Abstract
Microgreens are considered products of high biological value because they contain natural and beneficial metabolites and antioxidants in high amounts; also, consumers appreciate them very much for their aromas. In this work, we focused our attention on the volatile organic compounds (VOCs) emitted from whole fresh leaves of two Chinese basil varieties (Perilla frutescens var. frutescens and var. crispa) at the microgreens stage; to show that the emission is microgreens specific we tested whether this capacity remains during subsequent growth of the plants. We found differences between the VOCs produced by the leaves of the two varieties at the microgreens stage and significantly reduced emission after development (additional four weeks of growth) particularly for the green variety (var. frutescens). The main volatiles emitted by whole leaves were D-Limonene for the red variety (crispa) and 2-Hexanoylfuran for the green one. In addition, the total phenolic content (TPC) and antioxidant power increase in adult leaves. These results clearly indicate that the particular smell of microgreens Perilla leaves depends on the specific variety and is not related to the amount of total phenols or antioxidant capacity of the leaves.
1. Introduction
Microgreens are young vegetable seedlings harvested generally after the complete development of the cotyledons and/or the formation of the first leaves; they are considered innovative and emerging foods. In recent years they have aroused interest from consumers thanks to their sensory and visual attributes, as well as nutritional due to the high contents of bioactive molecules, such as polyphenols, carotenoids, vitamins and other antioxidant compounds [1,2,3,4]. In addition, they add colors and flavor to foods. Microgreens is a marketing term used to describe a product category distinct from sprouts that have no specific legal definition.
Perilla frutescens (L.) Britt, commonly called perilla, perilla mint, or Chinese basil in western countries, zisu in China and shiso in Japan [5], is an annual herbaceous plant of the Lamiaceae family widely cultivated in Asia and used as an edible vegetable for its pleasant taste and as a traditional medicinal plant for its many health benefits, as well as for coloring and the cosmetic industry (skin creams, soaps, etc.) [5,6,7]. In fact, recent pharmacological studies have shown that the leaves of P. frutescens carry rich bioactive components, like phenolics, flavonoids, anthocyanins, tannins, and exhibits a variety of activities, including antioxidant, antiallergy, anti-inflammation, antitumor, and antibacterial activities [8,9,10,11,12] and its essential oil is promising for the treatment of disorders caused by depression [13].
P. frutescens var. frutescens and var. crispa, which are characterized by green and red leaves, respectively, have recently gained an increasingly broad acceptance as a novel crop so that they were recently studied by Rouphael et al. [14] to test the effects of moderate salt stress on the content of bioactive compounds and secondary metabolites.
Given also the increasing interest in microgreens, the aim of the present work was essential to evaluate the aromatic profile of intact leaves of P. frutescens var. frutescens and var. crispa at the microgreens stage and at an adult stage (after 4 weeks of further growth) to show if the VOCs emission is a peculiar characteristic of the microgreens and the differences between the two varieties To our knowledge, VOCs produced by the whole (intact, not processed or dehydrated or subject to the extraction of essential oils) Perilla leaves are being analyzed for the first time to represent the odor perceived by consumers of microgreens before chewing.
2. Materials and Methods
2.1. Plant Culture Conditions
Microgreens of P. frutescens var. crispa and var. frutescens (Figure 1 and Figure 2) were obtained from the Ortogourmet company (Laterza, Taranto, Italy) where microgreens were grown in greenhouse leaving seeds germinate in brown peat (23 °C, 95% RH) in presence of half-strength Hoagland nutrient solution. The seeds come from the Chiltern Seeds Company (Wallingford, England, UK). It took about 20 days from sowing to the microgreens stage, at an optimum temperature of 30–32 °C. The microgreens were subsequently divided and planted singularly in 3 L pots, diameter 13 cm, height 13 cm in a mix of turf:vermiculite 3:1 plus slow-release N/P/K fertilizer, and placed in the greenhouse where the plants were periodically irrigated. The average temperature was 27 °C (minimum 22 °C, maximum 32 °C).

Figure 1.
Trays of microgreens of P. frutescens var. crispa (a), and var. frutescens (b).

Figure 2.
Leaves of microgreens of P. frutescens var. crispa (a,b), and var. frutescens (c,d). Upper leaf pages are shown in (a,c), lower leaf pages in (b,d), respectively.
Fresh P. frutescens leaves were analyzed at the microgreens stage and after further 4 weeks of growth (as representative of an adult stage). The dry weight was determined by placing the leaves in an oven at a temperature of 105 °C until constant weight.
