Morphological and Molecular Characterization of Nothotylenchus medians and N. similis (Nematoda: Anguinidae) from Southern Alberta, Canada
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Morphological Studies
2.2. DNA Extraction, PCR, and Sequencing
2.3. Phylogenetic Analyses
3. Results
3.1. Description of Nothotylenchus medians
3.2. Description of Nothotylenchus similis
3.3. Host Association and Distribution of the Species in Genus Nothotylenchus
3.4. Molecular Characterization and Phylogenetic Relationships of Nothotylenchus medians and Nothotylenchus similis with Related Anguinid Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicoll, W.; Vermes, V.I. Rhabditida. Anguinidae. Zool. Rec. 1935, 72, 105. [Google Scholar]
- Siddiqi, M. Order Tylenchida. In Tylenchida: Parasites of Plants and Insects, 2nd ed.; CABI: Wallingford, UK, 2000; pp. 86–121. [Google Scholar]
- Filipjev, I. On the classification of the Tylenchinae. Proc. Helminthol. Soc. Wash. 1936, 3, 80–82. [Google Scholar]
- Scopoli, G.A. Introductio Ad Historiam Naturalem Sistens Genera Lapidum, Plantarum et Animalium: Hactenus Detecta, Caracteribus Essentialibus Donata, in Tribus Divisa, Subinde Ad Leges Naturae; Apud Wolfgangum Gerle: Prague, Czech Republic, 1777. [Google Scholar]
- Decraemer, W.; Hunt, D.J. Structure and Classification, 2nd ed.; CABI Publishing: Wallingford, UK, 2013; pp. 3–32. [Google Scholar]
- Thorne, G. Some nematodes of the family Tylenchidae which do not possess a valvular median esophageal bulb. Great Basin Nat. 1941, 2, 37–85. [Google Scholar]
- Fortuner, R.; Maggenti, A.R. A reappraisal of Tylenchina (Nemata). 4. The family Anguinidae Nicoll, 1935 (1926). Rev. Nématol. 1987, 10, 163–176. [Google Scholar]
- Brzeski, M.W. Review of the genus Ditylenchus Filipjev, 1936 (Nematoda: Anguinidae). Rev. Nématol. 1991, 14, 9–59. [Google Scholar]
- Sturhan, D.; Brzeski, M.W. Stem and bulb nematodes, Ditylenchus spp. In Manual of Agricultural Nematology; CRC Press: Boca Raton, FL, USA, 1991; pp. 423–464. [Google Scholar]
- Hashemi, K.; Karegar, A. New and known species of Nothotylenchus Thorne, 1941 (Nematoda: Anguinidae) from Iran with an updated list of species. Zootaxa 2020, 4729, 482–500. [Google Scholar] [CrossRef] [Green Version]
- Massey, C.L. Biology and Taxonomy of Nematode Parasites and Associates of Bark Beetles in the United States; Forest Service, US Department of Agriculture: Washington, DC, USA, 1974. [Google Scholar]
- Gagarin, V. Nematode fauna in rotten wood of birch, aspen, and pine in Borok (Yaroslavl’district, Central Russia). Zool. Zhurnal 1999, 78, 146–157. [Google Scholar]
- Esmaeili, M.; Heydari, R.; Ye, W. Description of a new anguinid nematode, Nothotylenchus phoenixae n. sp.(Nematoda: Anguinidae) associated with palm date trees and its phylogenetic relations within the family Anguinidae. J. Nematol. 2017, 49, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Jalalinasab, P.; Hosseini, M.N.; Heydari, R. Nothotylenchus andrassy n. sp.(Nematoda: Anguinidae) from Northern Iran. J. Nematol. 2018, 50, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-G.; Kim, S.-H.; Lee, J.-H. Ditylenchus acris (Thorne, 1941) Fortuner and Maggenti 1987, A New Strawberry Nematode in Korea. Plant Pathol. J. 2005, 21, 83–85. [Google Scholar] [CrossRef] [Green Version]
- KHAN, S.H. Nothotylenchus acutus n. sp. and N. basiri n. sp.(Nematoda: Nothotylenchinae) from North India. Proc. Helminthol. Soc. Wash. 1965, 32, 90–93. [Google Scholar]
- Mulvey, R. Nematodes of the family Neotylenchidae (Tylenchida: Nematoda) from the Canadian high Arctic. Can. J. Zool. 1969, 47, 1261–1268. [Google Scholar] [CrossRef]
- Kheiri, A. Two new species of Nothotylenchus Thorne, 1941 from Iran and a redescription of N. affinis Thorne, 1941 (Nematoda, Neotylenchidae) with a key to the species of the genus. Nematologica 1971, 16, 591–600. [Google Scholar] [CrossRef]
- Mahajan, R. Scutellonema petersi n. sp. and Nothotylenchus fotedari n. sp., two new nematodes from India. Riv. Parassitol. 1977, 38, 334–337. [Google Scholar]
- Maqbool, M. Three new species of the super family Neotylenchoidea (Nematoda: Tylenchida) from Pakistan. J. Nematol. 1982, 14, 317–323. [Google Scholar]
