Abstract
The genus Vaccinium contains about 400 species distributed worldwide, but only a few species and varieties have had their phenolic composition and biological activity documented. In this study, phenols, flavonoids, anthocyanins, antioxidant and antimicrobial activity of methanolic extracts of Vaccinium stenophyllum Steud. fruits: 1-totally immature, 2-immature, 3-immature/close to maturity and 4-mature, were determined using UV-Visible spectrometry and HPTLC. The totally immature fruit extract showed the highest content of total phenols (19.153 ± 0.175 mg GAE/g DW), chlorogenic acid (20.867 ± 0.240 mg CAE/g DW), and the highest antioxidant activity by ABTS●+ (196.761 ± 0.641 µM TE/g DW) and DPPH● (146.580 ± 6.466 µM TE/g DW). Immature, immature/close to maturity and mature fruits extracts, exhibited the lowest MIC (9.37 mg/mL) and MBC (18.75 mg/mL) against Escherichia coli, Salmonella choleraesuis, and Shigella flexneri. The mature fruits extract exhibited the highest content of total anthocyanins (0.141 ± 0.004 mg CE/g DW) and cyanidin-3-glucoside (19.230 ± 0.309 mg CGE/g DW). The content of phenols, flavonoids and anthocyanins was higher than that reported for other Vaccinium species. These results showed the relevance of Vaccinium stenophyllum Steud. for breeding purposes to enhance the bioactivity of cultivars, or as a source of natural additives for the food industry, among others.
1. Introduction
The fruits of the genus Vaccinium are characterized by their red, blue, or almost black color, and are commonly known as blueberries, although they have other common names such as cranberry, bilberry, lingonberry, “ráspano”, “frutilla”, “madroñito”, and “pingüica”, among others [1,2]. There are an estimated 450 species belonging to the genus Vaccinium, which have a wide geographical distribution comprising the northern hemisphere and the mountains of tropical Asia, Central America, South America, and southern Africa. About 35% of the species are native to the Americas, including 25% in North America and 10% in South and Central America [2,3].
Blueberries are widely cultivated and consumed, as there is a market demand for foods rich in secondary metabolites that aid in the prevention of chronic diseases [4,5]. The secondary metabolites reported in blueberries include flavonols (predominantly derived from quercetin), phenolic acids (caffeic, p-coumaric, ferulic, and chlorogenic), and anthocyanins, which are considered to be nutraceuticals with potential as both preventive agents of diabetes, cancer, cardiovascular diseases and neurodegenerative diseases, and antimicrobial agents; this has been attributed to their biological activities, including antioxidant function [6,7,8].
For example, fruits of the variety ‘Blue Rose’ showed a trolox equivalent antioxidant capacity measured by the DPPH method of 2.11 mg of trolox equivalent per gram of fresh weight [9], while fruits of Vaccinium meridionale Sw., reached 143.68 µg of trolox equivalent per gram of dry weight [10]. On the other hand, studies on Vaccinium floribundum Kunth. Fruits, showed an inhibition percentage of 43% on Escherichia coli ATCC 25922 with an extract concentration of 10 mg of dry weight per mililiter [11]. In addition, studies on blueberries of the ‘Elliott’ variety showed a Minimum Inhibitory Concentration of 450 mg of dry weight per milliliter on Salmonella enteritidis CMCC50041 [12].
Moreover, it has been shown that there is a higher content of secondary metabolites in wild blueberries than in cultivated ones; for example, it was shown that the content of glycosylated peonidin and delphinidin in Vaccinium myrtillus L. (wild), was up to 3 times higher than that found in Vaccinium corymbosum L. (cultivated) [13].
Wild berry species, such as blackberry, strawberry, and blueberry, have been reported to possess greater biological activity as compared to cultivated species [14,15,16]. Therefore, one of the current methods of plant breeders is the identification of wild species, which are rich in secondary metabolites and have a greater biological activity, to be used as resources to achieve improved bioactivity in berries [17,18].
In Mexico, wild blueberry species are distributed in central and southeastern areas of the country. In the state of Michoacán, the native species Vaccinium stenophyllum Steud. has been reported upon, but there are no studies focused on its phytochemical or specific biological activity [19,20,21]. Therefore, the aim of the present work was to determine the phenolic content and the antioxidant and antimicrobial activity of Vaccinium stenophyllum Steud. fruit during ripening.
