How to Get More Silver? Culture Media Adjustment Targeting Surge of Silver Nanoparticle Penetration in Apricot Tissue during in Vitro Micropropagation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plan Material, Maintenance, and Proliferation in Semisolid or Liquid Medium
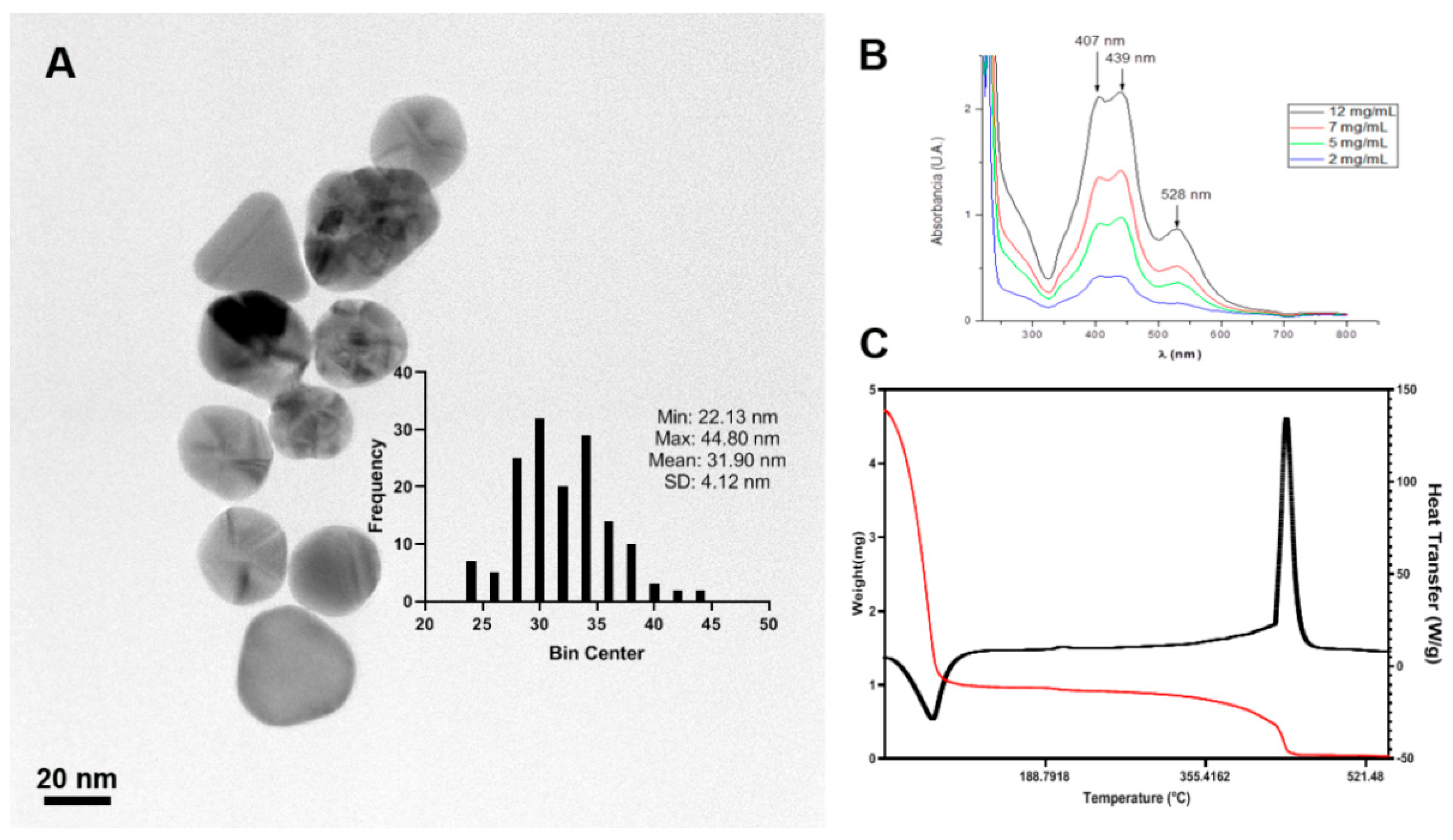
2.2. Physicochemical Characteristics of AgNPs
2.3. Addition of AgNPs to Different Media Compositions
2.4. Micropropagation Evaluation
2.5. Determination of Silver Content in the Plant Tissues
2.6. Statistical Analyses
3. Results
3.1. Physicochemical Characterization
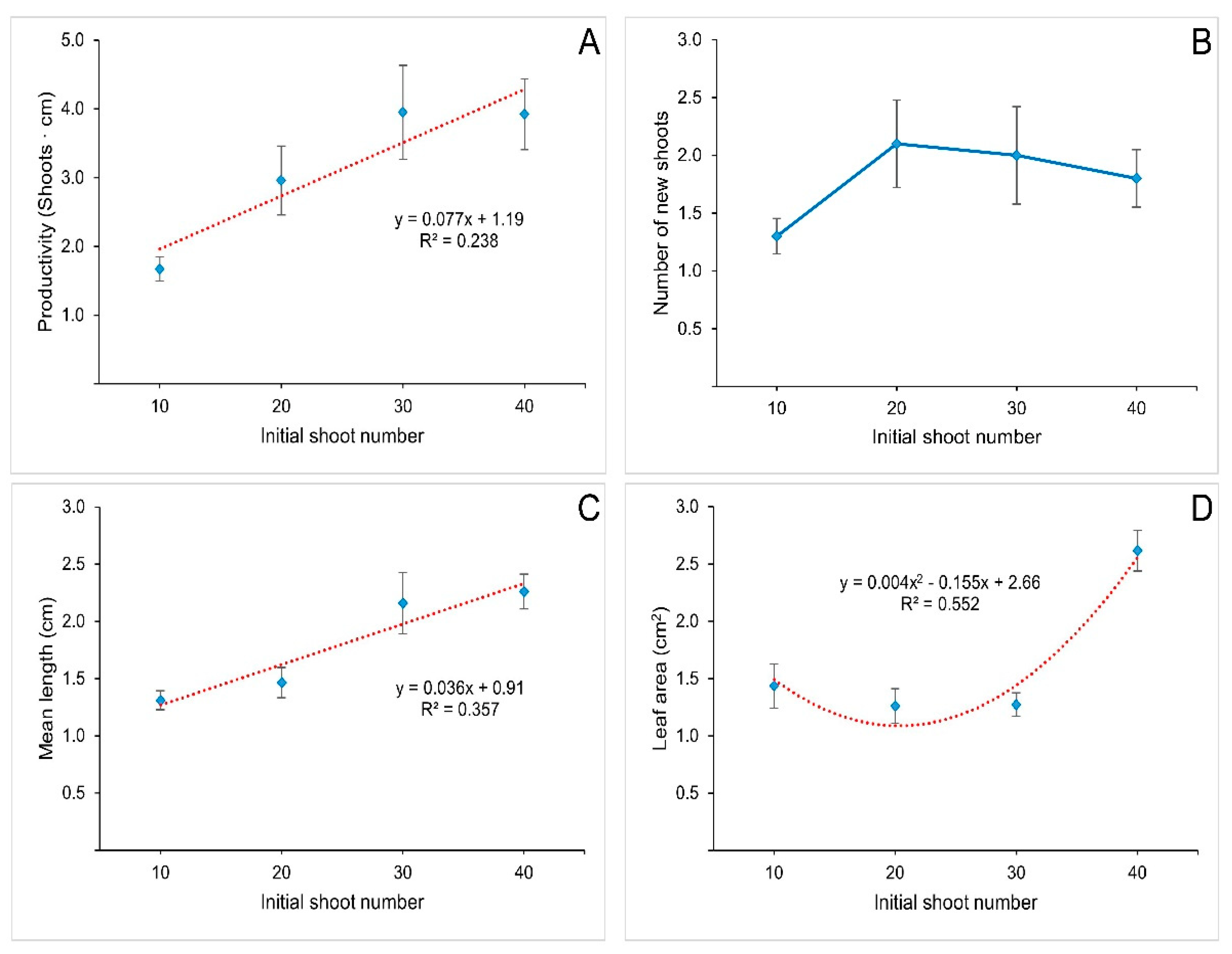
3.2. Effect of Initial Shoot Density
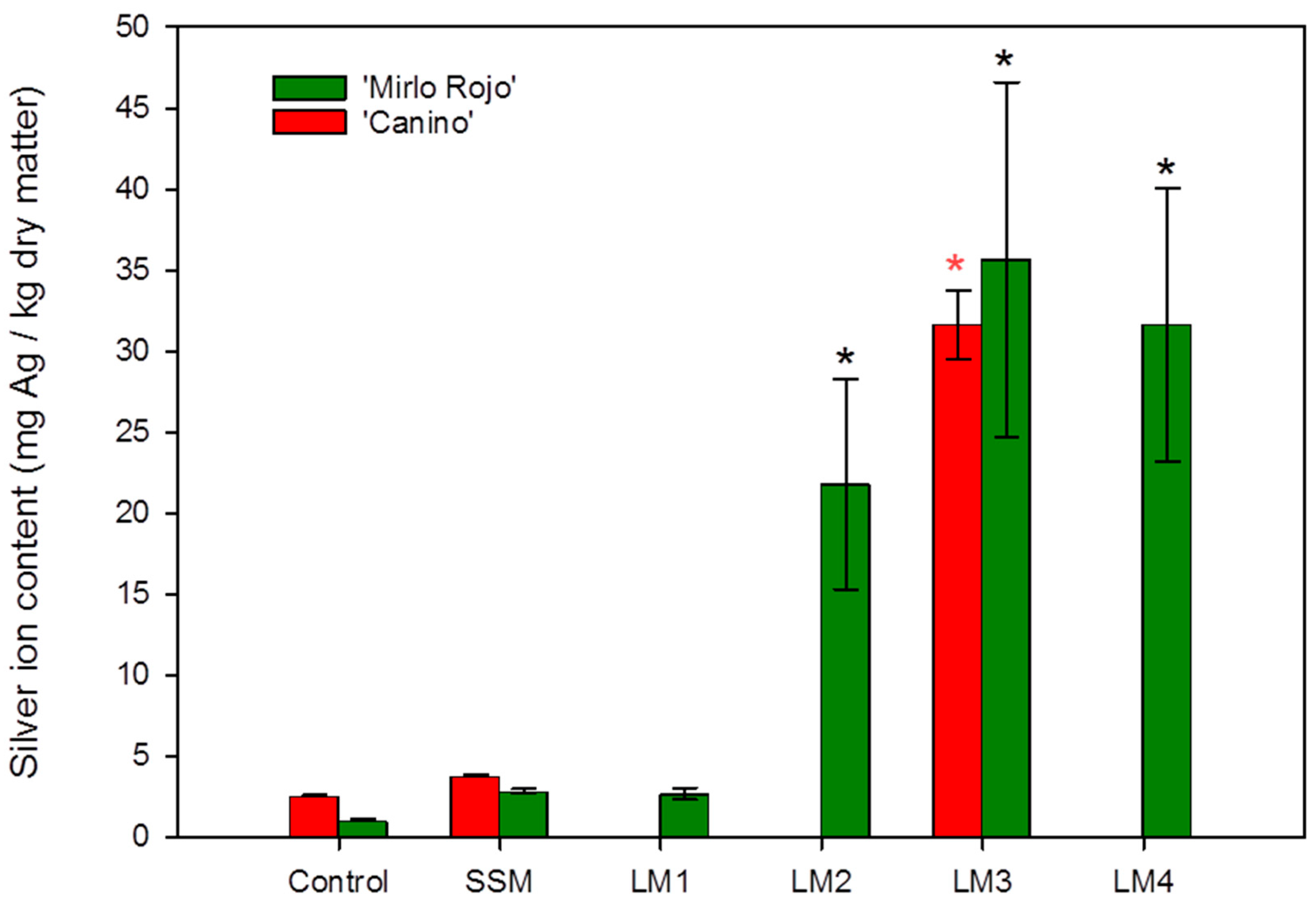
3.3. Silver Content within the Plants
3.4. Effects of AgNPs on Apricot Micropropagation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver Nanoparticles as Potential Antiviral Agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Kon, K.; Ingle, A.; Duran, N.; Galdiero, S.; Galdiero, M. Broad-Spectrum Bioactivities of Silver Nanoparticles: The Emerging Trends and Future Prospects. Appl. Microbiol. Biotechnol. 2014, 98, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, M.; Mostafavi, E.; Vincent, S.; Negash, H.; Andavar, R.; Perumal, V.; Chandra, N.; Narayanasamy, S.; Kalimuthu, K.; Barabadi, H. Nanotechnology-Based Approaches for Emerging and Re-Emerging Viruses: Special Emphasis on COVID-19. Microb. Pathog. 2021, 156, 104908. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.A.C. Global Plant Virus Disease Pandemics and Epidemics. Plants 2021, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Upadhyaya, C.P.; Singh, A.; Abd-Elsalam, K.A.; Prasad, R. Applications of Silver Nanoparticles in Plant Protection. In Nanobiotechnology Applications in Plant Protection; Springer: Cham, Switzerland, 2018; pp. 247–265. [Google Scholar] [CrossRef]
- Jones, R.A.C.; Naidu, R.A. Global Dimensions of Plant Virus Diseases: Current Status and Future Perspectives. Annu. Rev. Virol. 2019, 6, 387–409. [Google Scholar] [CrossRef] [PubMed]
- Farooq, T.; Adeel, M.; He, Z.; Umar, M.; Shakoor, N.; Da Silva, W.; Elmer, W.; White, J.C.; Rui, Y. Nanotechnology and Plant Viruses: An Emerging Disease Management Approach for Resistant Pathogens. ACS Nano 2021, 15, 6030–6037. [Google Scholar] [CrossRef] [PubMed]
- Avila-Quezada, G.D.; Golinska, P.; Rai, M. Engineered Nanomaterials in Plant Diseases: Can We Combat Phytopathogens? Appl. Microbiol. Biotechnol. 2022, 106, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Gopal, J.; Sivanesan, I. Nanomaterials in Plant Tissue Culture: The Disclosed and Undisclosed. RSC Adv. 2017, 7, 36492–36505. [Google Scholar] [CrossRef]
- Spinoso-Castillo, J.L.; Chavez-Santoscoy, R.A.; Bogdanchikova, N.; Pérez-Sato, J.A.; Morales-Ramos, V.; Bello-Bello, J.J. Antimicrobial and Hormetic Effects of Silver Nanoparticles on in Vitro Regeneration of Vanilla (Vanilla Planifolia Jacks. Ex Andrews) Using a Temporary Immersion System. Plant Cell Tissue Organ Cult. 2017, 129, 195–207. [Google Scholar] [CrossRef]
- Bello-Bello, J.J.; Chavez-Santoscoy, R.A.; Lecona-Guzmán, C.A.; Bogdanchikova, N.; Salinas-Ruíz, J.; Carlos Gómez-Merino, F.; Pestryakov, A. Hormetic Response by Silver Nanoparticles on In Vitro Multiplication of Sugarcane (Saccharum Spp. Cv. Mex 69-290) Using a Temporary Immersion System. Dose-Response 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Food and Agriculture Organization of the United Nations 2022. Available online: https://www.fao.org/faostat/es/#data/QCL (accessed on 15 May 2022).
