Abstract
The different pre- and post-treatments are critical in cryopreservation procedures and affect the shoot tip regrowth after freezing. In the present study, the long-term storage of four citrus cultivars [Bodrum Mandarin (Citrus deliciosa Ten.); Klin Mandarin (Citrus nobilis Lauriro); White grapefruit and Red grapefruit (Citrus paradisi L.)] were carried out by droplet vitrification methods, and the critical points for effective cryopreservation of these species were determined. In this study, we investigated the effect of explant size, cold hardening treatments, sucrose concentrations, and media combinations on shoot regrowth after cryopreservation. The highest shoot tip regrowth, ranging from 13.3 to 33.3%, was achieved when they were obtained from 0.3 to 0.7 mm explants excised from cold hardened seedlings at 4 °C for three days that were then precultured in a medium containing 0.25 M of sucrose and treated with PVS2 at 0 °C for 45 min. In addition, it has been determined that a regeneration medium containing boric acid (H3BO3) or ferric ethylenediaminetetraacetate (FeEDDHA) increased the regeneration up to 33.3% after cryopreservation.
1. Introduction
Cryopreservation is the most suitable preservation process for living cells, tissues, and organs in liquid nitrogen (LN, −196 °C) for long periods of time as the mitotic and metabolic activities are reduced at a basal level [1]. It allows for the use of different parts of plants, such as the shoot tips, seeds, nodal and dormant buds, pollen grains, somatic and zygotic embryos, calli, and cell suspensions. The genetic stability also maintains for many years during cold storage [2,3]. However, during and after cryopreservation, the cells, tissues, and organs can suffer from freezing damage and even lose their viability, resulting in failed cryopreservation. Therefore, the selection and pretreatment of excised explants are often needed as the first step of cryopreservation [4]. In addition, fatal ice crystals can form inside the cells, as freezing occurs during the liquid nitrogen treatment [5,6]. The reason for this is the amount of water in the cell. During the frozen storage of plant materials, the water content of the cell should be reduced, and ice crystal formation should be prevented. Plant cells cannot survive LN treatment without the use of cryoprotective solutions [7,8,9]. Therefore, in order to prevent the cells and shoot tips from freezing and to provide vitrification with liquid nitrogen, dehydration should be performed with cryoprotective solutions [10].
Cryoprotectants are generally divided into penetrating cryoprotectors and non-penetrating cryoprotectors [11]. Penetrating solutions pass through the cell membrane to maintain extracellular and intracellular balance, and non-penetrating solutions accumulate in the extracellular solution without passing through the cell membrane [11]. Penetrating cryoprotectants contain dimethyl sulfoxide (DMSO), glycerol, and amino acids such as proline, while non-penetrating cryoprotectants contain sugars and alcohols [12]. When these solutions were examined, it was determined that DMSO and glycerol were the most efficient cryoprotectants when used appropriately [13]. DMSO is often preferred because of its rapid penetration into cells, but it also has a toxic effect. Therefore, the PVS with an optimized concentration of DMSO is often required in vitrification-based methods [14].
Among various PVSs, PVS2 proved applicable to the shoot tip cryopreservation of a wide range of plant genera. However, it also poses osmotic stress and chemical toxicity to plant tissues, resulting in excessive damage to the cells and poor shoot regrowth after cryopreservation [15,16]. Therefore, the sucrose preculture and loading (with 2 M glycerol + 0.4 M sucrose) are often applied to induce osmotolerance prior to PVS2 treatment [16]. In addition, the duration of PVS2 exposure and temperature should be optimized for the establishment of an optimized vitrification-based protocol [17]. It was observed that shoot tips were formed at different rates in the samples treated with PVS2 at different times [18].
Citrus germplasms, which are taken under protection in their natural habitats and collection gardens, are suppressed by biotic stresses such as insects, nematodes, viruses, bacteria, and fungi, and abiotic stresses such as extreme heat and cold, soil salinity, and acidity [19]. The application of in vitro techniques on these species has some limitations as they have phenolic compounds, similar to some other woody species. Therefore, it is of great value to apply cryopreservation strategies for the safe conservation of Citrus germplasm [20,21,22]. In the literature, there are some protocols applied to the cryopreservation of citrus species. In a study applying shoot tip cryopreservation, nine different citrus species from greenhouse stock plants in Fort Collins (CO, USA) were cryopreserved using the droplet vitrification technique [23]. The same research group further established a droplet vitrification technique for cryopreservation of three Citrus accessions [24]. In both of these studies, the thawed shoot tips after liquid nitrogen were recovered using the micrografting method [22,23]. Droplet vitrification combines the use of aluminum foils that facilitate ultra-rapid freezing and thawing with the PVS vitrification and has been widely applied in shoot tip cryobanking [24,25,26]. Compared to other vitrification techniques, higher cooling and warming rates are observed in the droplet vitrification technique since the samples are placed on aluminum foil with very high thermal conductivity [27,28]. The use of aluminum foil allows explants to reach ultra-low temperatures quickly during contact with liquid nitrogen. Thus, the chance of reaching the vitrified state of the cytoplasm during ultra-fast freezing is increased [29,30].
In this context, the present study aimed to investigate the critical points such as shoot tip size, cold hardening, sucrose preculture, PVS2 treatment time, and culture media on four different citrus cultivars [Bodrum Mandarin (Citrus deliciosa Ten.); Klin Mandarin (Citrus nobilis Lauriro); cultivars of white grapefruit and red grapefruit (Citrus paradisi L.)]. The direct post-thaw culture was tested without micrografting to facilitate the easier operation of droplet vitrification.