2.2. Analysis of Volatile Organic Compounds
The analyses were carried out by solid-phase microextraction (SPME) methodology essentially as described by Negro et al. [15]. The analyses were repeated three times and the leaves were taken from 4–5 plants for microgreens and 1–2 plants for the 4-week stage. Approximately 1 g (FW) of leaves was sealed into 20 mL SPME vials (Agilent Technologies, Palo Alto, CA, USA) by metal screw-caps with pre-notched Teflon silicone septa. The vials were then placed at 40 °C for 10 min in a thermostatically controlled bath to allow the evaporation of the compounds; hereafter, an SPME syringe was inserted and the fiber (50/30 µm Divinylbenzene/Carboxen/Polydimethylsiloxane, Supelco/Merck KGaA, Darmstadt, Germany), which was previously conditioned for 5 min at 235 °C in the gas chromatograph injector, was exposed for 10 min to absorb the volatile compounds. Subsequently, the fiber was inserted into the injector port of a gas chromatograph with a mass spectrometry detector (Agilent 7890B coupled with MS single quadrupole Agilent 5977A) and the desorption of the volatile compounds was performed at 235 °C for 4 min. At this point, the chromatographic run was started with an Agilent HP-5 ms column (30 m × 0.25 mm, 0.25 µm) (which temperature was raised from 60 °C to 230 °C with a constant increase of 3 °C/minute) with helium (purity > 99.999%) and a constant flow of 1.0 mL/min. Compounds were identified by library search and analytical standards if available. The mass spectrum of an unknown compound was searched in the data processing system [16]. Substances with a score above 800, both in terms of identity and purity, were considered to be identified after comparing the detected compound with the one in the NIST Computational Chemistry Comparison and Benchmark database [16]. Retention Index (RI) was obtained essentially as reported by Zhao et al. [17] employing as reference the retention times of a series of C8–C20 alkanes separated under the GC-MS conditions mentioned above, and applying the following formula:
where, ta is the retention time of the unknown peak a; tn the retention time of n-alkane Cn; and tn + 1 the retention time of n-alkane Cn + 1; n = carbon number of the alkane which elutes before the unknown peak a.
The semi-quantitative analysis of volatile compounds was carried out as reported by Zhao et al. [17] with some modifications. The compound 1,7,7-Trimethylbiciclo [2.2.1] 2-Heptanone was chosen as internal standard; 2 μL of a solution 1.25 μg/mL of internal standard in hexane were added to the samples. The calculation of the amount of VOCs was determined with the following formula: Qc = (Qs × Ac)/As where Qc is the amount of VOC in the sample, Qs the amount of standard, Ac is the peak area of the VOC in the sample and As the peak area of the standard.
2.3. Total Phenols Determination
For phenols, determination samples were finely powdered with mortar and pestle in presence of liquid nitrogen and subjected to extraction in a ratio of 1:20 FW/V with a solution of methanol:water (75:25) acidified with formic acid 0.1% for 20 min in constant agitation in an ultrasonic bath. Then the extract was centrifuged, and extraction was repeated on the pellet. The total phenolic content (TPC) was determined using the spectrophotometric Folin-Ciocalteau method [18] measuring the absorbance, after a 1:10 dilution, with a JASCO (Tokyo, Japan) V-550 UV/VIS spectrophotometer at a wavelength of 765 nm; data were expressed as gallic acid equivalent (GAE)·per mg/g dry weight (DW).
2.4. Antioxidant Activity Determination
The evaluation of the antioxidant activity was carried out by testing three aspects: scavenger, reducing and quenching capacity.
DPPH Assay. Antioxidant activity was determined in vitro by evaluation of the free radical scavenging activity using 2,2-diphenyl-1-picrylhydrazyl (DPPH•) (DPPH assay) [19]. Inhibition of free radical DPPH• was expressed as Trolox (6-hydroxy-2,5,7,8-tetra-methylchroman-2-carboxylic acid) equivalents (TE) per g DW.
Ferric Reducing Antioxidant Power (FRAP). The ferric reducing ability was determined by the FRAP method [20]. The absorption of the reaction mixture was measured at 593 nm using Perkin Elmer (Waltham, MA, USA) 2030 Multilabel reader Victor X5 after 3 min of incubation at 37 °C. The samples were measured in triplicate, and the FRAP was expressed as Trolox equivalents (TE)/g DW.
Superoxide anion scavenging activity assay. The assay was carried out according to Beauchamp and Fridovich [21]. The photo-induced reactions were performed using fluorescent lamps (200 W at 1 m). All samples were measured in triplicate, and the superoxide anion scavenging activity was expressed as g DW corresponding to half-maximal inhibitory concentration (IC50).
2.5. Statistics
All data were reported as the mean ± standard deviation (SD), with at least three replications for each sample. Statistical evaluation was conducted by Duncan’s test to discriminate among the mean values. All statistical analyses were performed using the software Statistica (StatSoft, Tulsa, OK, USA).
