BrPARP1, a Poly (ADP-Ribose) Polymerase Gene, Is Involved in Root Development in Brassica rapa under Drought Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Evolution and Gene Collinearity Analysis
2.3. Gene Structure Protein Motif Identification and Protein Functional Domain Analysis
2.4. Subcellular Location
2.5. Histochemical Analysis of GUS Activity
2.6. Drought Stress Conditions and Phenotypic Observation
2.7. Determination of Relative Water Content (RWC)
2.8. Analysis of Proline, Malondialdehyde (MDA), and NAD+ Content
2.9. Quantitative Real-Time PCR Analysis
2.10. Statistical Analysis
3. Results
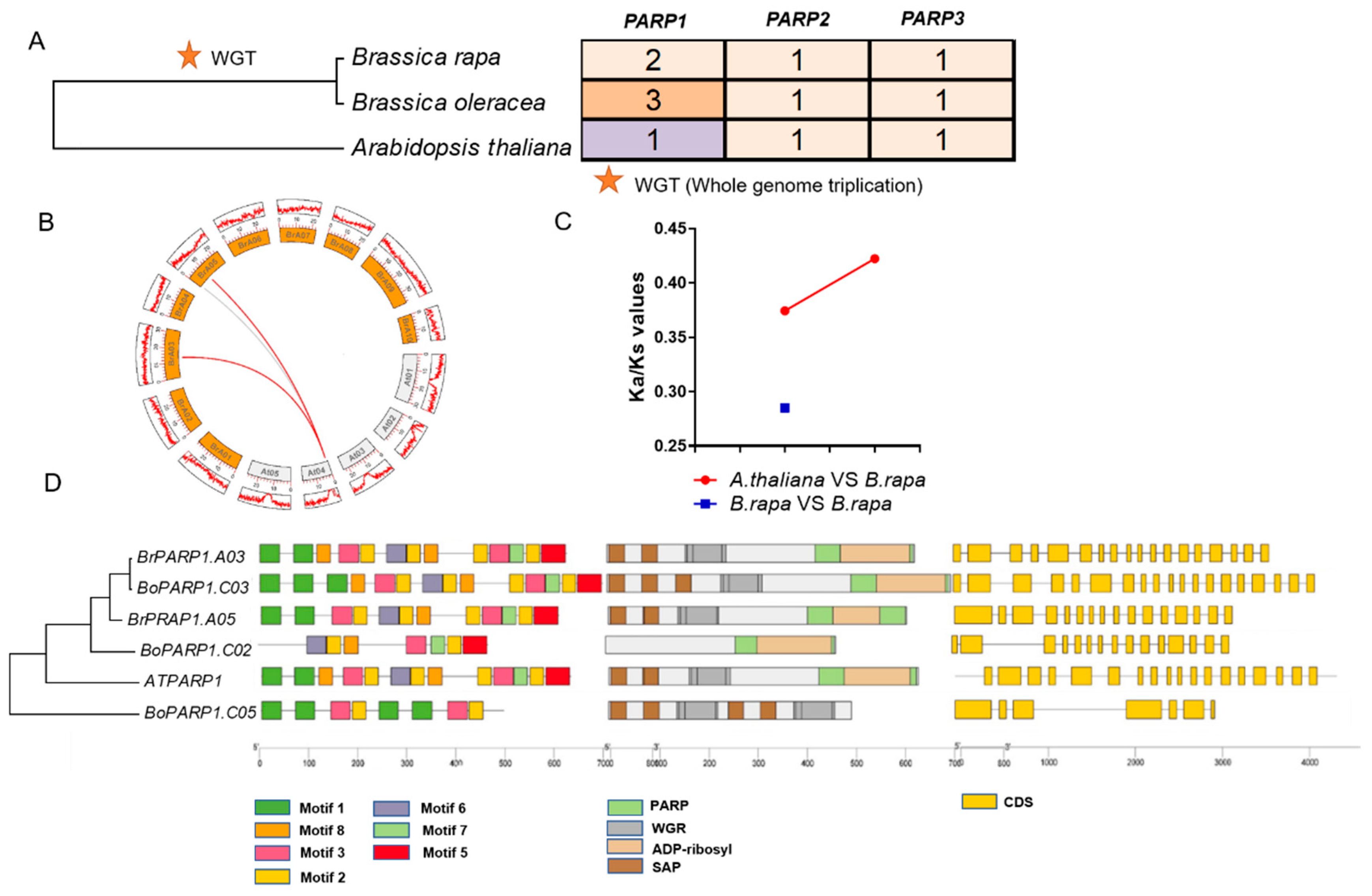
3.1. Conservation of BrPARP Genes following Whole-Genome Triplication Event in B. rapa
3.2. Expression Pattern and Subcellular Localization of BrPARP1 in B. rapa
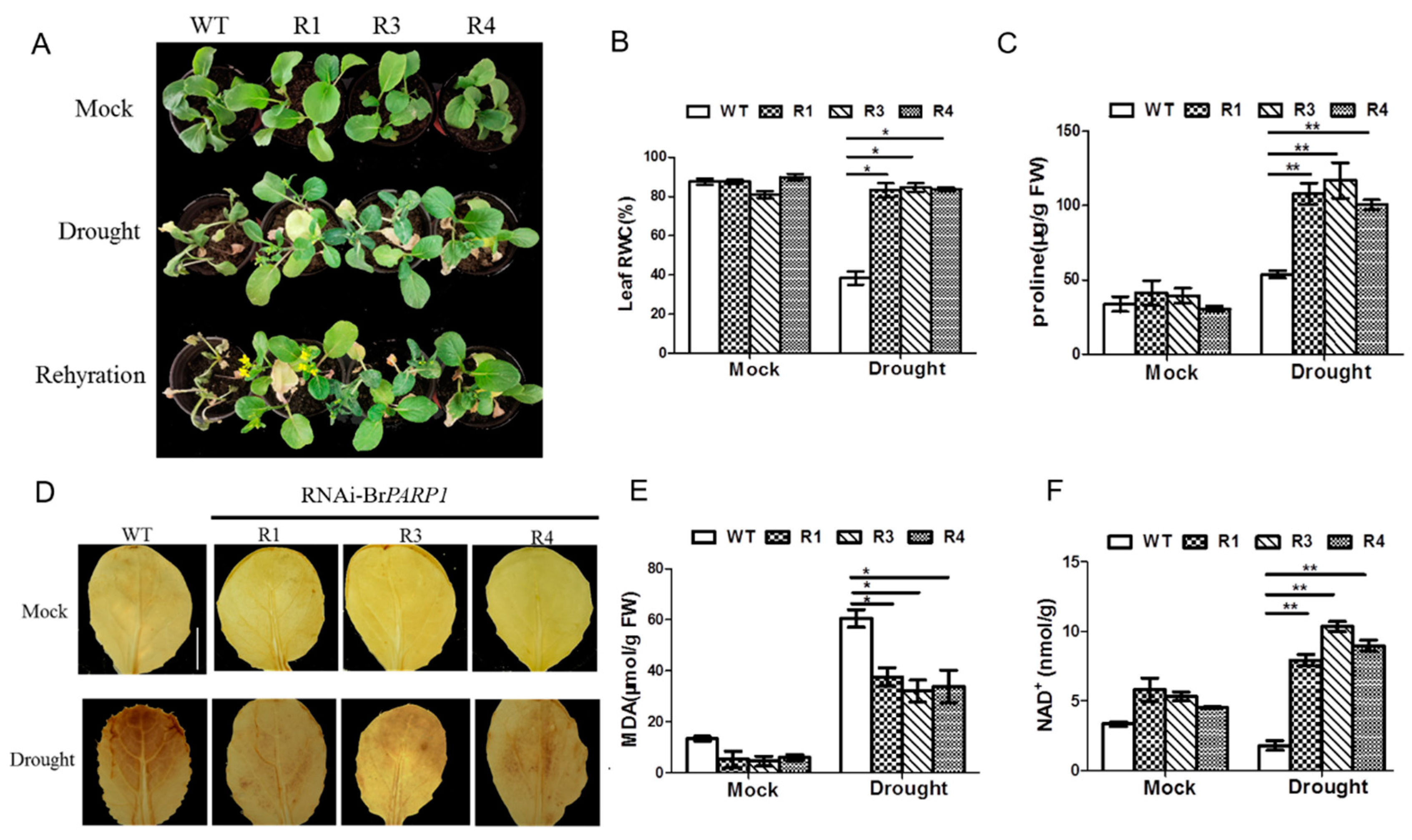
3.3. Silencing of BrPARP1 Enhanced Plant Tolerance in B. rapa under Drought Stress
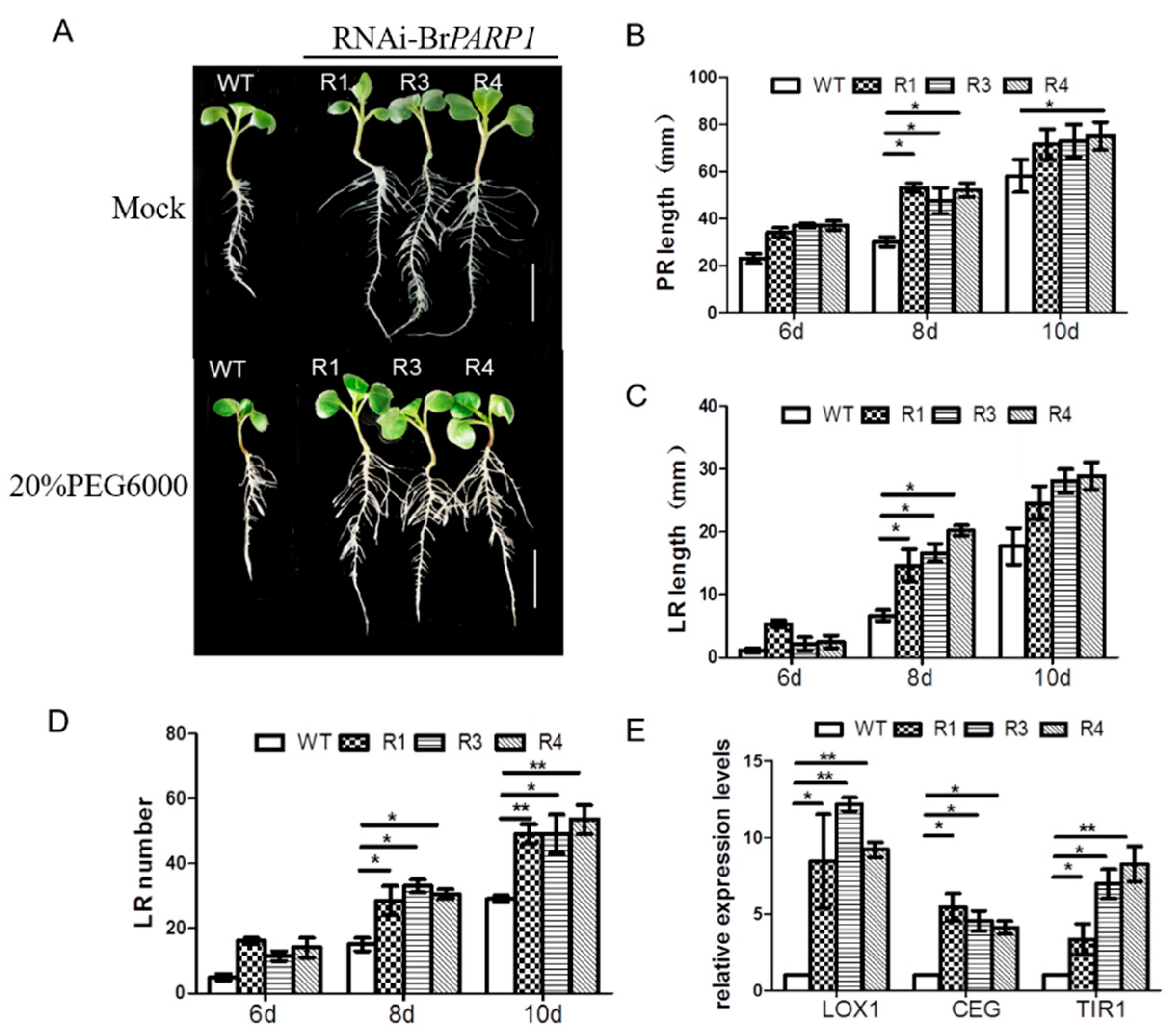
3.4. BrPARP1 Regulated Root Developments in B. rapa under Drought Stress
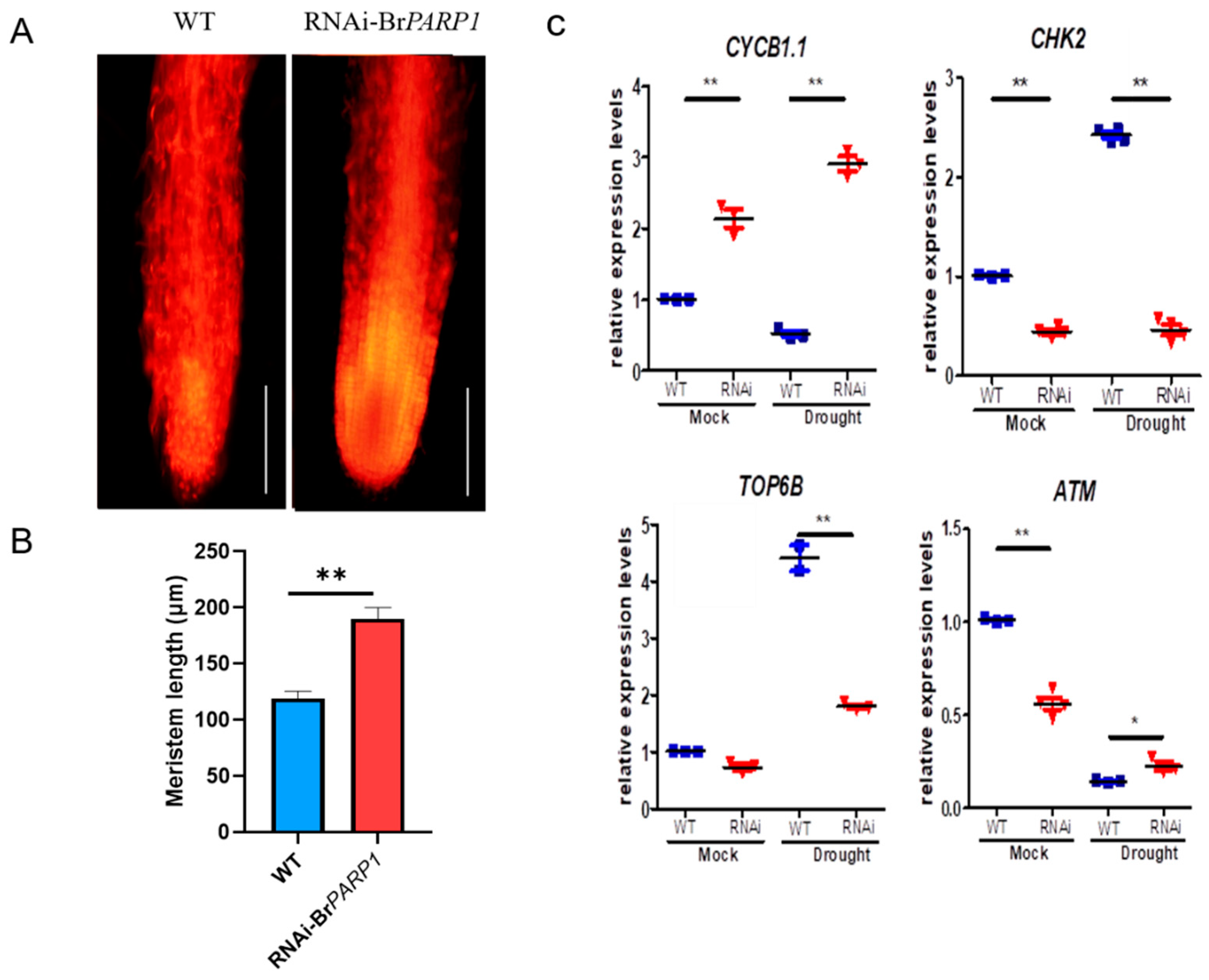
3.5. BrPARP1 Affected Root Cell Division in B. rapa
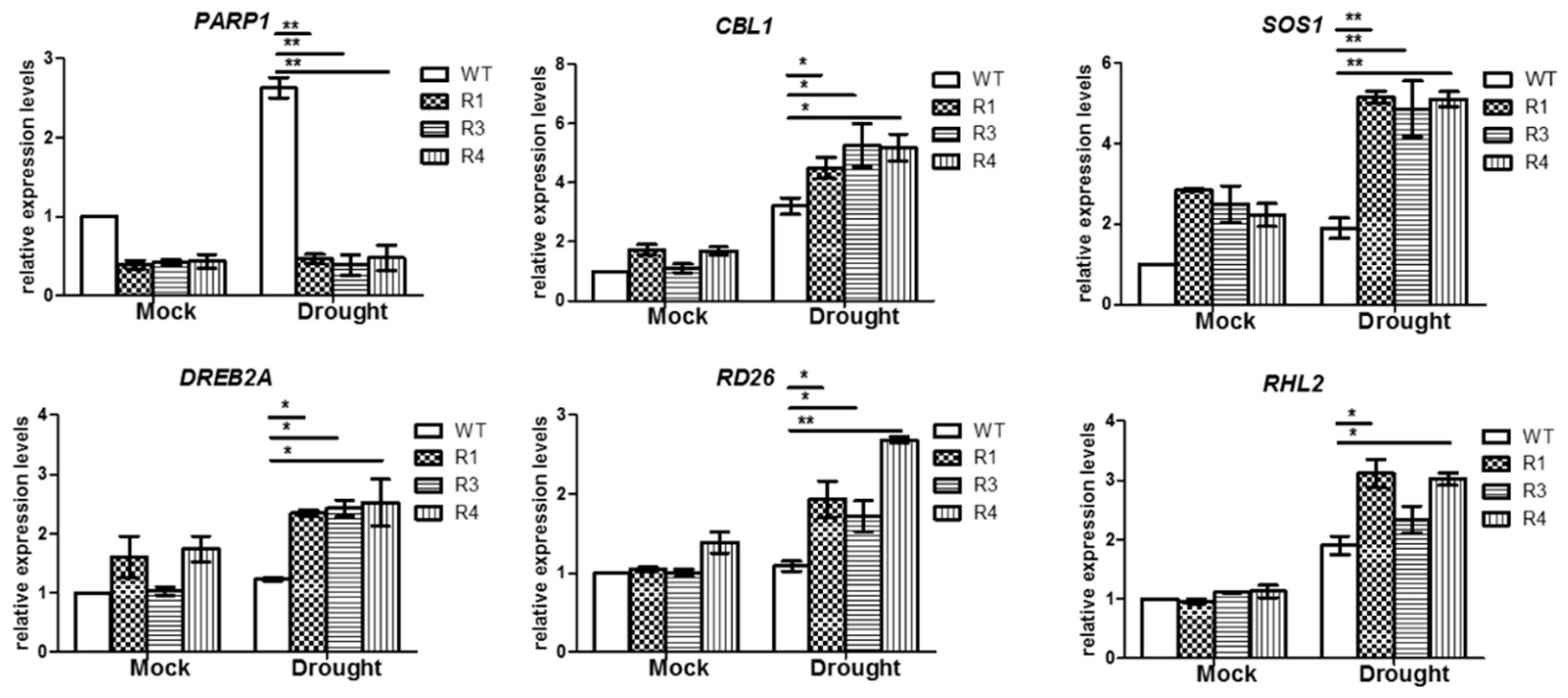
3.6. BrPARP1 Regulated Expression of Stress-Related Genes in B. rapa under Drought Stress
4. Discussion
4.1. BrPARP1 Probably Functions as a Single Copy Gene in B. rapa during Evolution
4.2. BrPARP1 Likely Regulated Root Development Responsive to Drought Stress
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agurla, S.; Gahir, S.; Munemasa, S.; Murata, Y.; Raghavendra, A.S. Mechanism of stomatal closure in plants exposed to drought and cold stress. Adv. Exp. Med. Biol. 2018, 1081, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Kosar, F.; Akram, N.A.; Ashraf, M. Exogenously-applied 5-aminolevulinic acid modulates some key physiological characteristics