- Das, D.; Bajaj, H.K. New and known species of Ditylenchus Filipjev, 1936 from Haryana, India. Indian J. Nematol. 2005, 35, 11–23. [Google Scholar]
- Esmaeili, M.; Heydari, R. New record of three species of Ditylenchus Filipjev, 1936 (Nematoda: Anguinidae), with a key to the species reported from Iran. J. Crop Prot. 2016, 5, 565–579. [Google Scholar] [CrossRef] [Green Version]
- Forge, T.A.; Larney, F.J.; Kawchuk, L.M.; Pearson, D.C.; Koch, C.; Blackshaw, R.E. Crop rotation effects on Pratylenchus neglectus populations in the root zone of irrigated potatoes in southern Alberta. Can. J. Plant Pathol. 2015, 37, 363–368. [Google Scholar] [CrossRef]
- Munawar, M.; Yevtushenko, D.P.; Palomares-Rius, J.E.E.; Castillo, P. Species diversity of pin nematodes (Paratylenchus spp.) from potato growing regions of southern Alberta, Canada. Plants 2021, 10, 188. [Google Scholar] [CrossRef]
- Munawar, M.; Yevtushenko, D.P.; Castillo, P. Integrative taxonomy, distribution, and host associations of Geocenamus brevidens and Quinisulcius capitatus from southern Alberta, Canada. J. Nematol. 2021, 53, e2021-15. [Google Scholar] [CrossRef]
- Munawar, M.; Yevtushenko, D.P.; Castillo, P. Overview of the Genus Boleodorus and First Reports of Boleodorus thylactus and B. volutus from Southern Alberta, Canada. Animals 2021, 11, 1760. [Google Scholar] [CrossRef]
- Munawar, M.; Yevtushenko, D.P.; Castillo, P. First Report of Three Tylenchidae Taxa from Southern Alberta, Canada. Horticulturae 2021, 7, 449. [Google Scholar] [CrossRef]
- Thorne, G.; Malek, R.B. Nematodes of the northern Great Plains. Part I. Tylenchida (Nemata: Secernentea). Tech. Bull. S. Dakota Agric. Exp. Stn 1968, 1–111. Available online: https://openprairie.sdstate.edu/cgi/viewcontent.cgi?article=1001&context=agexperimentsta_tb (accessed on 28 November 2021).
- Jenkins, W. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Dis. Report. 1964, 48, 692. [Google Scholar]
- Seinhorst, J. A rapid method for the transfer of nematodes from fixative to anhydrous glycerin. Nematologica 1959, 4, 67–69. [Google Scholar] [CrossRef] [Green Version]
- De Grisse, A.T. Redescription ou modifications de quelques technique utilis [a] es dan l’etude des n [a] ematodes phytoparasitaires. Meded. Rijksfakulteit Landbowwetenschappen Gent. 1969, 34, 351–369. [Google Scholar]
- Maria, M.; Powers, T.; Tian, Z.; Zheng, J. Description and distribution of three criconematids nematodes from Hangzhou, Zhejiang Province, China. J. Nematol. 2018, 50, 183–206. [Google Scholar]
- Holterman, M.; van der Wurff, A.; van den Elsen, S.; van Megen, H.; Bongers, T.; Holovachov, O.; Bakker, J.; Helder, J. Phylum-wide analysis of SSU rDNA reveals deep phylogenetic relationships among nematodes and accelerated evolution toward crown clades. Mol. Biol. Evol. 2006, 23, 1792–1800. [Google Scholar] [CrossRef]
- De Ley, P.; Felix, M.-A.; Frisse, L.; Nadler, S.; Sternberg, P.; Thomas, W.K. Molecular and morphological characterisation of two reproductively isolated species with mirror-image anatomy (Nematoda: Cephalobidae). Nematology 1999, 1, 591–612. [Google Scholar] [CrossRef]
- Ferris, V.; Ferris, J.; Faghihi, J. Variation in spacer ribosomal DNA in some cyst-forming species of plant parasitic nematodes. Fundam. Appl. Nematol. 1993, 16, 177–184. [Google Scholar]
- Curran, J.; Driver, F.; Ballard, J.; Milner, R. Phylogeny of Metarhizium: Analysis of ribosomal DNA sequence data. Mycol. Res. 1994, 98, 547–552. [Google Scholar] [CrossRef]
- Esmaeli, M.; Heydari, R.; Castillo, P.; Palomares-Rius, J.E. Nothotylenchus persicus n. sp.(Nematoda: Anguinidae) from Kermanshah province, Iran. Nematology 2016, 18, 29–37. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, T. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef] [Green Version]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [Green Version]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [Green Version]
- Rambaut, A. FigTree v1. 4.2, a Graphical Viewer of Phylogenetic Trees. 2014. Java. 2018. Available online: https://github.com/rambaut/figtree (accessed on 10 November 2021).