3. Results
3.1. Phenolic Content and Antioxidant Activity
We measured the total phenolic content (TPC) in the leaves of Chinese red and green basil at the microgreen stage and after further four weeks to give more information concerning the secondary metabolites produced by young P. frutescens plantlets; Table 1 shows that, despite the modest values, Chinese red basil leaves contain a slightly higher level of TPC than the green variety at both stages. However, total phenols increase after 4 weeks of growth from the microgreens stage.

Table 1.
Total phenolic content of red (var. crispa) and green (var. frutescens) Chinese basil at the microgreen stage and after further 4 weeks of growth.
To evaluate the antioxidant activity of the P. frutescens we employed three different tests (DPPH, FRAP and superoxide anion scavenging activity assay) and data, expressed as Trolox equivalent (TE)/g DW or as g DW corresponding to IC50, were included in Table 2. Both red and green Chinese basil adult leaves showed a slightly higher antioxidant activity than microgreens leaves but this was statistically confirmed only by FRAP and superoxide anion scavenging activity test results (Table 2).

Table 2.
Antioxidant activity of the P. frutescens var. crispa (red) and var. frutescens (green) expressed as Trolox equivalent (TE µmol/g DW) for DPPH and FRAP, and as IC50 (g DW) for the superoxide anion scavenging activity assay.
3.2. Volatile Organic Compounds Emitted by the Two Varieties of Chinese Basil
The VOCs released by the leaves of Chinese red basil leaves at the two growth stages are listed in Table 3 where their amounts are shown as area % of the peaks obtained after GC and as values in ng/g FW obtained through a semi-quantitative procedure.

Table 3.
Volatile organic compounds produced at microgreen stage by Chinese red basil (var. crispa) and their semi-quantitative determination (ng/g FW).
At the microgreens stage, the compound present in greater quantity is D-Limonene, about 121 ng/g FW, followed by a not identified compound (No. 8), approximately 32 ng/g, then 2-Allyl-1,4-dimethoxybenzene (16.2 ng/g), 3-Carene (14.3 ng/g), α-Perilla aldehyde (9.2 ng/g), Isocaryophyllene (9.1 ng/g) and p-Mentha-1(7),8-diene (8.5 ng/g), for a total of around 237 ng/g FW of leaf tissue.
After 4 weeks of further growth, the total amount of VOCs drops by approximately half (to 127 ng/g FW); all single volatile compounds also decrease significantly with the exception of Isocaryophyllene (about 25 ng/g) which becomes the second most abundant compound after D-Limonene (about 76 ng/g) reaching almost 20% of the total compared to the 59.63% of D-Limonene.
The GC/MS chromatogram of Figure 3 shows graphically the previous data as the peaks representing the more abundant volatiles appear higher at the microgreens stage than at 4 weeks stage, except for the Isocaryophyllene peak (compound No. 15).
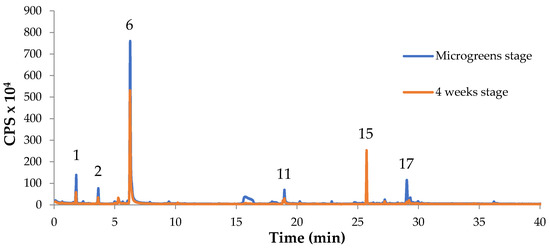
Figure 3.
Comparison of VOCs of Chinese red basil (var. crispa) at microgreens stage and after further 4 weeks. The numbers of the peaks correspond to the volatile compounds identified (see Table 3).
The leaves of Chinese green basil (var. frutescens) microgreens produce fewer VOCs but in larger quantities; in fact, as shown in Table 4, we have detected a total of approximately 1135 ng/g FW of volatiles among which are present, in descending order of quantity, 639.1 ng/g FW of 2-Hexanoylfuran, 154.8 ng/g of Perillene, 68 ng/g of an unknown compound (No. 6), 57.1 ng/g of β-Caryophyllene and 55.6 ng/g of trans-Methyl-Isoeugenol. Surprisingly, after 4 weeks of further growth, we were unable to identify VOCs emitted by Chinese green basil leaves above the instrument detection threshold (data not shown). This means that the leaves of P. frutescens var. frutescens lose their ability to produce volatile compounds during growth.

Table 4.
Volatile organic compounds produced at microgreen stage by Chinese green basil (var. frutescens) and their semi-quantitative determination (ng/g FW).
Thus, the two Chinese basil varieties, red and green, behave differently during the growth after the microgreens stage not only in quantitative but also in qualitative terms with regards to the production of volatile compounds. In fact, only one of the main volatile compounds is common between the two varieties at the microgreens stage, the cis-Methyl isoeugenol.
