Acid in Arabidopsis Leaf Senescence. Plant Physiol. 2002, 128, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Feng, Y.Q.; Zhang, X.W.; Zhang, Y.Y.; Bi, H.G.; Ai, X.Z. Salicylic Acid Is Involved in Rootstock–Scion Communication in Improving the Chilling Tolerance of Grafted Cucumber. Front. Plant Sci. 2021, 12, 693344. [Google Scholar] [CrossRef]
- Team R Development Core. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org (accessed on 5 August 2021).
- Ghozali, I. Structural Equation Modeling; Alternative Method with Partial Least Square (PLS), 2nd ed.; Badan Penerbit Universitas Diponegoro: Semarang, Indonesia, 2008. [Google Scholar]
- Fernandes, A. Structural Equation Modeling Approach PLS and SEM; Application of Smart PLS and AMOS Software; Laboratory of Statistics, Faculty of Mathematics and Natural Sciences, Brawijaya University: Malang, Indonesia, 2008. [Google Scholar]
- Krall, J.; Uthoff, V.; Harley, J. A Step-up Procedure for Selecting Variables Associated with Survival. Biometrics 1975, 31, 49–57. [Google Scholar] [CrossRef]
- SAS Institute. SAS System for Windows 9.4; SAS Institute, Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Raivo, K. Package “Pheatmap”: Prety Heatmap. Available online: https://cran.r-project.org/web/packages/pheatmap/index.html (accessed on 6 September 2021).
- Salloum, M.S.; Guzzo, M.C.; Velazquez, M.S.; Sagadin, M.B.; Luna, C.M. Variability in Colonization of Arbuscular Mycorrhizal Fungi and Its Effect on Mycorrhizal Dependency of Improved and Unimproved Soybean Cultivars. Can. J. Microbiol. 2016, 62, 1034–1040. [Google Scholar] [CrossRef]
- Sawers, R.J.H.; Gebreselassie, M.N.; Janos, D.P.; Paszkowski, U. Characterizing Variation in Mycorrhiza Effect among Diverse Plant Varieties. Theor. Appl. Genet. 2010, 120, 1029–1039. [Google Scholar] [CrossRef]
- Mishra, V.; Ellouze, W.; Howard, R.J. Utility of Arbuscular Mycorrhizal Fungi for Improved Production and Disease Mitigation in Organic and Hydroponic Greenhouse Crops. J. Hortic. 2018, 5, 1000237. [Google Scholar] [CrossRef]
- Tawaraya, K. Arbuscular Mycorrhizal Dependency of Different Plant Species and Cultivars. Soil Sci. Plant Nutr. 2003, 49, 655–668. [Google Scholar] [CrossRef]
- Janos, D.P. Plant Responsiveness to Mycorrhizas Differs from Dependence upon Mycorrhizas. Mycorrhiza 2007, 17, 75–91. [Google Scholar] [CrossRef]
- Matsunaga, H.; Saito, T.; Saito, A. Evaluation of Resistance to Bacterial Wilt and Phytophthora Blight in Capsicum Genetic Resources Collected in Myanmar. J. Jpn. Soc. Hortic. Sci. 2011, 80, 426–433. [Google Scholar] [CrossRef][Green Version]
- Mimura, Y.; Kageyama, T.; Minamiyama, Y.; Hirai, M. QTL Analysis for Resistance to Ralstonia Solanacearum in Capsicum Accession “LS2341”. J. Jpn. Soc. Hortic. Sci. 2009, 78, 307–313. [Google Scholar] [CrossRef]
- Aslam, M.N.; Mukhtar, T.; Hussain, M.A.; Raheel, M. Assessment of Resistance to Bacterial Wilt Incited by Ralstonia solanacearum in Tomato Germplasm. J. Plant Dis. Prot. 2017, 124, 585–590. [Google Scholar] [CrossRef]
- Matsunaga, H.; Monma, S. Sources of Resistance to Bacterial Wilt in Capsicum. J. Jpn. Soc. Hort. Sci. 1999, 68, 753–761. [Google Scholar] [CrossRef]
- Pawaskar, J.; Kadam, J.; Navathe, S.; Kadam, J. Response of Chilli Varieties and Genotypes to Bacterial Wilt Caused by Ralstonia solanacearum and Its Management. Indian J. Sci. 2014, 11, 66–72. [Google Scholar]
- Mimura, Y.; Yoshikawa, M.; Hirai, M. Pepper Accession LS2341 Is Highly Resistant to Ralstonia solanacearum Strains from Japan. HortScience 2009, 44, 2038–2040. [Google Scholar] [CrossRef]
- Tripodi, P.; Kumar, S. The Capsicum Crop: An Introduction. In Compendium of Plant Geomes; Springer Nature Switzerland AG: Basel, Switzerland, 2019; pp. 1–8. [Google Scholar] [CrossRef]
- Cameron, D.D.; Neal, A.L.; van Wees, S.C.M.; Ton, J. Mycorrhiza-Induced Resistance: More than the Sum of Its Parts? Trends Plant Sci. 2013, 18, 539–545. [Google Scholar] [CrossRef]
- Jung, S.C.; Martinez-Medina, A.; Lopez-Raez, J.A.; Pozo, M.J. Mycorrhiza-Induced Resistance and Priming of Plant Defenses. J. Chem. Ecol. 2012, 38, 651–664. [Google Scholar] [CrossRef]
- Liu, H.; Wu, M.; Liu, J.; Qu, Y.; Gao, Y.; Ren, A. Tripartite Interactions Between Endophytic Fungi, Arbuscular Mycorrhizal Fungi, and Leymus Chinensis. Microb. Ecol. 2020, 79, 98–109. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, J.; George, T.S.; Limpens, E.; Feng, G. Arbuscular Mycorrhizal Fungi Conducting the Hyphosphere Bacterial Orchestra. Trends Plant Sci. 2021, 27, 402–411. [Google Scholar] [CrossRef]
- Paszkowski, U. Mutualism and Parasitism: The Yin and Yang of Plant Symbioses. Curr. Opin. Plant Biol. 2006, 9, 364–370. [Google Scholar] [CrossRef]
- Van Wees, S.C.; Van der Ent, S.; Pieterse, C.M. Plant Immune Responses Triggered by Beneficial Microbes. Curr. Opin. Plant Biol. 2008, 11, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Pozo, M.J.; Azcón-Aguilar, C. Unraveling Mycorrhiza-Induced Resistance. Curr. Opin. Plant Biol. 2007, 10, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Shi, Y.; Zou, L.; Huang, J.; Shen, L.; Wang, Y.; Guan, D.; He, S. Pepper CaMLO6 Negatively Regulates Ralstonia solanacearum Resistance and Positively Regulates High Temperature and High Humidity Responses. Plant Cell Physiol. 2020, 61, 1223–1238. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008; Available online: https://www.scirp.org/(S(351jmbntvnsjt1aadkozje))/reference/referencespapers.aspx?referenceid=1398632 (accessed on 5 August 2021).
- Kaur, S.; Suseela, V. Unraveling Arbuscular Mycorrhiza-Induced Changes in Plant Primary and Secondary Metabolome. Metabolites 2020, 10, 335. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).