and antioxidative defense system in spring wheat (Triticum aestivum L.) seedlings under water stress. S. Afr. J. Bot. 2015, 96, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Kamanga, R.M.; Mbega, E.; Ndakidemi, P. Drought Tolerance Mechanisms in Plants: Physiological Responses Associated with Water Deficit Stress in Solanum lycopersicum. Adv. Crop. Sci. Technol. 2018, 6, 1–8. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, X.; Yang, Y.; Liu, C.; Zhou, G.; Wan, H.; Cheng, Y. Tomato WRKY81 acts as a negative regulator for drought tolerance by modulating guard cell H2O2–mediated stomatal closure. Environ. Exp. Bot. 2020, 171, 103960. [Google Scholar] [CrossRef]
- Cadet, J.; Richard Wagner, J. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb. Perspect. Biol. 2013, 5, a012559. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [Green Version]
- Janiak, A.; Kwaśniewski, M.; Szarejko, I. Gene expression regulation in roots under drought. J. Exp. Bot. 2015, 67, 1003–1014. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Xiang, Y.; He, N.; Liu, X.; Liu, H.; Fang, L.; Zhang, F.; Sun, X.; Zhang, D.; Li, X.; et al. Enhanced Vitamin C Production Mediated by an ABA-Induced PTP-like Nucleotidase Improves Plant Drought Tolerance in Arabidopsis and Maize. Mol. Plant 2020, 13, 760–776. [Google Scholar] [CrossRef]
- Gibson, B.A.; Kraus, W.L. New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nat. Rev. Mol. Cell Biol. 2012, 13, 411–424. [Google Scholar] [CrossRef]
- Briggs, A.G.; Bent, A.F. Poly(ADP-ribosyl)ation in plants. Trends Plant Sci. 2011, 16, 372–380. [Google Scholar] [CrossRef]