Figure 4 confirms graphically that the volatile profile of Chinese green basil is drastically reduced at a level close to zero after 4 weeks of growth.
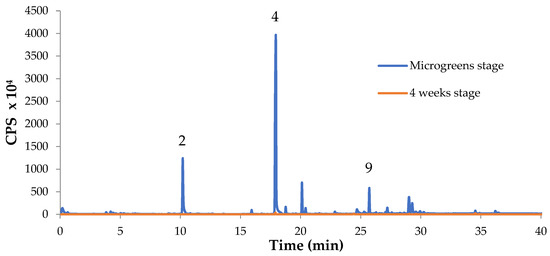
Figure 4.
Comparison of VOCs of Chinese green basil (var. frutescens) at microgreens stage and after 4 weeks of further growth. The numbers of the peaks correspond to the volatile compounds identified (see Table 4).
As summarized in Table 5 and Table 6, the two Chinese basils produce volatiles that results in a different perception of their odor/aroma. The Chinese red basil leaves emit mainly citrusy, spicy and woody odors and flavors, while the leaves of Chinese green basil produce compounds that tend to have fruity, sweet, spicy and herbaceous odors and flavors.

Table 5.
Perception of the aromatic compounds of Chinese red basil.

Table 6.
Perception of the aromatic compounds of Chinese green basil.
4. Discussion
Total phenolic content in our samples is low if compared with data obtained by Ahmed and Tavaszi- Sarosi [7] or Radacsi et al. [27], up to approximately 1/10. Indeed, among ten accessions of P. frutescens Ahmed and Tavaszi-Sarosi [7] detected a total polyphenol content between 137.5 and 234.2 mg GAE/g DW, while Radacsi et al. [27] found values between 84.7 and 204.3 mg GAE/g DW in leaves of five different accessions of P. frutescens analyzing them in two consecutive years. Additionally, recent publications have indicated values around 200 mg GAE/g DW for both varieties (green and red) of P. frutescens [28] and even values of more than 400 mg GAE/g DW in leaves of an unspecified Chinese basil variety [29].
The explanation for the low content of total phenols is that both at the microgreens stage and during the next four weeks the plants are not subject to particular stress so that the young Perilla leaves even compared to Pelargonium flowers [15] contain 1/10 of the total phenols (for DW) of the latter. Instead, the increase in TPC values in Perilla leaves during plant growth could be explained considering that the plants, after the microgreens stage, come out of a protected growth phase into a transplanted greenhouse environment with natural cycles of light and temperature, and periodical irrigation; therefore, leaves of mature Perilla plants are somehow “hardened” in comparison to microgreens. Thus, the increase in TPCs is related to a different developmental stage and different growth conditions as for example recently demonstrated by other authors for Amaranthus caudatus [30] or for African Cabbage [31].
According to the correlation between the level of phenolic compounds and antioxidant capacity [32], the low TPC content is probably the reason for the low levels of antioxidant capacity which can hardly be directly compared with other authors due to the lack of uniformity in the unit of measurement used to express the data, e.g., for the DPPH assay, from % [28] down to µg of Trolox/mg DW [29] or the IC50 [33].
Concerning the volatiles emitted by fresh Chinese basil leaves we have found a total of 19 compounds for crispa variety (Table 3) and 16 compounds for the frutescens variety (Table 4) but only the main seven and nine volatiles were identified, respectively. For the Chinese red basil (var. crispa) at the microgreens stage, the main volatile compounds were D-Limonene (51%), followed by 2-Allyl-1,4-dimethoxybenzene (7%) and 3-Carene (6%) (Table 3); Chinese green basil (var frutescens) microgreens emitted mainly 2-Hexanoylfuran (56%), Perillene (14%), β-Caryophyllene (5%) and trans-Methyl-isoeugenol (4.9%) (Table 4). It is important to note the peculiarity that the microgreens leaves of the two varieties have in common the emission of only one compound cis-Methyl-isoeugenol which represents a very small proportion of the total compounds emitted in both varieties, 2.3% for the green variety and 1.3% for the red one. In addition, at the microgreens stage, Chinese green basil produces VOCs almost four times as much (1135.80 vs. 236.83 ng/g FW).
Thus, the two varieties not only have a very distinct emission profile but also behave very differently during growth after the microgreens stage as leaves of the Perilla frutescens var. frutescens practically do not emit VOCs at 4 weeks stage (Figure 4). A reduction in the emission of compounds also occurs in Chinese red basil leaves, but the reduction is just under 50%, with D-Limonene, the most abundant compound, increasing from 51% to almost 60% in relation to the total volatile compounds emitted. (Table 3).