- Lee, J.-H.; Park, S.-J.; Choi, S.-Y. An Unrecorded Species Ditylenchus myceliophagus and Descripton of Ditylenchus acutus (Nematoda: Anguinidae) from Korea. Korean J. Appl. Entomol. 2010, 49, 171–174. [Google Scholar] [CrossRef] [Green Version]
- Sykes, G. A new species of Nothotylenchus (Nematoda: Neotylenchoidea) from England. Syst. Parasitol. 1980, 1, 237–239. [Google Scholar] [CrossRef]
- Ahmadi, S.; Mahdikhani Moghaddam, E.; Baghaee Davari, S. Identification of plant parasitic nematode collected from pulse fields in Northern Khorasan province. Iran. J. Pulses Res. 2014, 5, 111–118. [Google Scholar]
- Andrássy, I. Zur taxonomie der Neotylenchiden. Nematologica 1961, 6, 25–36. [Google Scholar] [CrossRef]
- Tikyani, M.; Khera, S. Nothotylenchus bhatnagari n. sp. from the rhizosphere of great millet (Sorghum vulgare Pers.). Zool. Anz. 1969, 182, 87–91. [Google Scholar]
- Varaprasad, K.; Khan, E.; Lal, M. Paurodontus solani sp. n. and P. citri sp. n.(Nematoda: Neotylenchoidea) with a key to the species of Paurodontus Thorne, 1941. Indian J. Nematol. 1980, 10, 182–188. [Google Scholar]
- Siddiqi, M. Tylenchida: Parasites of Plants and insects—Commonwealth Agricultural Bureaux; CABI: Slough, UK, 1986; p. 645. [Google Scholar]
- Dhanachand, C.; Gambhir, R. One new and two known species of Tylenchida from Manipur, India. Curr. Nematol. 1991, 2, 33–38. [Google Scholar]
- Khan, A.M.; Siddiqi, M.R. Three new species of Nothotylenchus (Nematoda: Neotylenchidae) from North India. Nematologica 1968, 14, 369–376. [Google Scholar]
- Andrássy, I. Beiträge zur Kenntnis der Freilebenden Nematoden Chinas; Annales Historico-Naturales Musei Nationalis Hungarici; 1960; pp. 202–216. [Google Scholar]
- Rühm, W. Die Nematoden der Ipiden. Parasitologische Schriftenreihe; Fischer: Jena, Germany, 1956. [Google Scholar]
- Andrássy, I. Freilebende nematoden aus Rumänien. Sect. Biol. 1959, 2, 3–27. [Google Scholar]
- Mathur, V.; Khan, E.; Prasad, S. Boleodoroides oryzae n. g., n. sp.(Nematoda: Boleodorinae) from Bihar, India. Nematologica 1966, 12, 448–452. [Google Scholar] [CrossRef]
- Das, V.; Shivaswamy, V. Paurodontus brassicae n. sp. and Nothotylenchus singhi n. sp. from South India. Proc. Indian Acad. Parasitol. 1980, 1, 62–65. [Google Scholar]
- Husain, S.; Khan, A. Three new species of neotylenchid nematodes from north India. Indian J. Nematol. 1974, 4, 81–87. [Google Scholar]
- Zeidan, A.; Geraert, E. The Genus Ditylenchus Filipjev, 1936 in Sudan (Nematoda: Tylenchida). Afro-Asian J. Nematol. 1991, 1, 5–14. [Google Scholar]
- Andrássy, I. Erd- und Süsswassernematoden aus Bulgarien. Acta Zool. Acad. Sci. Hung. 1958, 4, 1–88. [Google Scholar]
- Eliashvili, T.; Vacheishvili, L. New species of the nematode Nothotylenchus truncatus sp. nov.(Nematoda: Tylenchida) from Eastern Georgia. Soobshcheniia Akad. Nauk. Gruz. SSR. Bull. Acad. Sci. Georgian SSR 1980, 98, 177–180. [Google Scholar]
- Yokoo, T. Nematological studies on the yellow patch of green grass of the golf link: II. On the nemic-fauna in the green grass of International Golf Link of Isahaya, Nagasaki Prefecture, with descriptions on new species of Neotylenchus (Nematoda: Neotylenchidae). Agric. Bull. Saga Univ. 1968, 26, 9–19. [Google Scholar]
- Truskova, G.; Eroshenko, A. The nematode fauna of herbaceous and ligneous plants in the pine plantations of the Primor’yal. Tr. Biol. Inst. Novaya Seriya 1977, 47, 35–49. [Google Scholar]
- Gagarin, V. Two new species of the genus Nothotylenchus (Nothotylenchidae: Nematoda) and description of males of Tylocephalus auriculatus and Chronogaster typicus. Tr. Gelan 1974, 24, 30–35. [Google Scholar]
- Kumar, P. Nothotylenchus websteri n. sp.(Nematoda: Neotylenchoidea) from Lucknow. Indian J. Parasitol. 1983, 7, 105–107. [Google Scholar]
- Vera, I.C.D.P.; Maggenti, A.R. A new gall-forming species of Anguina Scopoli, 1777 (Nemata: Anguinidae) on bluegrass, Poa annua L., from the coast of California. J. Nematol. 1984, 16, 386–392. [Google Scholar]
- Vovlas, N.; Troccoli, A.; Palomares-Rius, J.E.; De Luca, F.; Liébanas, G.; Landa, B.B.; Subbotin, S.A.; Castillo, P. Ditylenchus gigas n. sp. parasitizing broad bean: A new stem nematode singled out from the Ditylenchus dipsaci species complex using a polyphasic approach with molecular phylogeny. Plant Pathol. 2011, 60, 762–775. [Google Scholar] [CrossRef]
- Greeff, R. Über Nematoden in Wurzelanschwellungen (Gallen) verschiedener Pflanzen. Sitz. Der Ges. Zür Beförderung Der Gesamten Nat. Zu Marburg 1872, 11, 172–174. [Google Scholar]
- Paramanov, A. A Critical Review of the Suborder Tylenchina (Filipjev, 1934)(Nematoda: Secernentea). Akad. Nauk. SSSR Tr. Gel’mint. Lab. 1967, 18, 78–101. [Google Scholar]
- Steinbuch, J.G. Das Grasaelchen, Vibrio Agrostis; Der Naturforscher: Halle, Germany, 1799. [Google Scholar]
- Chitwood, B. Nomenclatorial notes, I. Proc. Helminthol. Soc. Wash. 1935, 2, 51–54. [Google Scholar]
- Heydari, R.; Esmaeili, M. A New Anguinid Nematode from Iran. Available online: https://www.ncbi.nlm.nih.gov/nucleotide/MH243748.1?report=genbank&log$=nucltop&blast_rank=1&RID=U7MNHB1D01R (accessed on 28 November 2021).
- Mejia-Madrid, H.H. Soil Nematode Abundance and Diversity from Four Vegetation Types in Central Mexico. Available online: https://www.ncbi.nlm.nih.gov/nucleotide/KY750848.1?report=genbank&log$=nucltop&blast_rank=1&RID=U7MR16XR01R (accessed on 28 November 2021).