2. Materials and Methods
2.1. Plant Material, Surface Sterilization and In Vitro Propagation of Citrus Micro-Shoots
The seeds of Citrus deliciosa Ten. cv. “Bodrum Mandarin”, C. nobilis Lauriro cv. “Klin Mandarin”, C. paradisi L. cv. “white grapefruit” and cv. “red grapefruit” were obtained from Mugla Metropolitan Municipality, Agricultural Services Department, Mugla Local Seed Bank collection. Surface sterilization was performed via the protocol of Ozudogru et al. [31]. Each of these four Citrus spp. cultivars were used to evaluate the effects of shoot tip size, cold hardening, and sucrose preculturing on shoot tip cryopreservation with at least three replications per treatment. The seeds were treated with 70% ethyl alcohol for 5 min, 10% H2O2, and twice with 20% commercial bleach for 10 min with active chlorine, and then they were washed with sterile distilled water until completely rinsed. After drying the seeds for 10 min in a laminar flow cabinet, they were transferred to solid Woody Plant Medium (WPM, Duchefa Biochemie) nutrient medium [32] supplemented with 20 g.L−1 and 7 g.L−1 agar (pH, 5.8) without any growth regulators and incubated in a growth room under standard conditions (16 h light/8 h dark conditions on 50 µmol–1m–2s–1 with white cool fluorescent light, 25 ± 2 °C). The four weeks old micro-shoots (Figure 1a) obtained from the germinated seeds were subcultured on WPM medium supplement with 10 g.L−1 charcoal, 1 mg.L−1 6-Benzylaminopurine (BAP), 7 g.L−1 agar (pH, 5.8) in Magenta™ vessel GA-7 (Sigma-Aldrich) to obtain a sufficient number of micro-shoots (16 explants were cultured per vessel) for cryopreservation applications.
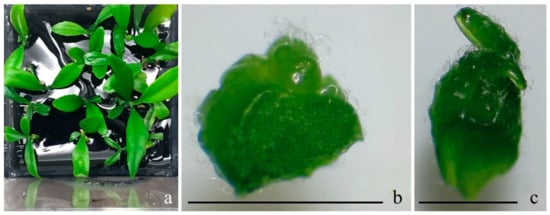
Figure 1.
The micro-shoots and shoot tips of Citrus spp. used in experiments. The micro-shoots of C. deliciosa cv. “Bodrum Mandarin” derived from in vitro clonal propagiton (a), the shoot tips in different sizes derived from micro-shoots of C. nobilis cv. “Klin Mandarin” (b,c), bars 1 mm.
In the preliminary trials, two different nutrient media, Murashige and Skoog (MS) nutrient medium [33] or WPM, were tested in combinations with or without charcoal for shoot tip regeneration. In these studies, the regeneration percentage, the average shoots formed from each shoot tip, and the shoot length were scored. The shoot-forming capacity index (SFC) was calculated via these obtained data [SFC = (average no of shoots per regenerating explant) × (% of regenerating explant)/100] [34]. In the light of this preliminary study, a WPM medium supplement with 10 g.L−1 charcoal, 1 mg.L−1 BAP, 7 g.L−1 agar (pH, 5.8), yielded the best stem-forming capacity index compared to other media combinations, and was decided as the regeneration medium for all of the shoot tips used in this study. In addition to this treatment, some combinations of additional chemical compounds were tested for the increased compacity of shoot tip regrowth.
2.2. Cold Hardening
The micro-shoots that belonged to four different Citrus spp. cultivars (16 micro-shoots were cultured per vessel) were reproduced in four-week subculture periods. They were covered with aluminum foil in GA-7 and cold-hardened at 4 °C in the dark following different incubation durations: 24 h, 3 days, and 7 days (Figure 2). To evaluate the effect of cold-hardening on shoot-tip viability, 15 shoot tips were excised from cold-hardened micro-shoots after the cold exposure. The cold-hardened shoot tips were then directly transferred to the regeneration medium and cultured for four weeks under the standard conditions of the control group.
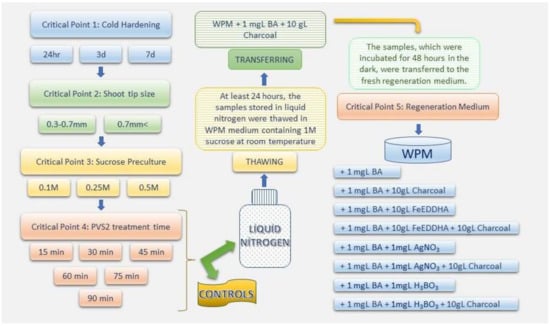
Figure 2.
Schematic representation of the stages that are crucial for regeneration before and after cryopreservation of four different Citrus spp. cultivars via droplet vitrification.
2.3. Isolation of Shoot Tips
For all pre- and post-cryopreservation treatments, two sizes of shoot tips belonging to four different Citrus spp. cultivars were used as control or liquid nitrogen groups. The shoot tips were cut between 0.3 and 0.7 mm (Figure 1b) or larger than 0.7 mm in size (Figure 1c). Then, 15 shoot tips of each size were then precultured with enriched sucrose levels and cryopreserved following a droplet-vitrification procedure.
2.4. Sucrose Preculture
The shoot tips excised from the cold-hardened micro-shoots were transferred separately to WPM media containing 0.1 M, 0.25 M, or 0.5 M sucrose, 7 g.L−1 agar (pH, 5.8), and they were incubated at growth room for 24 h. In order to evaluate the effect of each different concentration of sucrose on shoot tip regeneration, 15 shoot tips treated with WPM media containing 0.1 M, 0.25 M, or 0.5 M sucrose, 7 g.L−1 agar (pH, 5.8) for incubation of 24 h in a growth room.
2.5. Application of Droplet Vitrification Technique
For cryopreservation, a droplet vitrification technique, which is a more effective, easily applicable, and inexpensive method for many species, was applied [29]. After cold-hardening and sucrose preculture treatment, the shoot tips were transferred to aluminum foil strips (~0.5 × 2 cm in size) containing 3 µL PVS2 for each drop [35]. A total of five drops were dropped on the aluminum foil strip, and each shoot tip was placed on each drop (Figure 3a). Each shoot tip was treated with PVS2 for 15, 30, 45, 60, 75, or 90 min on ice (to prevent cell damage due to the rapid infiltration of osmotic agents). After treatment with PVS2, aluminum foils with shoot tips were plunged into liquid nitrogen by transferring them into cryovials in liquid nitrogen (Figure 3b). The control samples were treated with PVS2 for 15, 30, 45, 60, 75, or 90 min but not immersed in liquid nitrogen.