D-Limonene is one of the most common monoterpenes found in plants [34] main components of the essential oils present in citrus peels [35] invoking a sweet, orange, citrus and terpy flavor [23]. It is also widely used as a flavoring agent and adjuvant in the food industry, especially for beverages and cosmetics [36]. The major volatile produced by Chinese green basil is instead 2-Hexanoylfuran, which is characterized by a sweet, fruity, ketonic green apricot peach odor and flavors, followed by Perillene with a woody floral and citrus odor. So, Perilla microgreens leaves have a mild spicy odor somewhat more citrusy and minty for the crispa variety and more floral and with hints of peach and apricot for Chinese green basil (var. frutescens).
In the literature, most studies have focused on VOCs produced by Perilla essential oils or on leaf extract or crushed/powdered leaves; therefore, a comparison concerning volatiles emitted by whole Perilla leaves is practically impossible.
In fact, VOCs include all volatile compounds produced from plants, whether processed or unprocessed organs, extracts obtained with different solvents, plant parts homogenized as such or pulverized after dehydration up to essential oils extracted by distillation. This is confusing, of course, and makes also proper comparisons difficult unless a similar methodology has been used. In nature, VOCs emitted by plants, or their organs are chemical environmental mediators serving as informative signals and defense chemicals, largely compounds with reduced molecular mass which allows significant release into the air, without a distinctive smell to humans, [37,38,39].
So, volatile compounds produced by essential oils, or “volatile oil compounds”, do not fit into the above definition. A review of Ahmed [6] well described that the volatiles detected after the extraction of Perilla essential oil by various methods turn out to be more than 180. This confirms the enormous chemotypic variability that exists within the species and within VOCs. In addition, Tian et al. [40] collected Perilla frutescens samples from 11 different areas in China and found 119 different components present in a highly variable manner, with Limonene and 2-Hexanoylfuran (the main volatile compounds for the two varieties of Perilla frutescens analyzed in this work) found in only five and one of the 11 sampling sites, respectively. Similar data were recently collected by Ahmed and Al-Zubaidy [41] who individuated 63 components after GC/MS of essential oils extracted from 12 Perilla frutescens accessions.
On the other hand, volatile compounds of freeze-dried powdered Perilla leaves were investigated by Rouphael et al. [14] who found Perillaldehyde (41.6%) in P. frutescens var. crispa and Perilla ketone (51.5%) in P. frutescens var. frutescens as main components, while Benzaldehyde (26.7%) and cis-Jasmone (21.2%) were the second most abundant compounds in the red and green perilla, respectively. Benzaldehyde, Linalool and Caryophyllene were detected in both varieties and Perillene was specifically found exclusively in the green variety, the latter in accordance with our results (Table 4). A similar analysis was carried out by Chen et al. [42] crushing fresh and dried leaves of wild Perilla frutescens (L.) Britt. var. acuta (Thunb.) Kudo; after GC/MS they identified 23 volatile components of which the main ones were β-Caryophyllene (24.2–24.2%), Thujopsene (20.8–13.0%), Perillaldehyde (15.1–14.2%) and (Z)-β-Farnesene (10.9–3.3%), of which only Thujopsene was not identified as VOCs in Table 3 and Table 4. Additionally, Lee et al. [30] have identified a total of 142 volatile compounds from dried, roasted and then P. frutescens var. acuta Kudo; among them, Methyl benzoate and Limonene were predominant in terms of relative concentration at different roasting times suggesting that complex reactions take place during dehydration and roasting of the leaves.
Concerning attempts to characterize the odor or flavor of Perilla frutescens leaves, we found only two interesting articles, one paper analyzed the supernatant of finely cut leaves shaken with 10% NaCl employing a trained panel expressing a sensory evaluation; Perillaldehyde, Neral, Geranial, Eucalyptol, Methyl salicylate gave high positive correlation with the aromatic, fresh, perilla-like, green and minty-cool attributes [43]. The second paper of Laureati et al. [44] evidenced that panelists described infusions of green or red Perilla leaves as having a grassy and floral or an astringent and pungent odor, respectively, with green Perilla determining a higher level of odor intensity.
Regarding the reduction in the emission of volatile compounds from microgreens to adult leaves it can be explained either in terms of a transition from a juvenile leaf structure, and therefore, more “delicate” structure, to an adult organ characterized by a greater thickening of epidermis and cuticle, an increased presence of lignin, and fine stomatal control, as well as, hypothetically, to developmental stages for which VOCs may have a different role; for example, D-Limonene has antifeedant and antifungal properties and it is an attractant for pollinators [34], while Perillene is produced by a flower of several plant species and act as allomone/pheromone for Hymenoptera [45]. Of course, this second hypothesis requires further investigations that will be carried out starting from the data obtained in the present work.