- Subbotin, S.A.; Vovlas, N.; Crozzoli, R.; Sturhan, D.; Lamberti, F.; Moens, M.; Baldwin, J.G. Phylogeny of Criconematina Siddiqi, 1980 (Nematoda: Tylenchida) based on morphology and D2–D3 expansion segments of the 28S-rRNA gene sequences with application of a secondary structure model. Nematology 2005, 7, 927–944. [Google Scholar]
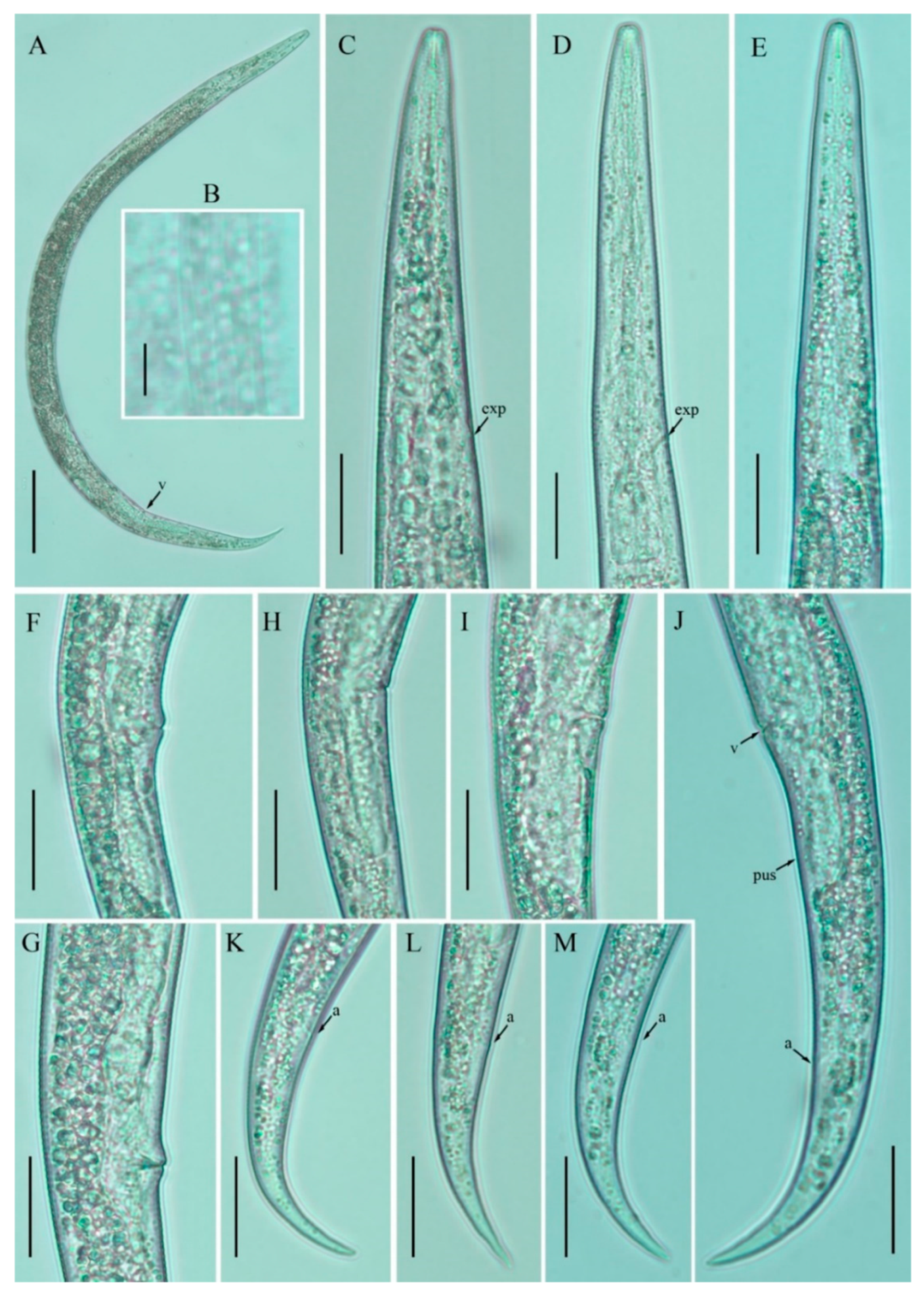
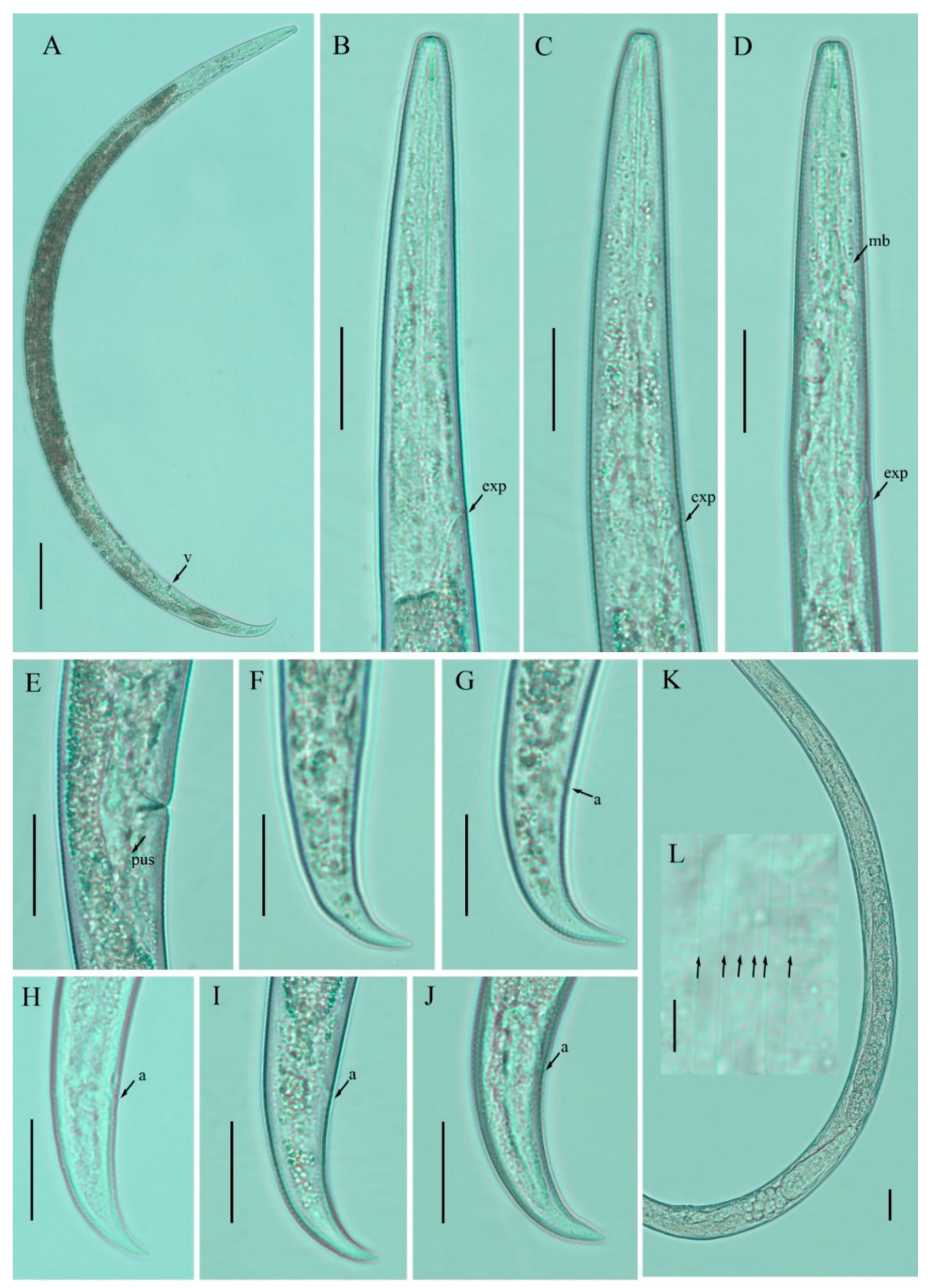
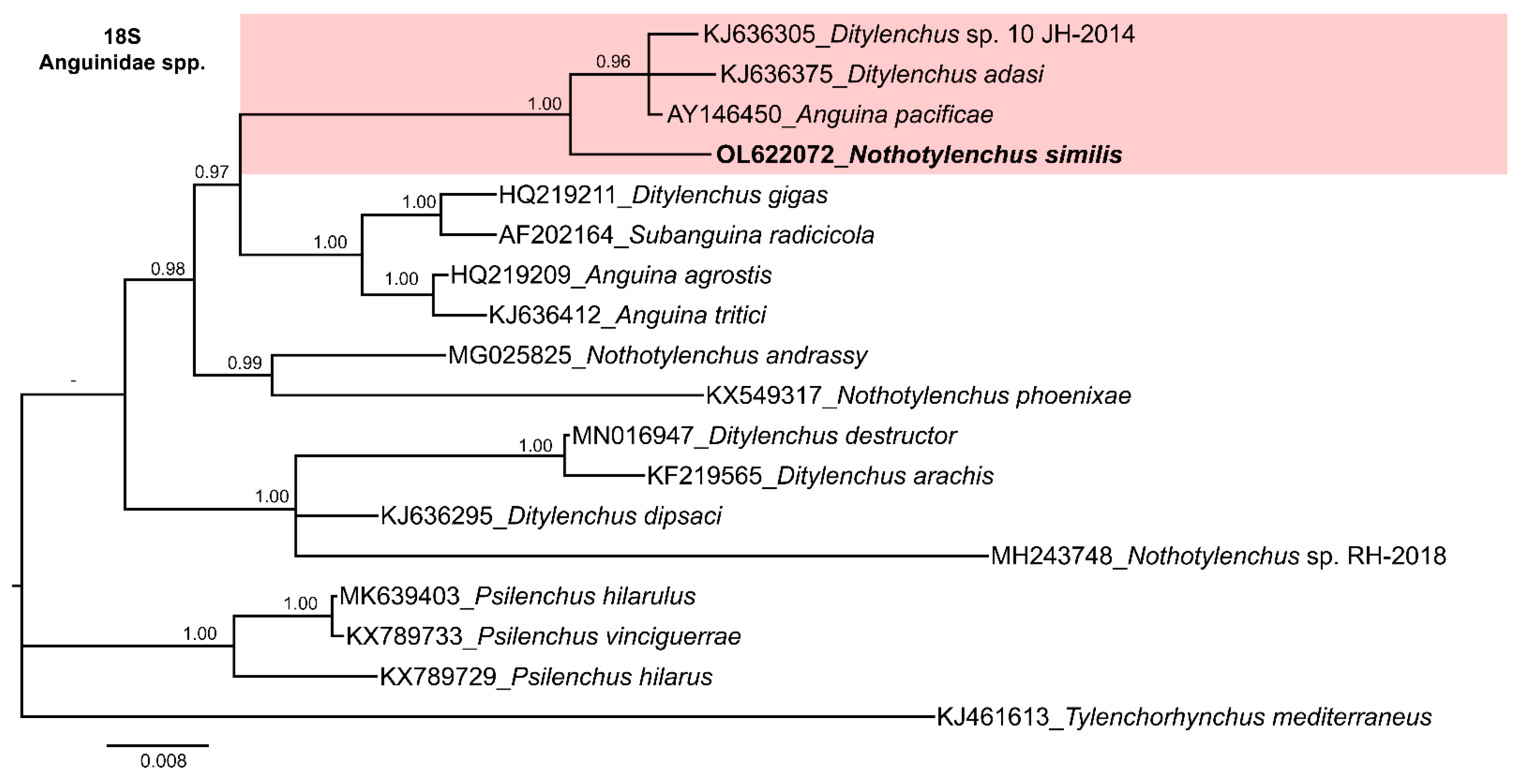
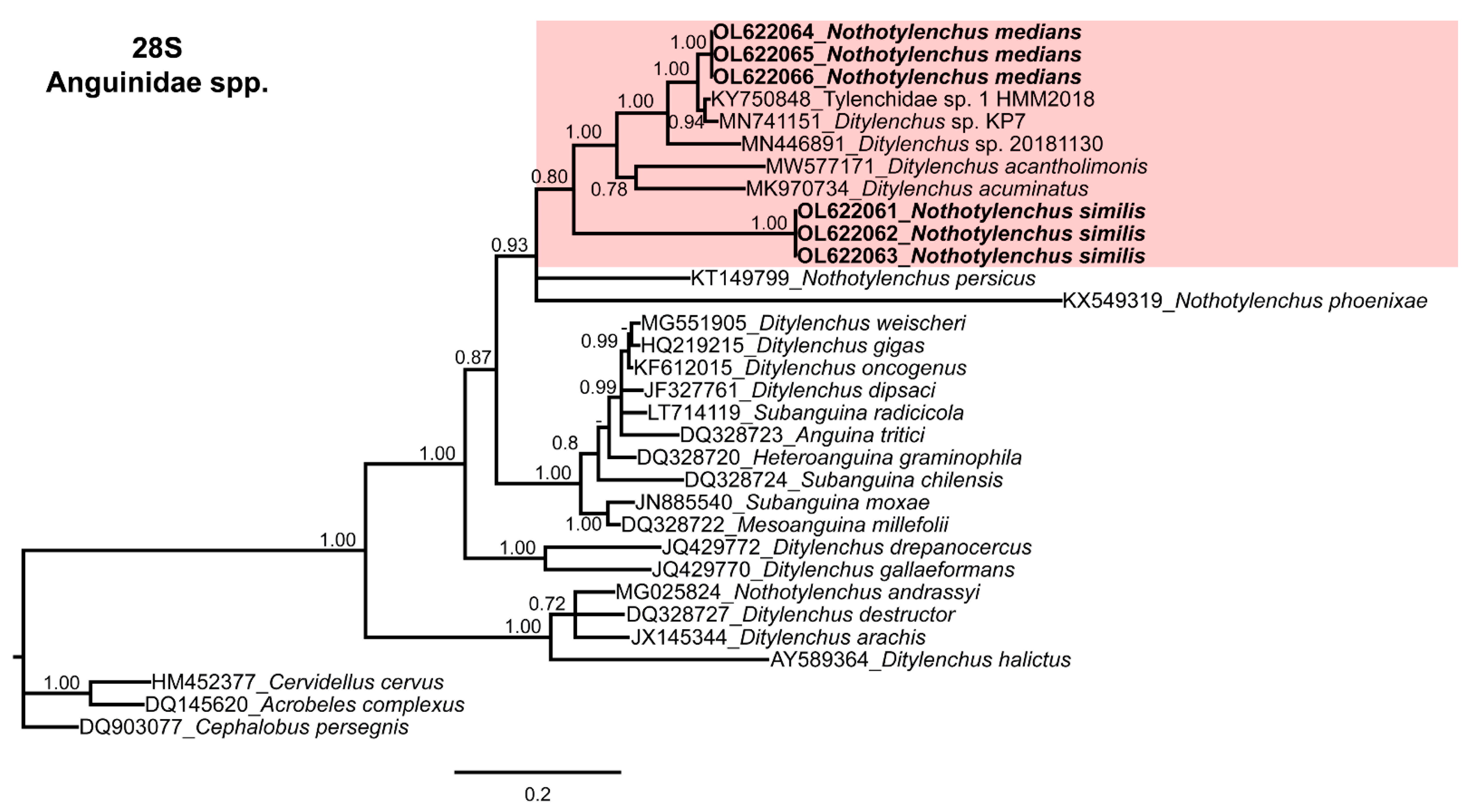
| Characters | This Study | Thorne and Malek [28] | Brzeski [8] | |
|---|---|---|---|---|
| Locality | Canadian population | USA | Poland | Syria |
| Host | Artiplex sp. | Cultivated field | Unknown | |
| n | 16 | – | 13 | 3 |
| Body length | 598.3 ± 48.9 (536.0–740.0) | 660 | 734 (660–803) | 612–624 |
| a | 25.5 ± 2.6 (21.3–32.2) | 32 | 31 (25–36) | 30–32 |
| b | 6.1 ± 0.3 (5.5–6.6) | 6.3 | 5.6 (5.0–6.0) | 4.7–5.2 |
| c | 11.0 ± 0.9 (9.4–12.0) | 14 | 12.5 (11.4–13.2) | 3.2–3.5 |
| c’ | 4.6 ± 0.4 (3.8–5.3) | 2.8–5.8 | 4.1 (3.7–4.6) | 13.8 (12.6–14.5) |
| MB | 42.0 ± 2.1 (35.3–45.0) | – | 39 (35–42) | 38–42 |
| V | 79.2 ± 1.0 (77.1–81.0) | 76 | 82 (79–83) | – |
| Lip height | 2.2 ± 0.1 (2.0–2.4) | – | – | – |
| Lip width | 5.3 ± 0.3 (5.0–5.8) | – | – | – |
| Stylet length | 8.1 ± 0.6 (7.3–9.5) | 8.0 | 7.3 (6.5–8.0) | 6.8 (6.0–7.5) |
| Anterior end to excretory pore | 82.6 ± 4.7 (79.0–98.0) | – | 99 (88–105) | 91–96 |
| Pharynx length | 98.0 ± 5.7 (88.0–116.0) | – | 131 (111–144) | 124 (119–130) |
| Maximum body width | 23.6 ± 2.4 (19.3–28.0) | – | – | – |
| Vulva body width | 21.4 ± 1.9 (18.3–25.0) | – | – | – |
| Post-vulval uterine sac (PUS) length | 30.9 ± 6.9 (20.0–44.0) | – | – | – |
| Distance from vulva to anus | 69.0 ± 6.7 (60.0–88.0) | – | – | – |
| Distance from vulva to tail terminus | 124.5 ± 10.5 (110.0–150.0) | – | – | – |