2. Materials and Methods
HPLC-grade methanol, hydrochloric acid (HCl), chlorogenic acid, and Trolox were obtained from Sigma-Aldrich® (St. Louis, MO, USA). All anthocyanin standards were obtained as HPLC-grade chloride salts. Cyanidin-3-glucoside, cyanidin-3-rutinoside, delphinidin-3-5-diglucoside, malvidin-3-glucoside and pelargonidin-3-glucoside were purchased from Sigma-Aldrich® (St. Louis, MO, USA). HPTLC Silica gel plates 60 F254 (20 × 10 cm, Art. 1.05729.0001) were supplied by Merck® (Darmstadst, Germany). For mobile phases, ethyl acetate, acetic acid, and formic acid purchased from Sigma-Aldrich® were used. Broth and Mueller–Hinton agar were purchased from BD Bioxon (Estado de Mexico, Mexico).
Certified strains of Escherichia coli ATCC 12792, Salmonella choleraesuis ATCC 12022, and Shigella flexneri ATCC 10708 were used.
Vaccinium stenophyllum Steud. fruits were collected in the locality of Los Gallineros, the Municipality of Cotija, Michoacán (19°40′16.0′′ N, 102°41′47.5′′ W at 1969 m.a.s.l.), in the autumn season of September 2020, from a total of 18 plants distributed in a triangular section, measuring 20, 29, and 35 m on each side; the plants were identified by M. en C. Ignacio García Ruíz (collection number 9971 and 9974). Specimens were deposited in the CIIDIR-MICHOACÁN Herbarium CIMI.
2.1. Fruit Preprocessing
The harvested fruits were classified according to their surface color in relation to their degree of ripeness, as reported by Arteaga Dalgo et al. [22], with some modifications. Color evaluation was performed using a colorimeter with a CIE L*, a*, b* scale (HFBTE, NR100, China), lightness (L*), and the color components a* (green-red) and b* (yellow-blue) were determined. In addition, the HUE-angle (h: hue) and chroma (C*: color saturation) were calculated from the a* and b* values. The classification was in four maturity stages as shown in Table 1.

Table 1.
Vaccinium stenophyllum Steud. fruit classification according to maturity stage.
2.2. Fruit Quality
The maturity index of each ripening stage was determined by means of the soluble solids/titratable acidity ratio based on the work of Santos et al. [23]. The test was carried out in triplicate.
2.3. Extract Preparation
The fruits were freeze dried (Lyophilizer, LABCON, Kansas City, MO, USA), and the freeze-dried tissue was pulverized with a pestle and mortar and stored at −20 °C until use. Methanolic extracts from each stage were prepared as reported by Cretu et al. [24]. A total of 1 g of samples was extracted with 50 mL of absolute methanol, using an ultrasonic processor (ULTRAsonik, DENSTPLY, NEYTECH, Yucaipa, CA, USA) at 55 ± 5 Hz for 30 min at room temperature. The extracts were vacuum filtered through a 0.45 μm cellulose filter, and 1.25 mL were stored in 40 1.5 mL microtubes at −20 °C.
2.4. Total Phenol Quantification
The quantification of total phenols in the extracts was performed by UV-Visible spectrophotometry as reported by Spinardi et al. [25]. The absorbance was measured at 700 nm using a spectrophotometer (PowerWave HT, Biotek, Biotek Instruments, Vermont, USA). Total phenol content was quantified based on a calibration curve (A700 = 0.6896 [gallic acid] + 0.0008, R2 = 0.9956) obtained from 10 concentrations (0.2–2.0 mg/mL) of gallic acid. The total content was expressed in milligram gallic acid equivalents per gram of dry weight (mg GAE/g DW). The assay was performed in triplicate.