5. Conclusions
The results presented show that the aroma profile of both Perilla varieties is higher at the microgreens stage than at the later adult stage (with green Chinese basil emitting practically no VOCs after 4 weeks) and that this profile is significantly different: the red variety produces a citrusy, spicy and woody odor and the green variety a fruity, sweet, spicy and herbaceous aroma at the microgreens stage. Thus, the different volatiles emitted differentiate the two varieties and justify the appreciation of both types of microgreens by consumers in Italy and Europe. This also confirms that microgreens are not overestimated in terms of their nutraceutical and hedonistic value even though both total phenolic and antioxidant power is higher at the adult stage.
Finally, it should be noted that this work represents the first analysis of VOCs emitted by whole Perilla leaves; in fact, Perilla VOCs have been studied extensively but starting from essential oils or dehydrated or homogenized organs, so as to simultaneously detect both VOCs produced following exposure of essential oils to air after cell rupture, and metabolites produced by contact between precursors and enzymes, as well as compounds generated in response to wounding/chewing. In the future, we intend to test whether the identified VOCs are constitutive or induced by particular conditions, e.g., different growing temperatures or lighting conditions so as to possibly further improve the Perilla microgreens product.
Author Contributions
Conceptualization, R.D., S.M.A. and L.D.B.; methodology, C.N. and R.A.; formal analysis, C.N. and D.G.; investigation, R.D., S.M.A., R.A. and C.N.; resources, L.D.B.; data curation, C.N. and A.L.; writing—original draft preparation, R.D., S.M.A. and A.L.; writing—review and editing: C.M., D.G. and L.D.B.; supervision, A.L. and L.D.B.; funding acquisition, A.L. and L.D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by Italian Ministry of Education, University and Research, Programma Operativo Nazionale FSE-FESR Ricerca e Innovazione 2014–2020, Asse I “Investimenti in Capitale Umano”, Azione I.1 “Dottorati Innovativi con caratterizzazione industriale”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All results are included within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of Vitamin and Carotenoid Concentrations of Emerging Food Products: Edible Microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef]
- Renna, M.; Di Gioia, F.; Leoni, B.; Mininni, C.; Santamaria, P. Culinary Assessment of Self-Produced Microgreens as Basic Ingredients in Sweet and Savory Dishes. J. Culin. Sci. Technol. 2017, 15, 126–142. [Google Scholar] [CrossRef]
- Caracciolo, F.; El-Nakhel, C.; Raimondo, M.; Kyriacou, M.C.; Cembalo, L.; De Pascale, S.; Rouphael, Y. Sensory Attributes and Consumer Acceptability of 12 Microgreens Species. Agronomy 2020, 10, 1043. [Google Scholar] [CrossRef]
- Di Gioia, F.; Reindeer, M.; Santamaria, P. Sprouts, microgreens and baby leaf vegetables. In Minimally Processed Refrigerated Fruits and Vegetables; Yildiz, F., Wiley, R.C., Eds.; Springer: Boston, MA, USA, 2017; pp. 403–432. [Google Scholar] [CrossRef]
- Ahmed, H.M. Ethnomedicinal, Phytochemical and Pharmacological Investigations of Perilla frutescens (L.) Britt. Molecules 2019, 24, 102. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Qiu, J.F.; Ma, L.J.; Hu, Y.J.; Li, P.; Wan, J.B. Phytochemical and Phytopharmacological Review of Perilla frutescens L. (Labiatae), a Traditional Edible-Medicinal Herb in China. Food Chem. Toxicol. 2017, 108, 375–391. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Tavaszi-Sarosi, S. Identification and Quantification of Essential Oil Content and Composition, Total Polyphenols and Antioxidant Capacity of Perilla frutescens (L.) Britt. Food Chem. 2019, 275, 730–738. [Google Scholar] [CrossRef] [PubMed]