- Luo, X.; Kraus, W.L. On PAR with PARP: Cellular stress signaling through poly(ADP-ribose) and PARP-1. Genes Dev. 2012, 26, 417–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Keppler, B.D.; Wise, R.R.; Bent, A.F. PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune Responses. PLoS Genet. 2015, 11, e1005200. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, Q.; Liu, W.; Gu, Z.; Wang, W.; Xu, P.; Ma, H.; Ge, X. Poly(ADP-ribose) polymerases regulate cell division and development in Arabidopsis roots. J. Integr. Plant Biol. 2017, 59, 459–474. [Google Scholar] [CrossRef]
- Fenton, A.L.; Shirodkar, P.; MacRae, C.J.; Meng, L.; Anne Koch, C. The PARP3-and ATM-dependent phosphorylation of APLF facilitates DNA double-strand break repair. Nucleic Acids Res. 2013, 41, 4080–4092. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-M.; Shall, S.; O’farrell, M. Poly(ADP-Ribose) Polymerase in Plant Nuclei. Eur. J. Biochem. 1994, 224, 135–142. [Google Scholar] [CrossRef]
- Tian, R.-H.; Zhang, G.-Y.; Yan, C.-H.; Dai, Y.-R. Involvement of poly(ADP-ribose) polymerase and activation of caspase-3-like protease in heat shock-induced apoptosis in tobacco suspension cells. FEBS Lett. 2000, 474, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Pham, P.A.; Wahl, V.; Tohge, T.; De Souza, L.R.; Zhang, Y.; Do, P.T.; Olas, J.J.; Stitt, M.; Araújo, W.L.; Fernie, A.R. Analysis of knockout mutants reveals non-redundant functions of poly(ADP-ribose)polymerase isoforms in Arabidopsis. Plant Mol. Biol. 2015, 89, 319–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams-Phillips, L.; Briggs, A.G.; Bent, A.F. Disruption of Poly(ADP-ribosyl)ation Mechanisms Alters Responses of Arabidopsis to Biotic Stress. Plant Physiol. 2010, 152, 267–280. [Google Scholar] [CrossRef] [Green Version]
- Ricci, J.E.; Waterhouse, N.; Green, D.R. Mitochondrial functions during cell death, a complex (I-V) dilemma. Cell Death Differ. 2003, 10, 488–492. [Google Scholar] [CrossRef] [PubMed]