2.5. Flavonoid Quantification
The quantification of the total flavonoids in the methanolic extracts was performed by UV-Visible spectrophotometry using the technique proposed by Woisky and Salatino [26]. The absorbance was measured at 425 nm using methanol as blank with a spectrophotometer PowerWave HT, Biotek, (Biotek Instruments, Vermont, VT, USA). Total flavonoid content was quantified based on the calibration curve (A425 = 1.0443 [quercetin] − 0.048, R2 = 0.9967) obtained from 10 concentrations (0.2–2.0 mg/mL) of quercetin. The total content was expressed as milligram quercetin equivalents per gram dry weight (mg QE/g DW). The assay was performed in triplicate.
2.6. Total Anthocyanin Quantification
The total anthocyanin content in methanolic extracts was determined by UV-Visible spectrophotometry based on the work of Abdel-Aal and Hucl [27]. The absorbance of each extract was read at 535 nm using a spectrophotometer PowerWave HT, Biotek. The assay was performed in triplicate. The total anthocyanin content was expressed as cyanidin-3-glucoside equivalent (mg CGE/g DW), and was calculated by the following formula:
2.7. General Chromatography Conditions
For the identification and quantification of chlorogenic acid using high-performance thin-layer chromatography (HPTLC); 10 × 20 cm silica gel plates 60 F 254 (Merck) were used. For pretreatment, the plates were activated in the plate heater (TLC Plate Heater 3, CAMAG, Muttenz, Switzerland) at 120 °C for 20 min. After cooling the plate, the samples were applied, and the plate was developed with the specific conditions for each compound to be quantified. Developed plates were derivatized with a 1% Natural Products (NP) methanolic solution (2-aminoethyl diphenyl borate, Sigma-Aldrich®, St. Louis, MO, USA) reagent, and the derivatization was performed in the immersion device (Chromatogram Immersion Device, CAMAG, Muttenz, Switzerland) using a vertical speed of 5 cm/s. After derivatization, the plate was dried under air flow for 3 min. Images of each plate in this study were documented with a TLC Visualizer (CAMAG, Muttenz, Switzerland), under visible light, at UV 254 nm and UV 366 nm. The obtained data were processed with the VisionCATS version 2.4 software (CAMAG, Muttenz, Switzerland).
2.7.1. Identification and Quantification of Chlorogenic Acid
In order to identify the chlorogenic acid in the methanolic extracts using HPTLC, we used the technique reported by Cretu et al. [24]. The reference solution was prepared at 1 mg/mL methanol. The extracts and the reference solution were tested with an automatic sampler (ATS 4, CAMAG, Muttenz, Switzerland) using a 25 µL syringe; the bands were applied with a length of 6 mm on the different lanes of the plates, with a distance between lanes of 8.3 mm, a distance from the bottom edge of 8 mm, and a distance from the left side of 21.1 mm. A total of 2.0 µL of the standard and of each extract were applied at a constant application rate (150 nL/s).
Chromatography was performed in an automatic development chamber ADC 2 (CAMAG, Switzerland) at a relative humidity of 25 ± 2% and a mixture of ethyl acetate (10): formic acid (1.1): acetic acid (1.1): water (2.3) (v/v/v/v) was used as solvent separation system. For standardized separation, the plate humidity control unit activity was exposed for 4 min and equilibrated with a saturated potassium acetate solution (257.6 g/100 g H2O) to obtain a 37 ± 2% humidity content. The solvent front migration distance was 70 mm from the bottom edge of the plate and took 15 min. After development, the plate was dried under an air stream for 5 min.
After chromatography, the plate was heated on a TLC Plate Heater 3 (CAMAG, Muttenz, Switzerland) at 120 °C for 5 min and then derivatized. The images were documented and processed.
The chlorogenic acid content expressed as milligram chlorogenic acid equivalents per gram of dry weight (mg CAE/g DW) was obtained from the calibration curves (y = 2.745 × 10−4x − 5.225 × 10−2, R2 = 0.9900). Five different volumes (3, 6, 9, 12 and 15 µL) of the reference solution were applied to the plate, and 2.0 µL of the methanolic extracts were applied in triplicate at a constant application rate (150 nL/s). The chromatogram was obtained, and the data were processed with the VisionCATS software (version 2.4, CAMAG, Muttenz, Switzerland).