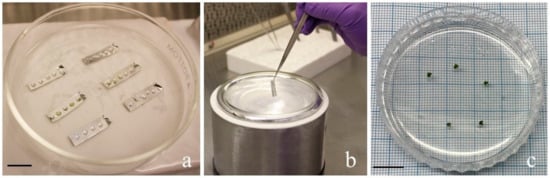
Figure 3.
(a) the demonstration of droplet-vitrification performed in this study. (b) The ultra-rapid freezing performed with aluminum foil as the carrier of shoot tips. (c) Post-thaw cultured shoot tips after cryopreservation.
2.6. Thawing and Post-Thaw Recovery
The samples of each cultivar were sucrose-precultured by removing the shoot tips in two different sizes after the cold preculture step. Afterward, the vitrification solution application (at the different treatment times mentioned above) and immersion in liquid nitrogen and thawing steps were applied to the shoot tips. The samples stored in liquid nitrogen for at least 24 h were thawed by direct transfer to a liquid WPM nutrient medium containing 1 M sucrose (pH, 5.8) at room temperature. For this process, aluminum foils containing shoot tips, which are treated with liquid nitrogen and the control group (not treated with liquid nitrogen) were removed from the cryovials and immersed in the sucrose liquid nutrient medium in Petri dishes. The shoot tips were washed in the same nutrient medium for 15 min and the PVS2 solution was diluted from the cells and tissues. Afterwards, the samples were transferred on solid WPM medium supplement with 10 g.L−1 charcoal, 1 mg.L−1 BAP and 7 g.L−1 agar (pH, 5.8) for 24 h (Figure 3c), and after 24 h, the samples were transferred to different nutrient media [WPM medium supplemented with 1 mg.L−1 BAP; 1 mg.L−1 BAP and 10 g.L−1 charcoal; 1 mg.L−1 BAP and 10 g.L−1 FeEDDHA; 1 mg.L−1 BAP, 10 g.L−1 FeEDDHA and 10 g.L−1 charcoal; 1 mg.L−1 BAP and 10 g.L−1 AgNO3; 1 mg.L−1 BAP, 10 g.L−1 AgNO3 and 10 g.L−1 charcoal; 1 mg.L−1 BAP and 10 g.L−1 H3BO3; 1 mg.L−1 BAP, 10 g.L−1 H3BO3 and 10 g.L−1 charcoal, and each media also contains 7 g.L−1 agar (pH, 5.8)] to observe their regeneration during the final recovery (Figure 2).
2.7. Data Analyses
A high percentage of regeneration, over 90%, was obtained from each control group sample (Table 1, Table 2, Table 3 and Table 4) of all critical point treatments except the sucrose preculture treatment on WPM medium supplemented with 0.5 M sucrose and different time PVS2 treatments. In this context, the study is based on the results obtained after liquid nitrogen treatments, and each critical point was studied in conjunction with the other. After thawing, shoot tips incubated in the dark for 48 h in regeneration nutrient medium (described before) were transferred to different nutrient media and incubated for 4 weeks under the above-mentioned standard conditions (Figure 2). After four weeks of incubation, at least one leaf formation from the shoot tips of incubation was determined as the shoot tip regeneration. In total, five different critical steps were evaluated in the present study. Four of these were the application parameters before cryopreservation, and one of them was applied after cryopreservation. A total of 15 shoot-tips were used for each parameter, which was performed in three replicates. Including the controls, 2 different sizes of shoot tips, 3 different sucrose concentrations containing sucrose preculture, 6 different times of cryoprotectant application, and finally, after 24 h of incubation after thawing, the shoot tips were transferred to 8 different nutrient media (described in Figure 2). In all these studies, 864 different parameters were tried in tree replicates, and a total of 6720 shoot tips were used for each cultivar, including controls. The percentages of regeneration after cryopreservation were calculated for each cultivar, and IBM® SPSS Statistics 24.0 was used for the statistical analysis of data.

Table 1.
The shoot tip regeneration, the number of shoots, and the shoot forming capacity (SFC) of four different Citrus spp. cultivars tested in different media combinations.

Table 2.
The shoot tip regeneration of four different Citrus spp. cultivars cold hardened in different treatment times. The shoot tips treated with PVS2 for 45 min were incubated in regeneration medium for the first 24 h, and then they transferred to WPM medium supplemented with 1 mg.L−1 BAP, 10 mg.L−1 activated charcoal, and 1 mg.L−1 H3BO3 medium for four weeks under standard culture conditions.

Table 3.
The regeneration of two differently sized shoot tips of four different Citrus spp. Cultivars. The shoot tips treated with PVS2 for 45 min were incubated in regeneration medium for the first 24 h, and then they transferred to WPM medium supplemented with 1 mg.L−1 BAP, 10 mg.L−1 activated charcoal, and 1 mg.L−1 H3BO3 medium for four weeks under standard culture conditions.

Table 4.
The shoot tip regeneration of four different Citrus spp. cultivars tested in different sucrose preculture media for 24 h. The shoot tips treated with PVS2 for 45 min were incubated in regeneration medium for the first 24 h, and then they transferred to WPM medium supplemented with 1 mg.L−1 BAP, 10 mg.L−1 activated charcoal, and 1 mg.L−1 H3BO3 medium for four weeks under standard culture conditions.
3. Results
3.1. The Determination of the Medium for Shoot Tip Regrowth
Pre-trials of the present study were conducted to optimize the optimal regeneration medium for the shoot tips of four different Citrus spp. cultivars. Two different nutrient media supplemented with 1 mg.L−1 BAP were tested with or without charcoal, and WPM supplemented with charcoal yielded the best results for the shoot tip regeneration. Very high regeneration percentages ranging between 93.3 and 100 were obtained in all of the tested media. However, in the tested media, the highest number of stems per shoot ranged between 3.1 and 5.3, was obtained from WPM supplemented with 1 mg.L−1 and 10 g.L−1 Charcoal, pH 5.8 (Table 1). For this reason, this medium was used as the regeneration medium in further trials based on the calculated Shoot Forming Capacity (SFC) index [34].