- Pérez-Tornero, O.; Burgos, L. Apricot Micropropagation. In Protocols for Micropropagation of Woody Trees and Fruits; Jain, S.M., Häggman, H., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 267–278. [Google Scholar]
- Vidal, N.; Sánchez, C. Use of Bioreactor Systems in the Propagation of Forest Trees. Eng. Life Sci. 2019, 19, 896–915. [Google Scholar] [CrossRef] [PubMed]
- Castro-González, C.G.; Sánchez-Segura, L.; Gómez-Merino, F.C.; Bello-Bello, J.J. Exposure of Stevia (Stevia Rebaudiana B.) to Silver Nanoparticles in Vitro: Transport and Accumulation. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- El-Gamal, A.Y.; Tohamy, M.R.; Abou-Zaid, M.I.; Atia, M.M.; El Sayed, T.; Farroh, K.Y. Silver Nanoparticles as a Viricidal Agent to Inhibit Plant-Infecting Viruses and Disrupt Their Acquisition and Transmission by Their Aphid Vector. Arch. Virol. 2022, 167, 85–97. [Google Scholar] [CrossRef]
- Elbeshehy, E.K.F.; Elazzazy, A.M.; Aggelis, G. Silver Nanoparticles Synthesis Mediated by New Isolates of Bacillus Spp., Nanoparticle Characterization and Their Activity against Bean Yellow Mosaic Virus and Human Pathogens. Front. Microbiol. 2015, 6, 453. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Kothari, S.L. Green Synthesis of Silver Nanoparticles and Their Application in Plant Virus Inhibition. J. Mycol. Plant Pathol. 2014, 44, 21–24. [Google Scholar]
- Podkopaev, D.O.; Shaburova, L.N.; Balandin, G.V.; Kraineva, O.V.; Labutina, N.V.; Suvorov, O.A.; Sidorenko, Y.I. Comparative Evaluation of Antimicrobial Activity of Silver Nanoparticles. Nanotechnologies Russ. 2014, 9, 93–97. [Google Scholar] [CrossRef]
- Nefedova, E.; Koptev, V.; Bobikova, A.S.; Cherepushkina, V.; Mironova, T.; Afonyushkin, V.; Shkil, N.; Donchenko, N.; Kozlova, Y.; Sigareva, N.; et al. The Infectious Bronchitis Coronavirus Pneumonia Model Presenting a Novel Insight for the Sars-Cov-2 Dissemination Route. Vet. Sci. 2021, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Bogdanchikova, N.; Vázquez-Muñoz, R.; Huerta-Saquero, A.; Pena-Jasso, A.; Aguilar-Uzcanga, G.; Picos-díaz, P.L.; Pestryakov, A. Silver Nanoparticles Composition for Treatment of Distemper in Dogs. Int. J. Nanotechnol. 2016, 13, 227–237. [Google Scholar] [CrossRef]
- Romo-Quiñonez, C.R.; Álvarez-Sánchez, A.R.; Álvarez-Ruiz, P.; Chávez-Sánchez, M.C.; Bogdanchikova, N.; Pestryakov, A.; Mejia-Ruiz, C.H. Evaluation of a New Argovit as an Antiviral Agent Included in Feed to Protect the Shrimp Litopenaeus Vannamei against White Spot Syndrome Virus Infection. PeerJ 2020, 8, e8446. [Google Scholar] [CrossRef] [PubMed]