| Anal body width | 12.2 ± 1.0 (10.0–13.6) | – | – | – |
| Tail length | 56.0 ± 5.8 (46.0–66.0) | – | 59 (52–63) | 42–64 |
| Characters | Canadian Population | Thorne and Malek [28] |
|---|---|---|
| Host | Grasses | Pasture grass |
| n | 16 | – |
| Body length | 783.0 ± 48.5 (693.0–885.0) | 900 |
| a | 30.0 ± 3.4 (25.0–38.4) | 24 |
| b | 6.4 ± 0.4 (5.8–7.3) | 4.3 |
| c | 16.5 ± 1.1 (14.5–18.5) | 17 |
| c’ | 2.9 ± 0.2 (2.4–3.3) | 2.9 |
| MB | 43.0 ± 2.0 (38.5–46.4) | – |
| V | 84.1 ± 0.9 (82.5–85.5) | 84 |
| Lip height | 2.7 ± 0.2 (2.3–2.9) | – |
| Lip width | 6.9 ± 0.5 (6.4–7.5) | – |
| Stylet length | 9.8 ± 0.5 (8.6–10.6) | 10 |
| Anterior end to excretory pore | 108.1 ± 5.5 (100.0–116.0) | – |
| Pharynx length | 123.1 ± 7.0 (112.0–136.0) | – |
| Maximum body width | 26.4 ± 2.8 (22.0–31.0) | – |
| Vulva body width | 23.3 ± 2.4 (20.0–27.0) | – |
| Post-vulval uterine sac (PUS) length | 25.7 ± 2.7 (21.0–31.0) | – |
| Distance from vulva to anus | 77.0 ± 6.1 (70.0–88.0) | – |
| Distance from vulva to tail terminus | 124.6 ± 6.9 (115.0–137.0) | – |
| Anal body width | 16.2 ± 0.9 (15.0–17.6) | – |
| Tail length | 47.6 ± 2.9 (40.0–52.0) | – |
| Species Name | Host | Location | Reference | |
|---|---|---|---|---|
| 1 | N. acris | Rhizosphere of Alfalfa crowns, red clover roots, and sugar beets. | USA | Thorne [6] |
| Native prairie sod. | USA | Thorne and Malek [28] | ||
| Grass roots growing in a dry pond bed. | Canada | Mulvey [17] | ||
| 2 | N. acutus | Rhizosphere of deciduous shrub (Plumeria acutifolia). | India | Khan [16] |
| Rhizosphere of wild hay. | Canada | Mulvey [17] | ||
| Cultivated sandy soil. | Poland | Brzeski [8] | ||
| Roots around dwarf shrub (Thuja orientalis L.). | Korea | Lee et al. [44] | ||
| 3 | N. adasi | Loamy sand around roots of Sugar beet (Beta vulgaris). | England | Sykes [45] |
| Wet sandy low peat soil. | Poland | Brzeski [8] | ||