- De Block, M.; Verduyn, C.; De Brouwer, D.; Cornelissen, M. Poly(ADP-ribose) polymerase in plants affects energy homeotasis, cell death and stress tolerance. Plant J. 2005, 41, 95–106. [Google Scholar] [CrossRef]
- Vanderauwera, S.; De Block, M.; Van De Steene, N.; Van De Cotte, B.; Metzlaff, M.; Van Breusegem, F. Silencing of poly(ADP-ribose) polymerase in plants alters abiotic stress signal transduction. Pro. Nat. Aca. Sci. USA 2007, 104, 15150–15155. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Nekrutenko, A.; Makova, K.D.; Li, W.H. The KA/KS ratio test for assessing the protein-coding potential of genomic regions: An empirical and simulation study. Genome Res. 2002, 12, 198–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, C.D.; Koch, M.A.; Mayer, M.; Mummenhoff, K.; O’Kane, S.L.; Warwick, S.I.; Windham, M.D.; Al-Shehbaz, I.A. Toward a global phylogeny of the Brassicaceae. Mol. Biol. Evol. 2006, 23, 2142–2160. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [Green Version]
- Sparkes, I.A.; Runions, J.; Kearns, A.; Hawes, C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 2006, 1, 2019–2025. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Q.; Guthrie, C.; Sarmast, M.K.; Dehesh, K. BBX19 interacts with CONSTANS to repress FLOWERING LOCUS T transcription, defining a flowering time checkpoint in Arabidopsis. Plant Cell 2014, 26, 3589–3602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, N.; Dasaradhi, P.V.N.; Mohmmed, A.; Malhotra, P.; Bhatnagar, R.K.; Mukherjee, S.K. RNA Interference: Biology, Mechanism, and Applications. Microbiol. Mol. Biol. Rev. 2003, 67, 657–685. [Google Scholar] [CrossRef] [Green Version]
- Riccardi, C.; Nicoletti, I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat. Protoc. 2006, 1, 1458–1461. [Google Scholar] [CrossRef]
- Browne, M.; Yardimci, N.T.; Scoffoni, C.; Jarrahi, M.; Sack, L. Prediction of leaf water potential and relative water content using terahertz radiation spectroscopy. Plant Direct 2020, 4, 1–16. [Google Scholar] [CrossRef]