- Borrego, B.; Lorenzo, G.; Mota-Morales, J.D.; Almanza-Reyes, H.; Mateos, F.; López-Gil, E.; de la Losa, N.; Burmistrov, V.A.; Pestryakov, A.N.; Brun, A.; et al. Potential Application of Silver Nanoparticles to Control the Infectivity of Rift Valley Fever Virus in Vitro and in Vivo. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Almanza-Reyes, H.; Moreno, S.; Plascencia-López, I.; Alvarado-Vera, M.; Patrón-Romero, L.; Borrego, B.; Reyes-Escamilla, A.; Valencia-Manzo, D.; Brun, A.; Pestryakov, A.; et al. Evaluation of Silver Nanoparticles for the Prevention of SARS-CoV-2 Infection in Health Workers: In Vitro and in Vivo. PLoS ONE 2021, 16, e0256401. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Petri, C.; Burgos, L.; Alburquerque, N. Phosphomannose-Isomerase as a Selectable Marker for Transgenic Plum (Prunus Domestica L.). Plant Cell. Tissue Organ Cult. 2013, 113, 189–197. [Google Scholar] [CrossRef]
- Quoirin, M.; Lepoivre, P. Improved Media for in Vitro Culture of Prunus Sp. Acta Hortic. 1977, 78, 437–442. [Google Scholar] [CrossRef]
- Driver, J.A.; Kuniyuki, A.H. In Vitro Propagation of Paradox Walnut Rootstock. HortScience 1984, 19, 507–509. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Arab, M.M.; Yadollahi, A. Effects of Antimicrobial Activity of Silver Nanoparticles on in Vitro Establishment of G x N15 (Hybrid of Almond x Peach) Rootstock. J. Genet. Eng. Biotechnol. 2014, 12, 103–110. [Google Scholar] [CrossRef]
- Moradpour, M.; Aziz, M.A.; Abdullah, S.N.A. Establishment of in Vitro Culture of Rubber (Hevea Brasiliensis) from Field-Derived Explants: Effective Role of Silver Nanoparticles in Reducing Contamination and Browning. J. Nanomed. Nanotechnol. 2016, 7, 2. [Google Scholar] [CrossRef]
- Ochoa-Meza, A.R.; Álvarez-Sánchez, A.R.; Romo-Quiñonez, C.R.; Barraza, A.; Magallón-Barajas, F.J.; Chávez-Sánchez, A.; García-Ramos, J.C.; Toledano-Magaña, Y.; Bogdanchikova, N.; Pestryakov, A.; et al. Silver Nanoparticles Enhance Survival of White Spot Syndrome Virus Infected Penaeus Vannamei Shrimps by Activation of Its Immunological System. Fish Shellfish Immunol. 2019, 84, 1083–1089. [Google Scholar] [CrossRef]
- El-Dougdoug, N.K.; Bondok, A.M.; El-Dougdoug, K.A. Evaluation of Silver Nanoparticles as Antiviral Agent against ToMV and PVY in Tomato Plants. Middle East J. Appl. Sci. 2018, 8, 100–111. [Google Scholar]
- Narayanan, J.; Xiong, J.Y.; Liu, X.Y. Determination of Agarose Gel Pore Size: Absorbance Measurements Vis a Vis Other Techniques. J. Phys. Conf. Ser. 2006, 28, 83–86. [Google Scholar] [CrossRef]
- Carvalho, L.S.O.; Ozudogru, E.A.; Lambardi, M.; Paiva, L.V. Temporary Immersion System for Micropropagation of Tree Species: A Bibliographic and Systematic Review. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 269–277. [Google Scholar] [CrossRef]
- Etienne, H.; Berthouly, M. Temporary Immersion Systems in Plant Micropropagation. Plant Cell. Tissue Organ Cult. 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Rojas-Martínez, L.; Visser, R.G.F.; Klerk, G. De The Hyperhydricity Syndrome: Waterlogging of Plant Tissues as a Major Cause. Propag. Ornam. Plants 2010, 10, 169–175. [Google Scholar]
- Pérez-Tornero, O.; Egea, J.; Olmos, E.; Burgos, L. Control of Hyperhydricity in Micropropagated Apricot Cultivars. In Vitro Cell. Dev. Biol. Plant. 2001, 37, 250–254. [Google Scholar] [CrossRef]