3.2. The Evaluation of Cold-Hardening
Considering each treatment as an integrated (Figure 4), it was observed that shoot tip explants cut from shoots acclimatized to cold for 3 days had a significantly positive effect on regeneration after cryopreservation and the best regeneration percentages were 33.3%, 26.7%, 13.3%, and 26.7, respectively, in all four citrus cultivars (Table 2). It was observed that the regeneration of the non-acclimatized samples was significantly reduced, and they even died after cryopreservation, evidencing that this step is critical for the process.

Figure 4.
The shoot tip regrowth of Citrus spp. after optimized cryopreservation. After three days of cold-hardening application, shoot tip explants cut in 0.3–0.7 mm size were taken to the sucrose preculture stage on solid WPM nutrient medium containing 0.25 M sucrose. Then, they were kept in droplets containing 3 µL PVS2 for 45 min, and they were incubated on solid WPM nutrient medium containing 1 mg.L−1 BAP and 10 mg.L−1 charcoal for 24 h. Then, they were transferred to WPM solid nutrient medium supplemented with 1 mg.L−1 BAP, 1 mg.L−1 H3BO3, and 10 mg.L−1 charcoal (a) cv. Bodrum Mandarin; (b) cv. Klin Mandarin; (c) white grapefruit and (d) red grapefruit after four weeks incubation; (e) cv. Bodrum Mandarin; (f) cv. Klin Mandarin; (g) red grapefruit after eight weeks incubation, bars 1 cm.
Local necrosis formations and etiolated shoots were observed in the one-week-cold-hardened micro-shoots of all Citrus spp. cultivars. For this reason, the healthy shoot tips could not be cut from them. On the other hand, in the control groups of cold hardening, all of the shoot tips of each cultivar cut from 24 h- or three-days cold hardened micro-shoots obtained 100% regeneration. However, in the post-cryo applications, the regeneration was just only observed in three-day cold-hardened shoot tips (Figure 4), and the viability was not observed in shoot tips obtained from 24 h-cold hardened micro-shoots (Table 2).
3.3. The Effect of Shoot Tip Size on Cryopreservation
In the control groups, 100% shoot regrowth was obtained from all four tested Citrus spp. After cryopreservation, no viability was obtained in the shoot tips larger than 0.7 mm. On the other hand, the regenerations were obtained from all four different Citrus spp. cultivars from the applications where 0.3–0.7 mm cut shoot tips were used (Table 3).
3.4. The Evaluation of Sucrose Preculture
As for the optimized shoot tip preculture with elevated sucrose levels, the shoot tips were transferred to solid WPM medium containing sucrose at three different concentrations (0.1 M, 0.25 M, and 0.5 M) and incubated for 24 h at the standard growth room conditions described above and then they subjected to other cryogenic applications. For the shoot tips tested in the control group, 100% regeneration was obtained after preculture with 0.1 and 0.25 M sucrose for 24 h. However, a significant decrease was observed in the shoot tip regrowth of those precultured with 0.5 M sucrose. Of these three parameters, the samples precultured in a nutrient medium containing 0.25 M sucrose provided the best results in recovery after cryopreservation (Table 4).
3.5. The Evaluation of Cryoprotectant Treatment Time
The PVS2 treatment was also optimized in this study with exposure durations ranging from 0 to 90 min. In the control groups without freeze–thaw cycles, high shoot regrowth levels (83.3–100%) were obtained. However, when the samples were cryopreserved after various PVS2 exposures, the highest shoot regrowth levels ranging from 13.3% to 33.3% were obtained after the 45 min of PVS2 treatment for all four tested genotypes (Table 5, Figure 5 and Figure 6).

Table 5.
The shoot tip regeneration of four different Citrus spp. cultivars tested in different PVS2 treatment times. After PVS2 treatment, the shoot tips were incubated in regeneration medium for the first 24 h, and then they transferred to WPM medium supplemented with 1 mg.L−1 BAP, 10 mg.L−1 activated charcoal, and 1 mg.L−1 H3BO3 medium for four weeks under standard culture conditions.
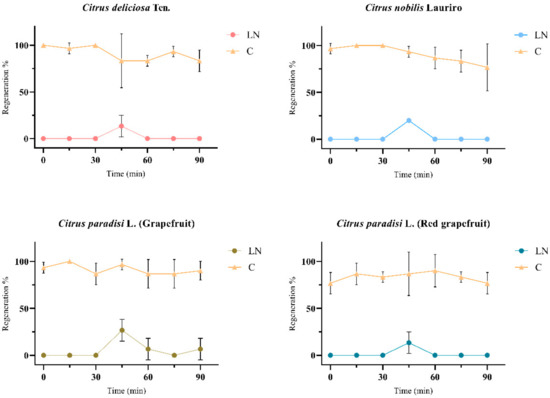
Figure 5.
The shoot-tips regenerations of liquid nitrogen (LN) and control (C) groups of four different Citrus spp. cultivars after cryogenic applications on solid WPM medium supplemented with 1 mg.L−1 BAP, 10 mg.L−1 activated charcoal, and 1 mg.L−1 FeEDDHA. The graphs were formed according to the shoot tip regeneration data, which obtained the best regeneration in the optimized cryogenic applications and then treated with PVS2 at different times. In addition, shoot tips untreated with PVS2 (0 min) were also given as the control group of PVS2 treated with different time in graph. The graphs represent the mean ± SD (p < 0.05).
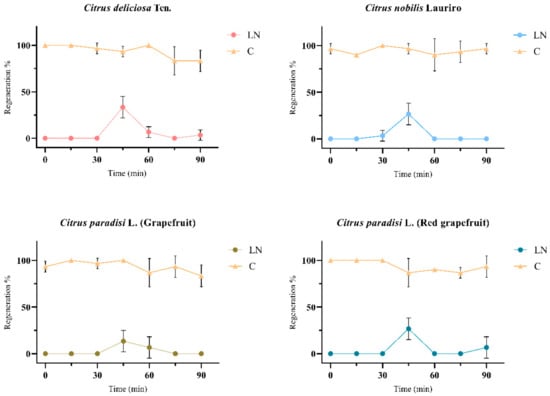
Figure 6.