- Leclercq, J.; Martin, F.; Sanier, C.; Clément-Vidal, A.; Fabre, D.; Oliver, G.; Lardet, L.; Ayar, A.; Peyramard, M.; Montoro, P. Over-expression of a cytosolic isoform of the HbCuZnSOD gene in Hevea brasiliensis changes its response to a water deficit. Plant Mol. Biol. 2012, 80, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Liu, F.; Zhang, C.; Zhang, J.; Feng, H. Non-destructive determination of Malondialdehyde (MDA) distribution in oilseed rape leaves by laboratory scale NIR hyperspectral imaging. Sci. Rep. 2016, 6, 35393. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Fan, Y.; Yu, M.; Liu, M.; Zhang, R.; Sun, W.; Qian, M.; Duan, H.; Chang, W.; Ma, J.; Qu, C.; et al. Genome-wide identification, evolutionary and expression analyses of the GALACTINOL SYNTHASE gene family in rapeseed and tobacco. Int. J. Mol. Sci. 2017, 18, 2768. [Google Scholar] [CrossRef] [Green Version]
- Turner, N.C.; Wright, G.C.; Siddique, K.H.M. Adaptation of grain legumes (pulses) to water-limited environments. Adv. Agron. 2001, 71, 193–231. [Google Scholar]
- Verma, S.; Mishra, S.N. Putrescine alleviation of growth in salt stressed Brassica juncea by inducing antioxidative defense system. J. Plant Physiol. 2005, 162, 669–677. [Google Scholar] [CrossRef]
- Hamani, A.K.M.; Wang, G.; Soothar, M.K.; Shen, X.; Gao, Y.; Qiu, R.; Mehmood, F. Responses of leaf gas exchange attributes, photosynthetic pigments and antioxidant enzymes in NaCl-stressed cotton (Gossypium hirsutum L.) seedlings to exogenous glycine betaine and salicylic acid. BMC Plant Biol. 2020, 20, 434. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Gharavi, R.; Pitta, M.; Gleichmann, M.; Mattson, M.P. Nicotinamide prevents NAD+ depletion and protects neurons against excitotoxicity and cerebral Ischemia: NAD+ consumption by sirt1 may endanger energetically compromised neurons. Neuromol. Med. 2009, 11, 28–42. [Google Scholar] [CrossRef] [Green Version]