- He, Y.K.; Yao, Y.Y.; Chang, Y.N. Characterization of Anthocyanins in Perilla frutescens Var. Acuta Extract by Advanced UPLC-ESI-IT-TOF-MSn Method and Their Anticancer Bioactivity. Molecules 2015, 20, 9155–9169. [Google Scholar] [CrossRef]
- Kim, J.; Kang, H.; Choi, H.; Jo, A.; Oh, D.R.; Kim, Y.; Im, S.; Lee, S.G.; Jeong, K.I.; Ryu, G.C.; et al. Aqueous Extract of Perilla frutescens Var. Acuta Relaxes the Ciliary Smooth Muscle by Increasing NO/CGMP Content In Vitro and In Vivo. Molecules 2018, 23, 1777. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, J.; Ju, J. Perilla frutescens Britton Var. Frutescens Leaves Attenuate Dextran Sulfate Sodium-Induced Acute Colitis in Mice and Lipopolysaccharide-Stimulated Angiogenic Processes in Human Umbilical Vein Endothelial Cells. Food Sci. Biotechnol. 2020, 29, 131–140. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, N.; Yeo, J.Y.; Seo, D.G.; Kim, S.; Lee, J.S.; Hwang, K.W.; Park, S.Y. Anti-Amyloidogenic Effects of Asarone Derivatives from Perilla frutescens Leaves against Beta-Amyloid Aggregation and Nitric Oxide Production. Molecules 2019, 24, 4297. [Google Scholar] [CrossRef]
- Hashimoto, M.; Tanabe, Y.; Hossain, S.; Matsuzaki, K.; Ohno, M.; Kato, S.; Katakura, M.; Shido, O. Intake of Alpha-Linolenic Acid-Rich Perilla frutescens Leaf Powder Decreases Home Blood Pressure and Serum Oxidized Low-Density Lipoprotein in Japanese Adults. Molecules 2020, 25, 2099. [Google Scholar] [CrossRef]
- Ji, W.W.; Li, R.P.; Li, M.; Wang, S.Y.; Zhang, X.; Niu, X.X.; Li, W.; Yan, L.; Wang, Y.; Fu, Q.; et al. Antidepressant-like effect of essential oil of Perilla frutescens in a chronic, unpredictable, mild stress-induced depression model mice. Chin. J. Nat. Med. 2014, 12, 753–759. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Carillo, P.; Pizzolongo, F.; Romano, R.; Sifola, M.I. Chemical Eustress Elicits Tailored Responses and Enhances the Functional Quality of Novel Food Perilla frutescens. Molecules 2019, 24, 185. [Google Scholar] [CrossRef] [PubMed]
- Negro, C.; Dimita, R.; Min Allah, S.; Miceli, A.; Luvisi, A.; Blando, F.; De Bellis, L.; Accogli, R. Phytochemicals and Volatiles in Developing Pelargonium ‘Endsleigh’ Flowers. Horticulturae 2021, 7, 419. [Google Scholar] [CrossRef]
- Johnson, R.D., III (Ed.) Standard Reference Database Number 101; Computational Chemistry Comparison and Benchmark Database. NIST: Gaithersburg, MD, USA, 2015. [CrossRef]
- Zhao, Y.Z.; Li, Z.G.; Tian, W.L.; Fang, X.M.; Su, S.K.; Peng, W.J. Differential Volatile Organic Compounds in Royal Jelly Associated with Different Nectar Plants. J. Integr. Agric. 2016, 15, 1157–1165. [Google Scholar] [CrossRef]
- Negro, C.; Aprile, A.; De Bellis, L.; Miceli, A. Nutraceutical Properties of Mulberries Grown in Southern Italy (Apulia). Antioxidants 2019, 8, 223. [Google Scholar] [CrossRef] [PubMed]
- Oki, T.; Kobayashi, M.; Nakamura, T.; Okuyama, A.; Masuda, M.; Shiratsuchi, H.; Suda, I. Changes in Radical-Scavenging Activity and Components of Mulberry Fruit during Maturation. J. Food Sci. 2006, 71, C18–C22. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Flavornet. Available online: http://flavornet.org (accessed on 25 November 2021).
- The Good Scents Company Information System. Available online: http://www.thegoodscentscompany.com (accessed on 25 November 2021).
- FooDB Resource. Available online: www.foodb.ca (accessed on 25 November 2021).