2.7.2. Identification and Quantification of Anthocyanins
In order to identify anthocyanins using HPTLC, we used the technique reported by Cretu et al. [24] with some modifications. Reference solutions of cyanidin-3-glucoside, cyanidin-3-rutinoside, delphinidin-3-5-diglucoside, malvidin-3-glucoside, and pelargonidin-3-glucoside were prepared at a concentration of 1.1 mg/mL methanol. For the separation of anthocyanins from blueberry extracts, a mixture of ethyl acetate (10): formic acid (1.1): acetic acid (1.1): water (2.3) (v/v/v/v) was used as the mobile phase. Fruit methanolic extract at development stage 4, i.e., the stage with the highest total anthocyanin content, was used. The standard and stage 4 extracts were applied on the plate, with an automatic sampler ATS 4 (CAMAG, Muttenz, Switzerland) using a 25 µL syringe. Then, 4 mm length bands were applied on the plates, with a distance between lanes of 6.4 mm, a distance from the bottom edge of 8 mm, and a distance from the left side of 10.4 mm. A total of 2.0 µL of the extracts and standards were applied at a constant application rate (150 nL/s).
Chromatographic separation was performed in an automatic development chamber (ADC 2, CAMAG, Muttenz, Switzerland) with a relative humidity of 25 ± 2% and a mixture of ethyl acetate (10): formic acid (1.1): acetic acid (1.1): water (2.3) (v/v/v/v) solvent separation system. For standardized separation, the plate was exposed for 4 min using a saturated potassium acetate solution (257.6 g/100 g H2O) in the humidity control unit at 37 ± 2%. The solvent front migration distance was 70 mm from the bottom edge of the plate and took 20 min. After development, the plate was dried under an air stream for 5 min.
After chromatography, the plate was heated on a TLC Plate Heater 3 at 120 °C for 5 min and immediately derivatized. The images were documented and processed with the use of TLC Visualizer and the obtained data were processed using the Win-CATS software under visible light, at UV 254 nm and UV 366 nm.
The cyanidin-3-glucoside content was expressed as milligram cyanidin-3-glucoside equivalents per gram of dry weight (mg CE/g DW), which was obtained from the calibration curve (y = −3.100 × 10−3x2 + 6.82 × 10−2x −1.480 × 10−2, R2 = 0.9998). Five volumes of the reference solution, i.e., 0.4, 1.0, 2.0, 4.0, and 5.5 µL, and 5.0 µL in triplicate of each methanolic extract were applied to each plate at a constant application rate (150 nL/s). The chromatogram was obtained, and the data were processed with the VisionCATS software.
2.8. Antioxidant Activity by ABTS Assay
The radical-scavenging capacity of the methanolic extracts was determined using the ABTS+- method, as reported by Hosu et al. [28]. The ABTS solution was prepared by dissolving 360 mg of ABTS (2,2′azinobis-(3-ethylbenzothiazdin-6-sulfonic acid) in 100 mL of distilled water, and 100 mL of ABTS solution was added to 100 mL of 2.45 mM potassium persulfate solution, for the activation of ABTS+•. The mixture reaction was conserved in the dark for 24 h and refrigerated. The absorbance was measured at 734 nm using a spectrophotometer (PowerWave HT, Biotek, Vermont, VT, USA) and was adjusted to about 0.760 ± 0.001 by dilution with distilled water. A total of 280 µL of ABTS solution were added to 20 µL of methanolic extracts and the mixture was kept in the dark for 15 min. The absorbance of the sample and the blank was measured at 734 nm using the PowerWave HT spectrophotometer. The antioxidant capacity of the extracts was expressed as μmoles Trolox equivalent/g DW (μmol TE/g DW) using a calibration curve (μmol TE = −5.4371 [A734nm] + 1.4357, R2 = 0.9852) with five different concentrations (0–0.60 μmol Trolox). The assay was performed in triplicate.
2.9. Antioxidant Activity by DPPH Assay
The antioxidant activity of the methanolic extracts was determined using the microplate DPPH● method previously described by Untea et al. [29], with some modifications. In 96-well microplates, 20 µL of methanolic extracts were mixed with 200 µL of DPPH● (1,1-diphenyl-2-picrylhydrazyl) (150 mmol, ethanolic solution). The plate was kept in complete darkness for 30 min. After this time, the absorbance was measured at 515 nm using an PowerWave HT spectrophotometer (BioTek®, Vermont, VT, USA). The antioxidant capacity of the extracts was expressed as μmol TE/g DW using a calibration curve (μmol TE = −2.8918 [A515nm] + 1.4357, R2 = 0.9849) with five concentrations (0–0.60 μmol Trolox). The assay was performed in triplicate.