| Pulse fields. | Iran | Ahmadi et al. [46] | ||
| 4 | N. affinis | Dying Alfalfa crowns. | USA | Thorne [6] |
| – | Iran | Kheiri [18] | ||
| 5 | N. andrassy | Moss samples (Sphagnum sp.). | Iran | Jalalinasab, Nassaj-Hosseini, and Heydari [14] |
| 6 | N. antricolus | Wet and rotten pieces of wood, that wood was found in the Kölyok cave located in Mànfa Southern Hungary. | Hungary | Andrássy [47] |
| 7 | N. attenuatus | Marshy area, wet grass, and moss, near Skeleton Lake. | Canada | Mulvey [17] |
| 8 | N. basiri | The soil around the roots of the mango tree (Mangifera indica L.). | India | Khan [16] |
| 9 | N. brzeskii | Rhizosphere of Alfafa sp. | Iran | Hashemi and Karegar [10] |
| 10 | N. bhatnagari | Rhizosphere of sorghum (Sorghum vulgare). | India | Tikyani and Khera [48] |
| 11 | N. bhattii | Rhizosphere of Bermuda grass (Cynodon dactylon L.). | India | (Das and Bajaj) Hashemi and Karegar [10,21] |
| 12 | N. boroki | Pine and Birchwood dead and rotten material. | Central Russia | Gagarin [12] |
| 13 | N. citri | Rhizosphere of Citrus sp. | India | (Varaprasad, Khan, and Lal) Siddiqi [49,50] |
| 14 | N. clavatus | Roots of paddy (Oryza sativa L.). | India | Dhanachand and Gambhir [51] |
| 15 | N. cylindricollis | Soil and organic debris at the base of Ananas sp. | Paraguay | Thorne [6] |
| 16 | N. cylindricus | Rhizosphere of cabbage (Brassica oleracea L.) and potato (Solanuna tuberorusum L.). | India | Khan and Siddiqi [52] |
| 17 | N. danubialis | Periphyton found in the Danube River. | Hungary | Andrássy [53] |
| 18 | N. drymocolus | Isolated from decaying organic matter of bark beetles (Orthotomicus suturalis; Hylaster ater, H. attenuatus) residing in spruce and pine trees, respectively. | Germany | Rühm [54] |
| 19 | N. fotedari | Fallow field soil, previously cultivated with Mung Bean (Phaseolus aureus). | India | Mahajan [19] |