- Braidy, N.; Grant, R. Kynurenine pathway metabolism and neuroinflammatory disease. Neural Regen. Res. 2017, 12, 39–42. [Google Scholar] [CrossRef]
- Rowe, J.H.; Topping, J.F.; Liu, J.; Lindsey, K. Abscisic acid regulates root growth under osmotic stress conditions via an interacting hormonal network with cytokinin, ethylene and auxin. New Phytol. 2016, 211, 225–239. [Google Scholar] [CrossRef] [Green Version]
- Kwon, Y.R.; Lee, H.J.; Kim, K.H.; Hong, S.W.; Lee, S.J.; Lee, H. Ectopic expression of Expansin3 or Expansinβ1 causes enhanced hormone and salt stress sensitivity in Arabidopsis. Biotechnol. Lett. 2008, 30, 1281–1288. [Google Scholar] [CrossRef]
- Huang, Z.; Tang, J.; Duan, W.; Wang, Z.; Song, X.; Hou, X. Molecular evolution, characterization, and expression analysis of SnRK2 gene family in Pak-choi (Brassica rapa ssp. chinensis). Front. Plant Sci. 2015, 6, 879. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.; Wu, J.; Wang, X. Genome triplication drove the diversification of Brassica plants. Hortic. Res. 2014, 1, 14024. [Google Scholar] [CrossRef] [Green Version]
- Paterson, A.H.; Chapman, B.A.; Kissinger, J.C.; Bowers, J.E.; Feltus, F.A.; Estill, J.C. Many gene and domain families have convergent fates following independent whole-genome duplication events in Arabidopsis, Oryza, Saccharomyces and Tetraodon. Trends Genet. 2006, 22, 597–602. [Google Scholar] [CrossRef]
- Gout, J.-F.; Kahn, D.; Duret, L. Paramecium Post-Genomics Consortium The Relationship among Gene Expression, the Evolution of Gene Dosage, and the Rate of Protein Evolution. PLoS Genet. 2010, 6, 20. [Google Scholar] [CrossRef]