The shoot-tips regenerations of liquid nitrogen (LN) and control (C) groups of four different Citrus spp. cultivars after cryogenic applications on solid WPM medium supplemented with 1 mg.L−1 BAP, 10 mg.L−1 activated charcoal, and 1 mg.L−1 H3BO3. The graphs were formed according to the shoot tip regeneration data, which obtained the best regeneration in the optimized cryogenic applications and then treated with PVS2 at different times. In addition, shoot tips untreated with PVS2 (0 min) were also given as the control group of PVS2 treated with different time in graph. The graphs represent the mean ± SD (p < 0.05).
3.6. The Evaluation of the Optimized Post Thaw Culture after Cryopreservation
In this study, cryopreserved shoot tips were first post-thaw cultured on solid WPM medium supplemented with 1 mg.L−1 BAP and 10 mg.L−1 charcoal before transferring to eight different nutrient mediums for final recovery (Figure 2). For the four tested cultivars, as the optimized explant size, preculture, PVS2 exposure, etc., have already been presented in the results, the only solid WPM nutrient medium containing 10 mg.L−1 activated charcoal and 1 mg.L−1 BAP supplemented with 1 mg.L−1 H3BO3 (Figure 5) or 1 mg.L−1 FeEDDHA (Figure 6) led to shoot regrowth after cryopreservation. Noticeably, the highest shoot regrowth levels, ranging from 13.3% to 33.3%, were obtained for all the tested cultivars after the post-thaw recovery with 1 mg.L−1 H3BO3 (Figure 6).
4. Discussion
In this study, five critical points following a droplet-vitrification protocol were evaluated for cryopreservation of four Citrus spp. Each critical point was evaluated in different parameters, and the optimum combination was obtained.
First, in the current work, the positive effects of cold-hardening for three days of cold-hardening proved necessary to obtain shoot regrowth after cryopreservation. It has been proven by studies that some genes for the adaptation of plants to low temperatures are expressed during cold adaptation [36]. It is known that proline synthesis is induced during cold hardening, especially in plants, and the accumulation of this amino acid in tissues increases the plant’s resistance to both osmotic stress and low temperatures [37]. In a study on the cryopreservation of the date plant, meristems were used, and it was proven that cold preculture increased the accumulation of proline in the tissues [38]. Similarly, in studies on the cryopreservation of blackberry [39], apple [40], and heaven bamboo [41] plants, it has been proven that cold preculture provides very successful results in recovery after cryopreservation. In our study, cold hardening led to successful shoot recovery after cryopreservation.
The size of the explant used in cryopreservation is another factor affecting the cryopreservation success, and the shoot tip size should be large enough for the tissue to regenerate during the recovery after cryopreservation but small enough to prevent excessive fatal ice crystallization due to the higher water content of the vacuole in the mature tissues during the treatment with liquid nitrogen [42,43]. In this study, successful shoot tip regrowth was only obtained with the use of small shoot tips (0.3–0.7 mm). The small shoot tips consist of more cells that could survive after cryogenic treatments due to the reduced number and size of the vacuoles [44,45]. In another study for cryopreservation of Vitis spp., four different shoot tips, 0.5, 1.0, 1.5, and 2.0 mm, in size, were used, and the best regeneration was obtained from meristems 1 mm in size. An earlier study made for citrus cryopreservation also found that explants cut to 1 mm in size presented better results [22,46]. Nevertheless, some other factors, such as the anatomical and morphological characteristics of the in vitro shoots may also affect the optimized explant size for shoot tip cryopreservation.
Preculturing the shoot tips with a high concentration of sucrose is important to ensure shoot tip recovery after cryopreservation. In this study, 0.25 sucrose treatment for 24 h resulted in improved shoot regrowth after cryopreservation. During the preculture, sugar accumulation in the extracellular compartments will ensure the transfer of the existing water in the vacuoles of the cells to the intercellular compartments [47,48,49]. The critical point to be considered here is to optimize the sugar concentration to be used for sucrose preculture so that it is both high enough to ensure maximum removal of water in the vacuole and low enough to not damage tissues and cells while removing water [20,50]. Similar to our study, the sucrose preculture at 0.25 M to 1.0 M for 24 h has proved effective in the successful cryopreservation of many species such as heavenly bamboo [38], eucalyptus [3], sweet orange [51], and pineapple [52].
In vitrification-based methods for shoot tip cryopreservation, the exposure time to the PVS is a critical point for recovery after cryopreservation [53]. For example, insufficient PVS2 dehydration can cause freezing injuries due to ice crystallization, while excessive PVS exposure would lead to greater osmotic stress and toxicity to the tissues [54]. In the present study, PVS2 exposure for 45 min proved optimal for obtaining shoot regrowth after cryopreservation for all of the tested cultivars. We also applied PVS2 exposure on ice as it could result in the slow penetration of cryoprotectants into the tissues for alleviated osmotic stress and toxicity. Similarly, a PVS2 exposure on ice for 30 min was applied in a droplet-vitrification protocol for the shoot tip cryopreservation of Citrus spp. [22].
In the present study, various chemical additives were tested in the recovery medium for improved shoot tip regrowth after cryopreservation. In the present study, it was observed that the medium containing activated charcoal yielded healthier shoots compared to those without (Table 1, Figure 4). Activated charcoal provides the retention of various chemicals, especially the phenolic components present in the environment. Thus it can prevent shoot tips from the excessive damage caused by pre- and post-cryopreservation applications from inhibiting shoot development by keeping these chemicals [28]. They act against this stress and limit the growth of the plant by passing into the nutrient medium [54]. Moreover, in the present study, successful shoot regrowth was obtained only after the addition of H3BO3 or FeEDDHA in the post-thaw recovery, whereas shoot tip micrografting was applied to assist in the regrowth of Citrus spp. Shoot tips cryopreservation [22]. H3BO3 is important in the recovery after liquid nitrogen may be due to its supporting effect on the cell wall [55]. Therefore, its deficiency can be a limiting factor for growth and development [55,56].In our study, the positive response of H3BO3 in, thus, the use of H3BO3 in cryogenic applications may have ensured the preservation of cell integrity due to the support it provides the cell wall. FeEDDHA, which also led to shoot recovery after cryopreservation, is effective in metabolic activities due to its chelating feature and the chlorophyll ratio in plants. Therefore, it is important to shoot growth [57]. In this context, the current study may suggest that FeEDDHA may have positive effects on post-cryo shoot development, and this is the first use of these substances in shoot tip cryopreservation.