- Li, Z.; Howell, K.; Fang, Z.; Zhang, P. Sesquiterpenes in Grapes and Wines: Occurrence, Biosynthesis, Functionality, and Influence of Winemaking Processes. Compr. Rev. Food Sci. Food Saf. 2020, 19, 247–281. [Google Scholar] [CrossRef]
- Chen, Q.-C.; Zhu, Y.; Yan, H.; Chen, M.; Xie, D.-C.; Wang, M.-Q.; Ni, D.-J.; Lin, Z. Identification of Aroma Composition and Key Odorants Contributing to Aroma Characteristics of White Teas. Molecules 2020, 25, 6050. [Google Scholar] [CrossRef] [PubMed]
- Radácsi, P.; Sárosi, S.; Szomor, L.Á.; Németh-Zámbori, É. Comparison of the Production and Chemical Constituents of Five Perilla frutescens (L.) Britt. Accessions. Acta Biol. Hung. 2017, 68, 453–465. [Google Scholar] [CrossRef][Green Version]
- Lin, K.H.; Jhou, Y.J.; Wu, C.W.; Chang, Y. Sen. Growth, Physiological, and Antioxidant Characteristics in Green and Red Perilla frutescens Varieties as Affected by Temperature- and Water-Stressed Conditions. Sci. Hortic. 2020, 274, 109682. [Google Scholar] [CrossRef]
- Wang, Z.; Tu, Z.; Xie, X.; Cui, H.; Kong, K.W.; Zhang, L. Perilla frutescens Leaf Extract and Fractions: Polyphenol Composition, Antioxidant, Enzymes (Alpha-Glucosidase, Acetylcholinesterase, and Tyrosinase) Inhibitory, Anticancer, and Antidiabetic Activities. Foods 2021, 10, 315. [Google Scholar] [CrossRef] [PubMed]
- Jimoh, M.O.; Afolayan, A.J.; Lewu, F.B. Antioxidant and phytochemical activities of Amaranthus caudatus L. harvested from different soils at various growth stages. Sci. Rep. 2019, 9, 12965. [Google Scholar] [CrossRef]
- Maina, S.; Ryu, D.H.; Bakari, G.; Misinzo, G.; Nho, C.W.; Kim, H.-Y. Variation in Phenolic Compounds and Antioxidant Activity of Various Organs of African Cabbage (Cleome gynandra L.) Accessions at Different Growth Stages. Antioxidants 2021, 10, 1952. [Google Scholar] [CrossRef] [PubMed]
- Piluzza, G.; Bullitta, S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef]
- Lee, J.; Kim, D.S.; Cho, J.; Hong, S.J.; Pan, J.H.; Kim, J.K.; Shin, E.C. Perilla frutescens Britton: A Comprehensive Study on Flavor/Taste and Chemical Properties during the Roasting Process. Molecules 2019, 24, 1–15. [Google Scholar] [CrossRef]
- Erasto, P.; Viljoen, A.M. Limonene—A Review: Biosynthetic, Ecological and Pharmacological Relevance. Nat. Prod. Commun. 2008, 3, 1934578X0800300728. [Google Scholar] [CrossRef]
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Amparo Blázquez, M.; Granell, A. Volatile Compounds in Citrus Essential Oils: A Comprehensive Review. Front. Plant Sci. 2019, 10, 1–18. [Google Scholar] [CrossRef]
- Ravichandran, C.; Badgujar, P.C.; Gundev, P.; Upadhyay, A. Review of Toxicological Assessment of D-Limonene, a Food and Cosmetics Additive. Food Chem. Toxicol. 2018, 120, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of Plant Volatiles. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef]
- Ameye, M.; Allmann, S.; Verwaeren, J.; Smagghe, G.; Haesaert, G.; Schuurink, R.C.; Audenaert, K. Green leaf volatile production by plants: A meta-analysis. New Phytol. 2018, 220, 666–683. [Google Scholar] [CrossRef]
- Pichersky, E.; Dudareva, N. Biology of Plant Volatiles, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar] [CrossRef]
- Tian, J.; Zeng, X.; Zhang, S.; Wang, Y.; Zhang, P.; Lü, A.; Peng, X. Regional variation in components and antioxidant and antifungal activities of Perilla frutescens essential oils in China. Ind. Crops Prod. 2014, 59, 69–79. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Al-Zubaidy, A.M.A. Exploring natural essential oil components and antibacterial activity of solvent extracts from twelve Perilla frutescens L. Genotypes. Arab. J. Chem. 2020, 13, 7390–7402. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, J.; Guo, Y. Analysis of Volatile Components of Fresh Perilla frutescens (L.) Britt. Var. Acuta (Thunb.) Kudo by Headspace Gc/Ms. J. Essent. Oil Res. 2004, 16, 435–436. [Google Scholar] [CrossRef]
- Tanaka, F.; Miyazawa, T.; Ujiie, Y. Effect of cultivation conditions on odor character and chemical profile of shiso (Perilla frutescens) flavor. In Proceedings of the 19th World Congress of Soil Science, Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010; Available online: https://www.iuss.org/19th%20WCSS/Symposium/pdf/1391.pdf (accessed on 25 November 2021).
- Laureati, M.; Buratti, S.; Bassoli, A.; Borgonovo, G.; Pagliarini, E. Discrimination and characterisation of three cultivars of Perilla frutescens by means of sensory descriptors and electronic nose and tongue analysis. Food Res. Int. 2010, 43, 959–964. [Google Scholar] [CrossRef]
- El-Sayed, A.M. The Pherobase: Database of Pheromones and Semiochemicals. 2021. Available online: http://www.pherobase.com (accessed on 25 November 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).