- Yang, L.; Gaut, B.S. Factors that Contribute to Variation in Evolutionary Rate among Arabidopsis Genes. Mol. Biol. Evol. 2011, 28, 2359–2369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pogson, B.J.; Woo, N.S.; Förster, B.; Small, I. Plastid signalling to the nucleus and beyond. Trends Plant Sci. 2008, 13, 602–609. [Google Scholar] [CrossRef] [PubMed]
- De Smet, R.; Adams, K.L.; Vandepoele, K.; Van Montagu, M.C.E.; Maere, S.; Van de Peer, Y. Convergent gene loss following gene and genome duplications creates single-copy families in flowering plants. Proc. Natl. Acad. Sci. USA 2013, 110, 2898–2903. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Han, Y.; Meng, Z.; Zhou, S.; Xiangzhu, K.; Wei, W. Overexpression of the wheat expansin gene TaEXPA2 improved seed production and drought tolerance in transgenic tobacco plants. PLoS ONE 2016, 11, e0153494. [Google Scholar] [CrossRef]
- Bandeppa, S.; Paul, S.; Thakur, J.K.; Chandrashekar, N.; Umesh, D.K.; Aggarwal, C.; Asha, A. Antioxidant, physiological and biochemical responses of drought susceptible and drought tolerant mustard (Brassica juncea L) genotypes to rhizobacterial inoculation under water deficit stress. Plant Physiol. Biochem. 2019, 143, 19–28. [Google Scholar] [CrossRef]
- Yue, Y.; Zhang, M.; Zhang, J.; Duan, L.; Li, Z. SOS1 gene overexpression increased salt tolerance in transgenic tobacco by maintaining a higher K+/Na+ ratio. J. Plant Physiol. 2012, 169, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, V.; Weinl, S.; Blazevic, D.; D’Angelo, C.; Batistic, O.; Kolukisaoglu, Ü.; Bock, R.; Schulz, B.; Harter, K.; Kudla, J. The calcium sensor CBL1 integrates plant responses to abiotic stresses. Plant J. 2003, 36, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, M.; Kim, J.Y.; Yoon, E.K.; Kim, J.A.; Lee, S.I. BrEXLB1, a brassica rapa expansin-like b1 gene is associated with root development, drought