There are two publications by the same researcher in the literature on citrus shoot tip cryopreservation. In one of the studies, shoot tips cryopreserved with PVS2 vitrification [22], and in the second study, shoot tips cryopreserved with droplet vitrification technique [23]; similar to our study, the micrografting method was used for the recovery of shoot tips after liquid nitrogen treatment. In our study, the shoot tips were transferred to different nutrient media with different contents for recovery after thawing (Figure 2). Our study, which is basically based on the same principle, aimed to determine the most suitable recovery medium by transferring the shoot tips to different nutrient media so that they can overcome these post-cryo stresses. However, the method we applied was relatively less time-consuming and easier to manipulate than the micrografting method. Thus, it was implemented more effectively during the study. In addition, with this application, the healthier growth of shoot tips was ensured in the recovery after cryopreservation.
5. Conclusions
The determination of the physical, molecular, and biochemical changes associated with successful regeneration after cryopreservation is a critical point for developing cryopreservation protocols [58,59,60,61]. In this study, an optimized explant size, preculture, and PVS2 exposure, and the addition of H3BO3 and FeEDDHA in the post-thaw recovery, are critical factors affecting the direct shoot tip regrowth of Citrus spp. after cryopreservation. We believe that the combined optimization of these critically important treatments in difficult-to-cryopreservation species, such as Citrus spp., would yield positive results in the cryopreserve of similar species or difficult woody species in the future. In this context, the present study will be a very useful resource for scientists working in similar fields for their future work.
Author Contributions
Conceptualization, E.K. and F.V.D.S.; methodology, D.E.O., E.K. and F.V.D.S.; validation, D.E.O., E.K. and F.V.D.S.; investigation, D.E.O. and E.K.; writing—original draft preparation, D.E.O., E.K. and F.V.D.S.; writing—review and editing, D.E.O., E.K. and F.V.D.S.; supervision, E.K. and F.V.D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Mugla Sitki Kocman University, Scientific Research Projects Coordination Unit (Mugla, Turkey, MSKU-BAP, Project Number: 19/076/08/1/2).
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the Muğla Local Seed Bank staff (Muğla Metropolitan Municipality, Agricultural Services Department) for providing the Citrus spp. seeds and to Naeem Abdul Ghafoor for helping the graph construction from the statistical data in the study.
Conflicts of Interest
The Authors declare to have no conflicts of interests.
References
- Thormann, I.; Dulloo, M.; Engels, J. Techniques for ex situ plant conservation. In Plant Conservation Genetics; Henry, R.J., Ed.; The Haworth Press: New York, NY, USA, 2006; pp. 7–36. [Google Scholar]
- Kaya, E.; Alves, A.; Rodrigues, L.; Jenderek, M.; Hernandez-Ellis, M.; Ozudogru, A.; Ellis, D. Cryopreservation of Eucalyptus genetic resources. CryoLetters 2013, 34, 608–618. [Google Scholar]
- Kaya, E.; Souza, F.V.D. Comparison of two PVS2-based procedures for cryopreservation of commercial sugarcane (Saccharum spp.) germplasm and confirmation of genetic stability after cryopreservation using ISSR markers. Vitr. Cell Dev. Biol. Plant. 2017, 53, 410–417. [Google Scholar] [CrossRef]
- Kalaiselvi, R.; Rajasekar, M.; Gomathi, S. Cryopreservation of plant materials-a review. Int. J. Chem. Stud. 2017, 5, 560–564. [Google Scholar]
- Sakai, A.; Yoshida, S. Survival of plant tissue at super-low temperature VI. Effects of cooling and rewarming rates on survival. Plant Physiol. 1967, 42, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Sakai, A. Cryopreservation of Germplasm of Woody Plants. In Cryopreservation of Plant Germplasm I; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1995; Volume 32, pp. 53–69. [Google Scholar] [CrossRef]
- Gnanapragasam, S.; Vasil, I.K. Ultrastructural changes in suspension culture cells of Panicum maximum during cryopreservation. Plant Cell Rep. 1992, 11, 169–174. [Google Scholar] [CrossRef]
- Volk, G.M.; Walters, C. Plant vitrification solution 2 lowers water content and alters freezing behavior in shoot tips during cryoprotection. Cryobiology 2006, 52, 48–61. [Google Scholar] [CrossRef]
- Panis, B. Sixty years of plant cryopreservation: From freezing hardy mulberry twigs to establishing reference crop collections for future generations. Acta Hortic. 2019, 1234, 1–8. [Google Scholar] [CrossRef]
- Sakai, A. Potentially valuable cryogenie procedures for cryopreservation of cultured plant meristems. In Conservation of Plant Genetic Resources In Vitro; Razdan, M.K., Cocking, E.C., Eds.; Science Publishers Inc.: Enfield, CT, USA, 1997; Volume 1, pp. 53–66. [Google Scholar]
- Reed, B.M. Cryopreservation—Practical Considerations. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; pp. 3–13. [Google Scholar] [CrossRef]
- Panis, B.; Lambardi, M. Status of cryopreservation technologies in plants (crops and forest trees). In The Role of Biotechnology in Exploring and Protecting Agricultural Genetic Resources, 2nd ed.; Ruane, J., Sonnino, A., Eds.; FAO: Rome, Italy, 2006; pp. 61–78. [Google Scholar]
- Nag, K.K.; Street, H.E. Freeze preservation of cultured plant cells: I. the pretreatment phase. Physiol. Plant. 1975, 34, 254–260. [Google Scholar] [CrossRef]
- Zamecnik, J.; Faltus, M.; Bilavcik, A. Vitrification Solutions for Plant Cryopreservation: Modification and Properties. Plants 2021, 10, 2623. [Google Scholar] [CrossRef]
- Kim, H.H.; No, N.Y.; Shin, D.J.; Ko, H.C.; Kang, J.H.; Cho, E.G.; Engelmann, F. Development of alternative plant vitrification solutions to be used in droplet-vitrification procedures. Acta Hortic. 2011, 908, 181–186. [Google Scholar] [CrossRef]
- Ping, K.S.; Poobathy, R.; Zakaria, R.; Subramaniam, S. Development of a PVS2 Droplet-vitrification Cryopreservation Technique for Aranda Broga Blue Orchid Protocorm-like Bodies (PLBs). CryoLetters 2017, 38, 290–298. [Google Scholar]
- Reed, B.M. Shoot Tips Cryopreservation Manual; USDA-ARS National Clonal Germplasm Repository: Corvallis, OR, USA, 2004; pp. 1–39. Available online: https://www.ars.usda.gov/ARSUserFiles/4630/cryopreservation/20.%20Cryopreservation%20Manual%20(2004).pdf (accessed on 25 October 2022).