2.10. Antioxidant Activity by HPTLC-DPPH
Antioxidant activity was determined using the HPTLC-DPPH● method as described by Orisini et al. [30], with some modifications. The DPPH● reagent was prepared by dissolving 40 mg of DPPH in 200 mL of methanol. The developed plates, as described in Section 2.7.1, were used and derivatized with the DPPH reagent, in a Chromatogram Immersion Device (CAMAG, Muttenz, Switzerland) using a vertical speed of 5 cm/s, for 1 s. The plates were then dried in the same way as described in Section 2.7.1 at room temperature in the dark for 30 min. Images of the plate were documented with the TLC Visualizer under visible light, at UV 254 nm and UV 366 nm. The obtained data were processed with the VisionCATS software.
2.11. Determination of Antimicrobial Activity
The antimicrobial activity was determined based on the method of Sun et al. [31]. The Minimum Inhibitory Concentrations (MIC) and Minimum Bactericidal Concentrations (MBC) were determined using the standard broth microdilution method. Thereafter, 100 μL aliquots of Mueller-Hinton broth were placed in sterile 96-well microtiter plates. Methanolic extracts were concentrated in a rotary evaporator (Rotavapor r-200, BUCHI, Zúrich, Switzerland), and resuspended in sterile deionized water. Extracts, 50 μL each, were adjusted to 75, 37.5, 28.12, 18.75, 14.06, 9.37, 7.03, 4.68, 3.51, and 2.34 mg/mL and were added to the wells. The following controls were considered: positive control, broth + bacteria; negative control, broth only; and antibiotic control, broth + bacteria + antibiotic. In the negative control, 200 μL of broth were placed in the corresponding wells. Moreover, 20 μL of the inocula adjusted to a density of 1 × 107 CFU/mL were also placed in the corresponding wells. The plates were incubated at 37 °C for 19 h. The strains tested were Eschericha coli ATCC 12792, Salmonella choleraesuis ATCC 12022, and Shigella flexneri ATCC 10708. After incubation, 20 μL of MTT tetrazolium salt were added, and the incubation continued for 45 min at 37 °C. Finally, the MIC was recorded. Subsequently, an aliquot of 50 μL from the wells without bacterial growth was spread on plates with Mueller–Hinton agar medium, and the MBC was evaluated after 24 h of incubation at 37 °C. MIC was defined as the lowest concentration of the extracts without visible bacterial growth and MBC as the lowest concentration that killed 100% of the bacteria. For each of the dilutions and controls, three replicates were performed. The tests were performed in triplicate on different dates.
2.12. Statistical Analysis
The results of all the different determinations are reported as the means ± standard deviations. An analysis of variance (ANOVA) was also performed, the differences between means were evaluated using Tukey’s test (p < 0.05), and Pearson’s correlations were determined using the R Studio software version 4.0.3.
3. Results and Discussion
3.1. Fruit Color
Table 2 shows the values that were obtained for the color parameters L*, a*, b*, h and C*. The highest lightness value (L*) was observed in stage 1, and it was between 0.21 and 2.63 times higher than those observed in the rest of the stages. In addition, stage 1 exhibited higher green (a*: −7.28) and yellow (b*: 44.44) coloring. Regarding the parameter a* with positive values, the highest value was observed in stage 3 (a*: 21.13), as this was where the red fruits were found. On the other hand, parameter b* exhibited a decrease as ripening progressed, due to a decrease in the yellow and an increase in blue color. A similar behavior concerning the L*, a*, and b* parameters was reported by Arteaga Dalgo et al. [22] in the ripening of Vaccinium floribundum fruit.

Table 2.
Color parameters L*, a*, b*, h and C* for the four ripening stages of Vaccinum stenophyllum Steud. fruit.