stress response, and seed germination. Genes 2020, 11, 404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, A.C.; Hill, L.J.; Ramsden, D.B. Nicotinamide, NAD(P)(H), and methyl-group homeostasis evolved and became a determinant of ageing diseases: Hypotheses and lessons from pellagra. Curr. Gerontol. Geriatr. Res. 2012, 2012, 302875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altmeyer, M.; Hottiger, M.O. Poly(ADP-ribose) polymerase 1 at the crossroad of metabolic stress and inflammation in aging. Aging 2009, 1, 458–469. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, G.; Jiang, W.; Shi, G.; Tian, Z.; Shang, J.; Xie, Z.; Chen, W.; Tian, B.; Wei, X.; Wei, F.; et al. BrPARP1, a Poly (ADP-Ribose) Polymerase Gene, Is Involved in Root Development in Brassica rapa under Drought Stress. Horticulturae 2022, 8, 78. https://doi.org/10.3390/horticulturae8010078
Cao G, Jiang W, Shi G, Tian Z, Shang J, Xie Z, Chen W, Tian B, Wei X, Wei F, et al. BrPARP1, a Poly (ADP-Ribose) Polymerase Gene, Is Involved in Root Development in Brassica rapa under Drought Stress. Horticulturae. 2022; 8(1):78. https://doi.org/10.3390/horticulturae8010078
Chicago/Turabian StyleCao, Gangqiang, Wenjing Jiang, Gongyao Shi, Zhaoran Tian, Jingjing Shang, Zhengqing Xie, Weiwei Chen, Baoming Tian, Xiaochun Wei, Fang Wei, and et al. 2022. "BrPARP1, a Poly (ADP-Ribose) Polymerase Gene, Is Involved in Root Development in Brassica rapa under Drought Stress" Horticulturae 8, no. 1: 78. https://doi.org/10.3390/horticulturae8010078
APA StyleCao, G., Jiang, W., Shi, G., Tian, Z., Shang, J., Xie, Z., Chen, W., Tian, B., Wei, X., Wei, F., & Gu, H. (2022). BrPARP1, a Poly (ADP-Ribose) Polymerase Gene, Is Involved in Root Development in Brassica rapa under Drought Stress. Horticulturae, 8(1), 78. https://doi.org/10.3390/horticulturae8010078