- Matsumoto, T.; Sakai, A.; Yamada, K. Cryopreservation of in vitro-grown apical meristems of wasabi (Wasabia japonica) by vitrification and subsequent high plant regeneration. Plant Cell Rep. 1994, 13, 442–446. [Google Scholar] [CrossRef]
- Roose, M.L.; Gmitter, F.G.; Lee, R.F.; Hummer, K.E. Conservation of citrus germplasm: An international survey. Acta Hortic 2015, 1101, 33–38. [Google Scholar] [CrossRef]
- Gonzalez-Arnao, M.T.; Engelmann, F. Cryopreservation of plant germplasm using the encapsulation-dehydration technique: Review and case study on sugarcane. CryoLetters 2006, 27, 155–168. [Google Scholar]
- Volk, G.M.; Bonnart, R.; Krueger, R.; Lee, R. Cryopreservation of citrus shoot tips using micrografting for recovery. CryoLetters 2012, 33, 418–426. [Google Scholar]
- Kaya, E.; Souza, F.; Yilmaz-Gokdogan, E.; Ceylan, M.; Jenderek, M. Cryopreservation of Citrus Seed via Dehydration Followedby Immersion in Liquid Nitrogen. Turk. J. Biol. 2017, 41, 242–248. [Google Scholar] [CrossRef]
- Volk, G.M.; Bonnart, R.; Shepherd, A.; Yin, Z.; Lee, R.; Polek, M.L.; Krueger, R. Citrus cryopreservation: Viability of diverse taxa and histological observations. Plant Cell Tissue Organ Cult. 2017, 128, 327–334. [Google Scholar] [CrossRef]
- Wang, M.R.; Lambardi, M.; Engelmann, F.; Pathirana, R.; Panis, B.; Volk, G.M.; Wang, Q.C. Advances in cryopreservation of in vitro-derived propagules: Technologies and explant sources. Plant Cell Tiss Organ Cult. 2021, 144, 7–20. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Bonnart, R.; Volk, G.M. Challenges in implementing plant shoot tip cryopreservation technologies. Plant Cell Tiss Organ Cult. 2021, 144, 21–34. [Google Scholar] [CrossRef]
- Kim, H.H.; Lee, Y.G.; Park, S.U.; Lee, S.C.; Baek, H.J.; Cho, E.G.; Engelmann, F. Development of Alternative Loading Solutions in Droplet-Vitrification Procedures. CryoLetters 2009, 30, 291–299. [Google Scholar]
- Cruz-Cruz, C.A.; González-Arnao, M.T.; Engelmann, F. Biotechnology and conservation of plant biodiversity. Resources 2013, 2, 73–95. [Google Scholar] [CrossRef]
- Kulus, D.; Zalewska, M. Cryopreservation as a tool used in long-term storage of ornamental species—A review. Sci. Hortic. 2014, 168, 88–107. [Google Scholar] [CrossRef]
- Panis, B.; Piette, B.; Swennen, R. Droplet vitrification of apical meristems: A cryopreservation protocol applicable to all Musaceae. Plant Sci. 2005, 168, 45–55. [Google Scholar] [CrossRef]
- Benelli, C.; De Carlo, A.; Engelmann, F. Recent advances in the cryopreservation of shoot-derived germplasm of economically important fruit trees of Actinidia, Diospyros, Malus, Olea, Prunus, Pyrus and Vitis. Biotechnol. Adv. 2013, 31, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Ozudogru, E.A.; Kirdok, E.; Kaya, E.; Capuana, M.; Benelli, C.; Engelmann, F. Cryopreservation of Redwood (Sequoia sempervirens (D. Don.) Endl.) in vitro Buds Using Vitrification-Based Techniques. CryoLetters 2011, 32, 99–110. [Google Scholar]
- Mc Cown, B.H.; Lloyd, G. Woody Plant Medium (WPM)—A Mineral Nutrient Formulation for Microculture of Woody Plant Species. Hort. Sci. 1981, 16, 453. [Google Scholar]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Plant Physiol. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lambardi, M.; Sharma, K.K.; Thorpe, T.A. Optimization of in vitro bud induction and plantlet formation from mature embryos of Aleppo pine (Pinus halepensis Mill.). Vitr. Cell Dev. Biol. Plant. 1993, 29, 189–199. [Google Scholar] [CrossRef]
- Sakai, A.; Kobayashi, S.; Oiyama, I. Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep. 1990, 9, 30–33. [Google Scholar] [CrossRef]
- Carpentier, S.C.; Vertommen, A.; Swennen, R.; Fortes, C.W.; Souza, M.T.; Panis, B. Sugar-Mediated Acclimation: The Importance Of Sucrose Metabolism in Meristems. J. Proteome Res. 2010, 9, 5038–5046. [Google Scholar] [CrossRef]
- Pociecha, E.; Plazek, A.; Janowiak, F.; Zwierzykowski, Z. ABA level, proline and phenolic concentration, and PAL activity induced during cold acclimation in androgenic Festulolium forms with contrasting resistance to frost and pink snow mould (Microdochium nivale). Physiol. Mol. Plant Pathol. 2009, 73, 126–132. [Google Scholar] [CrossRef]
- Fki, L.; Bouaziz, N.; Chkir, O.; Benjemaa-Masmoudi, R.; Rival, A.; Swennen, R.; Drira, N.; Panis, B. Cold hardening and sucrose treatment improve cryopreservation of date palm meristems. Biol. Plant 2012, 57, 375–379. [Google Scholar] [CrossRef]
- Reed, B.M. Cold Hardening vs ABA as a Pretreatment For Meristem Cryopreservation. Hort. Sci. 1990, 25, 1086. [Google Scholar] [CrossRef]