- Monette, P.L. Influence of Size of Culture Vessel on in Vitro Proliferation of Grape in a Liquid Medium. Plant Cell Tissue Organ Cult. 1983, 2, 327–332. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Fulazzaky, M.A.; Salmiati, S.; Kueh, A.B.H.; Fulazzaky, M.; Salim, M.R. Silver Nanoparticles Adsorption by the Synthetic and Natural Adsorbent Materials: An Exclusive Review. Nanotechnol. Environ. Eng. 2020, 5, 1. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]

| Media Codes | Basal Salts | CaCl2 (3 mM) | Other Components z | Agar (7 gL−1) |
|---|---|---|---|---|
| SSM | QL + DKW | Yes | a | Yes |
| LM1 | QL + DKW | Yes | a | No |
| LM2 | QL + DKW | No | b | No |
| LM3 | QL + DKW | No | a | No |
| LM4 | QL modified y + DKW | No | a | No |
| Treatment z | Productivity Shoots x cm | Proliferation Shoot Number | Shoot Length cm | Leaf Area cm2 |
|---|---|---|---|---|
| LM1 (no AgNPs) | 1.92 ± 0.41 | 1.40 ± 0.22 | 1.35 ± 0.11 | 2.53 ± 0.17 |
| LM (+AgNPs) | 4.95 ± 0.34 * | 3.57 ± 0.27 * | 1.50 ± 0.10 | 3.79 ± 0.50 * |
| LM2 (+ AgNPs) | 5.33 ± 0.65 ab | 3.08 ± 0.48 b | 2.06 ± 0.27 a | 7.21 ± 1.16 a |
| LM3 (+ AgNPs) | 6.10 ± 0.61 a | 4.58 ± 0.48 a | 1.35 ± 0.05 b | 2.50 ± 0.31 b |
| LM4 (+ AgNPs) | 3.92 ± 0.46 b | 3.22 ± 0.38 b | 1.23 ± 0.04 b | 2.38 ± 0.33 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Caselles, C.; Alburquerque, N.; Faize, L.; Bogdanchikova, N.; García-Ramos, J.C.; Rodríguez-Hernández, A.G.; Pestryakov, A.; Burgos, L. How to Get More Silver? Culture Media Adjustment Targeting Surge of Silver Nanoparticle Penetration in Apricot Tissue during in Vitro Micropropagation. Horticulturae 2022, 8, 855. https://doi.org/10.3390/horticulturae8100855
Pérez-Caselles C, Alburquerque N, Faize L, Bogdanchikova N, García-Ramos JC, Rodríguez-Hernández AG, Pestryakov A, Burgos L. How to Get More Silver? Culture Media Adjustment Targeting Surge of Silver Nanoparticle Penetration in Apricot Tissue during in Vitro Micropropagation. Horticulturae. 2022; 8(10):855. https://doi.org/10.3390/horticulturae8100855
Chicago/Turabian StylePérez-Caselles, Cristian, Nuria Alburquerque, Lydia Faize, Nina Bogdanchikova, Juan Carlos García-Ramos, Ana G. Rodríguez-Hernández, Alexey Pestryakov, and Lorenzo Burgos. 2022. "How to Get More Silver? Culture Media Adjustment Targeting Surge of Silver Nanoparticle Penetration in Apricot Tissue during in Vitro Micropropagation" Horticulturae 8, no. 10: 855. https://doi.org/10.3390/horticulturae8100855
APA StylePérez-Caselles, C., Alburquerque, N., Faize, L., Bogdanchikova, N., García-Ramos, J. C., Rodríguez-Hernández, A. G., Pestryakov, A., & Burgos, L. (2022). How to Get More Silver? Culture Media Adjustment Targeting Surge of Silver Nanoparticle Penetration in Apricot Tissue during in Vitro Micropropagation. Horticulturae, 8(10), 855. https://doi.org/10.3390/horticulturae8100855