It should be noted that the color saturation (C*) exhibited a decreasing trend as ripening progressed, as was reported in other Vaccinium species [22,32]. It is important to mention that h exhibited a descending trend as ripening progressed, since it started with a green fruit (stage 1) with a higher H than the red color present in the rest of the ripening stages. This was reported by Lin et al. [32] in the varieties ‘Bluecrop’ and ‘Northblue’.
3.2. Quality Attributes
The maturity index that was obtained from the ratio of soluble solid content to titratable acidity is shown in Figure 1. Soluble solids increased from 9 to 18% from ripening stage 1 to 4. The maturity index increased 9.3 times from ripening stages 1 to 4. Previous studies have reported the content of soluble solids in Vaccinium corymbosum cv. ‘Brigitta’ (14.3%) and ‘Rocio’ as being 1.23 and 1.19 times lower than those found in stage 4 fruit, respectively. On the other hand, titratable acidity present in the fruits of Vaccinium corymbosum cv. ‘Brigitta’ (0.83%) and ‘Rocio’ (0.55%) resulted 2.28 and 3.65 times lower than that found in the fruit of stage 4, respectively [33,34].
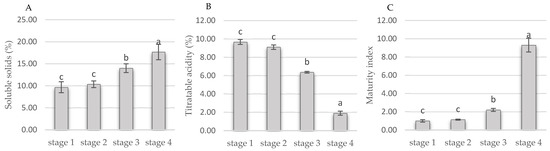
Figure 1.
Vaccinium stenophyllum Steud. fruit quality attributes during ripening. (A) Soluble solids; (B) titratable acidity; (C) maturity index. The mean ± standard deviation is shown; different letters indicate significant statistical differences. An analysis of variance (ANOVA) was performed, and means were separated using the Tukey test (p ˂ 0.05).
3.3. Total Phenols
The highest total phenol content was observed in the stage 1 extract (Table 3), and it was higher than those observed in the other stages by 13.06–28.56%. It was observed that the total phenol content decreased during ripening until the stage 3. This same trend was reported in fruits of the Vaccinium corymbosum L. [17,35,36], and could be due to a certain group of phenols requiring anthocyanin synthesis in the final stages of ripening [36]. Previous studies reported a total phenol content of 3.43 ± 0.07 mg GAE/g DW at stage 4 in Vaccinium myrtillus [37], which was 4.93 times lower than the content observed in this work. On the other hand, a content of 11.7 ± 2.44 mg GAE/g DW was previously reported in the variety ‘Nelson’ [35], which was 1.44 times lower than that found in stage 4 of Vaccinium stenophyllum Steud. fruits.

Table 3.
Total phenolic, flavonoid, and anthocyanin contents of methanolic extracts of Vaccinium stenophyllum Steud. fruits during ripening.
3.4. Total Flavonoids
The highest total flavonoid content was observed in maturity stage 1 (6.059 ± 0.185 mg QE/g DW) (Table 3), and the value exceeded the values of the other stages by an average of 10.8%. Previous studies reported an increase in flavonoid content as ripening increases [25,38], however in this study, only an increasing trend was found from the maturity stage 2 to 4. This could be attributed to the solvent system, which in this case was not acidified. Among the flavonoid compounds that are extracted and preserved at acidic pH, anthocyanins represent a high proportion. This is why, in studies using acidified solvents, there is an increasing trend in total flavonoid content as ripening progresses [25,38]. On the other hand, in previous studies, a total flavonoid content of 0.916 ± 0.001 mg QE/g DW was reported in Vaccinium arctostaphylos fruits at the ripe stage and 0.840 ± 0.001 mg QE/g DW was reported in the ‘Bluegold’ variety [39], but both of these values are lower than that reported in this study. It has been reported by HPLC that the main flavonoids present in cultivated blueberries are quercetin and catechin, with values ranging from two µg/g FW [40].
3.5. Total Anthocyanins
The highest total anthocyanin content was observed at stage 4 (Table 3), which was 4.5 times higher than that observed at stage 2. It was found that the total anthocyanin content increased as the ripening stage increased, which is a characteristic behavior of the genus Vaccinium during ripening [25,38]. On the other hand, previous studies reported 0.223 ± 0.003 mg CE/g DW at the mature stage in the variety ‘Misty’ and 0.916 ± 0.001 mg CGE/g DW in the species Vaccinium arctostaplylos [39], both values being higher than that observed at stage 4 in Vaccinium stenophyllum (by 1.58 and 6.49 times, respectively).