- Kushnarenko, S.V.; Romadanova, N.V.; Reed, B.M. Cold Acclimation Improves Regrowth of Cryopreserved Apple Shoot Tips. CryoLetters 2009, 30, 47–54. [Google Scholar]
- Ozudogru, A.; da Silva, D.P.C.; Kaya, E.; Dradi, G.; Paiva, R.; Lambardi, M. In vitro Conservation and Cryopreservation of Nandina domestica an Outdoor Ornamental Shrub. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 638–645. [Google Scholar] [CrossRef]
- Takada, S.; Tasaka, M. Embryonic Shoot Apical Meristem Formation in Higher Plants. J. Plant Res. 2002, 115, 411–417. [Google Scholar] [CrossRef]
- Benson, E.E. Cryopreservation Of Phytodiversity: A Critical Appraisal of Theory & Practice. CRC Crit. Rev. Plant Sci. 2008, 27, 141–219. [Google Scholar] [CrossRef]
- Helliot, B.; Swenen, R.; Poumay, Y.; Frison, E.; Lepoivre, P.; Panis, B. Ultrastructural Changes Associated with Cryopreservation of Banana (Musa spp.) Highly Proliferating Meristems. Plant Cell Rep. 2003, 21, 690–698. [Google Scholar] [CrossRef]
- Wang, Q.C.; Valkonen, J.P.T. Efficient elimination of sweetpotato little leaf phytoplasma from sweetpotato by cryotherapy of shoot tips. Plant Pathol. 2008, 57, 338–347. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Bonnart, R.; Shepherd, A.; Kretzschmar, A.A.; Volk, G.M. Modifications to a Vitis shoot tip cryopreservation procedure: Effect of shoot tip size and use of cryoplates. CryoLetters 2019, 40, 103–112. [Google Scholar]
- Panis, B.; Strosse, H.; Van Den Hende, S.; Swennen, R. Sucrose preculture to simplify cryopreservation of banana meristem cultures. CryoLetters 2002, 23, 375–384. [Google Scholar]
- Pinker, I.; Halmagyi, A.; Olbricht, K. Effects of sucrose preculture on cryopreservation by droplet-vitrification of strawberry cultivars and morphological stability of cryopreserved plants. CryoLetters 2009, 30, 202–211. [Google Scholar]
- Folgado, R.; Panis, B.; Sergeant, K.; Renaut, J.; Swennen, R.; Hausman, J.F. Unravelling the effect of sucrose and cold pretreatment on cryopreservation of potato through sugar analysis and proteomics. Cryobiology 2015, 71, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Panis, B.; Totte, N.; Van Nimmen, K.; Withers, L.A.; Swennen, R. Cryopreservation of banana (Musa spp.) meristem cultures after preculture on sucrose. Plant Sci. 1996, 121, 95–106. [Google Scholar] [CrossRef]
- Souza, F.V.D.; Kaya, E.; de Jesus Vieira, L.; da Silva Souza, A.; da Silva Carvalho, M.D.J.; Santos, E.B.; Alves, A.A.C.; Ellis, D. Cryopreservation of Hamilin sweet orange [(Citrus sinensis (L.) Osbeck)] embryogenic calli using a modified aluminum cryo-plate technique. Sci. Hortic. 2017, 224, 302–305. [Google Scholar] [CrossRef]
- Souza, F.V.D.; de Souza, E.H.; Kaya, E.; de Jesus Vieira, L.; da Silva, R.L. Cryopreservation of Pineapple Shoot Tips by the Droplet Vitrification Technique. Methods Mol. Biol. 2018, 815, 269–277. [Google Scholar] [CrossRef]
- Sakai, A.; Engelmann, F. Vitrification, encapsulation-vitrification and droplet-vitrification: A review. CryoLetters 2007, 28, 151–172. [Google Scholar]
- Thomas, T.D. The role of activated charcoal in plant tissue culture. Biotechnol. Adv. 2008, 26, 618–631. [Google Scholar] [CrossRef]
- Matoh, T. Boron in plant cell walls. Plant Soil 1997, 193, 59–70. [Google Scholar] [CrossRef]
- Matoh, T.; Kobayashi, M. Boron Function in Plant Cell Walls. In Boron in Plant and Animal Nutrition; Goldbach, H.E., Brown, P.H., Rerkasem, B., Thellier, M., Wimmer, M.A., Bell, R.W., Eds.; Springer: Boston, MA, USA, 2002; pp. 143–155. [Google Scholar] [CrossRef]
- Van der Salm, T.P.; Van der Toorn, C.J.; Hänisch ten Cate, C.H.; Dubois, L.A.; De Vries, D.P.; Dons, H.J. Importance of the iron chelate formula for micropropagation of Rosa hybrida L.‘Moneyway’. Plant Cell Tissue Organ Cult. 1994, 37, 73–77. [Google Scholar] [CrossRef]
- Kaviani, B. Conservation of plant genetic resources by cryopreservation. Aust. J. Crop. Sci. 2011, 5, 778–800. [Google Scholar]
- Kaya, E.; Souza, F.V.D.; Almeida dos Santos-Serejo, J.; Galatali, S. Influence of dehydration on cryopreservation of Musa spp. germplasm. Acta Bot Croat. 2020, 79, 99–104. [Google Scholar] [CrossRef]
- Reed, B.M.; Kovalchuk, I.; Kushnarenko, S.; Meier-Dinkel, A.; Schoenweiss, K.; Pluta, S.; Straczynska, K.; Benson, E.E. Evaluation of critical points in technology transfer of cryopreservation protocols to international plant conservation laboratories. CryoLetters 2004, 25, 341–352. [Google Scholar]
- Panis, B.; Nagel, M.; Van den houwe, I. Challenges and Prospects for the Conservation of Crop Genetic Resources in Field Genebanks, in In Vitro Collections and/or in Liquid Nitrogen. Plants 2020, 9, 1634. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).