| 20 | N. geraerti | Rhizosphere of potato (Solanum tuburosum L.) and wheat (Triticum aestivum L.). | Iran | Kheiri [18] |
| – | Iran | Hashemi and Karegar [10] | ||
| 21 | N. goldeni | Rhizosphere of apple (Pyrus malus). | Pakistan | Maqbool [20] |
| 22 | N. hexaglyphus | Rhizosphere of potato (Solanum tuberorum L.). | India | Khan and Siddiqi [52] |
| Soil near a water canal. | Poland | Brzeski [8] | ||
| Rhizosphere of palm. | Iran | Esmaeili and Heydari [22] | ||
| – | Iran | Hashemi and Karegar [10] | ||
| 23 | N. loksai | Grass rhizosphere (Sesleria sp.). | Romania | Andrássy [55] |
| 24 | N. medians | Cultivated field. | USA | Thorne and Malek [28] |
| Poland, Syria | Brzeski [8] | |||
| Artiplex sp. | Canada | This study | ||
| 25 | N. oryzae | Roots of paddy (Oryza sativa L.). | India | (Mathur, Khan, and Prasad) Siddiqi [50,56] |
| 26 | N. persicus | Rhizosphere of grapevine (Vitis vinifera sp.). | Iran | Esmaeili, Heydari, Castillo, and Palomares-Rius [37] |
| 27 | N. petilus | Associated with Dendroctomis terehrans in loblolly pine. (Pinus taeda L.). | USA | Massey [11] |
| 28 | N. phoenixae | Rhizosphere of palm trees (Phoenix dactylifera L.). | Iran | Esmaeili, Heydari, and Ye [13] |
| 29 | N. siddiqii | Rhizosphere of Alfalfa. | Iran | Hashemi and Karegar [10] |
| 30 | N. similis | Nature pasture grass. | USA | Thorne and Malek [28] |
| Grasses | Canada | This study | ||
| 31 | N. singhi | Rhizosphere of Cabbage (Brassica olearacea L.). | India | Das and Shivaswamy [57] |
| 32 | N. solani | Rhizosphere of eggplant (Solanum melongena L.). | India | (Varaprasad, Khan, and Lal) Siddiqi [49,50] |
| 33 | N. taylori | Rhizosphere of potato (Solanum tuberosum L.). | India | Husain and Khan [58] |
| Rhizosphere of Eucalyptus sp. | Sudan | Zeidan and Geraert [59] | ||
| 34 | N. tenuis | Pinewood dead and rotten material. | Central Russia | Gagarin [12] |