3.6. HPTLC
3.6.1. Identification and Quantification of Chlorogenic Acid by HPTLC
Chlorogenic acid was shown to be one of the most prevalent compounds in the extracts at an Rf = 0.500 (Figure 2). According to the quantification of chlorogenic acid, the highest chlorogenic acid content was observed at stage 1, i.e., 67% higher than in the rest of the ripening stages (Table 4). It should be noted that a decrease in chlorogenic acid as ripening increases was observed, as was the case for total phenol content (Table 3).
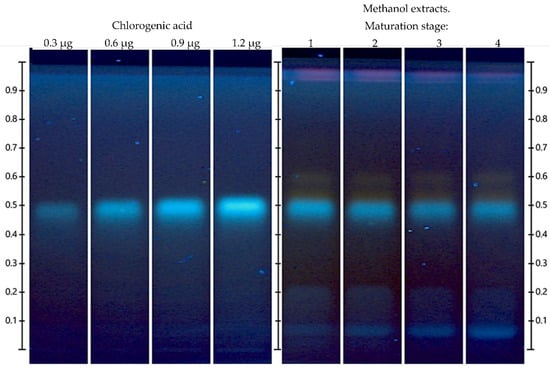
Figure 2.
HPTLC chromatogram performed for the identification and quantification of chlorogenic acid from Vaccinium stenophyllum Steud. fruit extracts, taken under UV light (366 nm) after derivatization with NP reagent. The mobile phase was a mixture of ethyl acetate (10): formic acid (1.1): acetic acid (1.1): water (2.3) (v/v/v/v). Chlorogenic acid was applied on different volumes as a 4-point calibrated (3, 6, 9 and 12 µL, equivalent to 0.3, 0.6, 0.9 and 1.2 µg; y = 2.745 × 10−4x − 5.225 × 10−2, R2 = 0.9900). Vaccinium stenophyllum Steud. fruit extract (stages 1–4) were applied in triplicate, volume = 2 μL (20 mg DW/mL).

Table 4.
Quantification of chlorogenic acid by HPTLC in methanolic extracts of Vaccinium stenophyllum Steud. fruits during ripening.
It is important to mention that this behavior is opposite to that reported by Guofang et al. [38], who performed an HPLC analysis and reported that chlorogenic acid increased as ripening progressed in four cultivars of rabbit’s eye blueberry (Vaccinium ashei Reade: ‘Powderblue’, ‘Gardenblue’, ‘Baldwin’, and ‘Bright Well’).
3.6.2. Identification and Quantification of Anthocyanins by HPTLC
With respect to the anthocyanin standards used, cyanidin-3-glucoside was found to be the most prevalent at stage 4 at Rf = 0.34 (Figure 3). Chung et al. [41] reported that the ripe fruit of Vaccinium corymbosum cv. Bluecrop contains cyanidin-3-glucoside, cyanidin-3-galactoside, cyanidin-3-arabinoside, cyanidin-3-(malonyl) glucoside, cyanidin-3-(6″-acetyl) galactoside, and cyanidin-3-(6″-acetyl)glucoside according to HPLC. The bands located at Rf = 0.44 may be a glycosylated cyanidin other than cyanidin-3-glucoside or cyanidin-3-rutinoside.
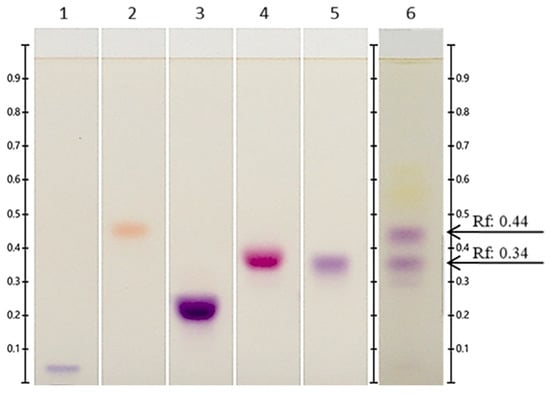
Figure 3.