| 35 | N. thornei | Moss collected from the forest. | Germany | Andrássy [60] |
| 36 | N. truncatus | Rhizosphere of apple and pear trees. | Georgia | Eliashvili and Vacheishvili [61] |
| 37 | N. tuberosus | Rhizosphere of potatoes (Solanum tuberosum L.). | Iran | Kheiri [18] |
| 38 | N. turfus | Rhizosphere of grasses. | Japan | (Yokoo) Siddiqi [50,62] |
| 39 | N. uniformis | Thin branches of a large tree. | Far Eastern Russia | Truskova and Eroshenko [63] |
| 40 | N. utschini | Wild rice (Zizania latifolia Griseb) growing on the bank of a water reservoir. | Central Russia | Gagarin [64] |
| 41 | N. websteri | Rhizosphere of pea (Pisum sativum L.). | India | Kumar [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munawar, M.; Rahman, A.U.; Castillo, P.; Yevtushenko, D.P. Morphological and Molecular Characterization of Nothotylenchus medians and N. similis (Nematoda: Anguinidae) from Southern Alberta, Canada. Horticulturae 2022, 8, 74. https://doi.org/10.3390/horticulturae8010074
Munawar M, Rahman AU, Castillo P, Yevtushenko DP. Morphological and Molecular Characterization of Nothotylenchus medians and N. similis (Nematoda: Anguinidae) from Southern Alberta, Canada. Horticulturae. 2022; 8(1):74. https://doi.org/10.3390/horticulturae8010074
Chicago/Turabian StyleMunawar, Maria, Atta Ur Rahman, Pablo Castillo, and Dmytro P. Yevtushenko. 2022. "Morphological and Molecular Characterization of Nothotylenchus medians and N. similis (Nematoda: Anguinidae) from Southern Alberta, Canada" Horticulturae 8, no. 1: 74. https://doi.org/10.3390/horticulturae8010074
APA StyleMunawar, M., Rahman, A. U., Castillo, P., & Yevtushenko, D. P. (2022). Morphological and Molecular Characterization of Nothotylenchus medians and N. similis (Nematoda: Anguinidae) from Southern Alberta, Canada. Horticulturae, 8(1), 74. https://doi.org/10.3390/horticulturae8010074