Identification of anthocyanins by HPTLC in extracts of Vaccinium stenophyllum Steud. fruits, taken under white light, after derivatization with NP reagent. Track 1: delphinidin-3-5-diglucoside; Track 2: pelargonidin-3-glucoside; Track 3: cyanidin-3-rutinoside; Track 4: malvidin-3-glucoside; Track 5: cyanidin-3-glucoside. Track 6: Vaccinium stenophyllum Steud. fruit extract (stage 4) was applied in triplicate, volume = 2 μL (20 mg DW/mL). Band from extract not identified with standard (Rf: 0.44). Band from extract identified as cyanidin-3-glucoside (Rf: 0.34).
On HPTLC plates developed for the quantification of cyanidin-3-glucoside, a sharp band separation of the cyanidin-3-glucoside standard was observed at a Rf = 0.34 and at similar Rf bands in the extracts from different maturity stages, which were found to be comparable with a calibration plate of cyanidin-3-glucoside standard (Figure 4). The highest cyanidin-3-glucoside content was observed at stage 4 (Table 5), which was higher than that in the other maturity stages (2.84–15.89%). It should be noted that the cyanidin-3-glucoside content increased as ripening progressed, as was the case with the quantification of total anthocyanins; see Table 3.
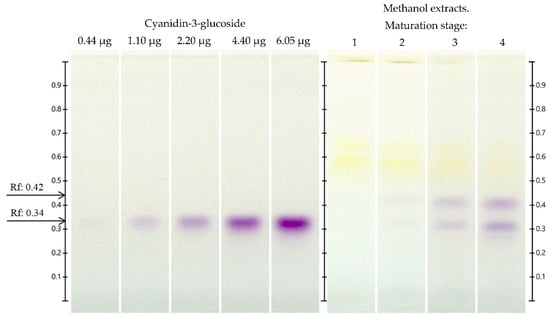
Figure 4.
Quantification of cyanidin-3-glucoside by HPTLC in extracts of Vaccinium stenophyllum Steud. fruits, taken under white light, after derivatization with NP. Cyanidin 3-glucoside was applicated on different volumes as a 5-point calibrated (0.4, 1.0, 2.0, 4.0, and 5.5 µL, equivalent to 0.44, 1.10, 2.20, 4.4 and 6.05 µg; y = −3.100 × 10−3x2 + 6.82 × 10−2x − 1.480 × 10−2, R2 = 0.9998). Vaccinium stenophyllum Steud. fruit extract (stages 1–4) were applied in triplicate, volume = 2 μL (20 mg DW/mL). Bands from extract not identified with standard (Rf: 0.42). Bands from extract identified as cyanidin-3-glucoside (Rf: 0.34).

Table 5.
HPTLC quantification of cyanidin-3-glucoside in methanolic extracts of Vaccinium stenophyllum Steud. fruits during ripening.
As reported by Silva et al. [42], the cyanidin-3-glucoside content increased at the late ripening stages of Vaccinium corymbosum L. cultivars (‘Duke’, ‘Bluecrop’, ‘Goldtraube’ and ‘Ozarkblue’).
3.7. Antioxidant Activity
3.7.1. DPPH● and ABTS●+ Assay
The highest antioxidant activity according to the ABTS●+ method was observed at stage 1, i.e., 15.9–47.8% higher than at the other the stages (Table 6). On the other hand, previous studies reported a value of 170.68 µM TE/g DW in ripe Vaccinium macrocarpon Aiton fruits [43], which is only 0.5% higher than the antioxidant activity (ABTS) at stage 4 in Vaccinium stenophyllum. Similarly, the highest antioxidant activity according to the DPPH● method was observed at stage 1, i.e., 15.4–19.55% higher than in the rest of the stages (Table 6). It should be noted that previous studies reported a value of 164.38 µmoles ET/g DW [44] in mature Vaccinium mirtillus fruits, which is 29.4% higher than the antioxidant activity (DPPH) of stage 4 Vaccinium stenophyllum Steud. The differences in results from the ABTS●+ and DPPH● methods are due to the fact that the ABTS●+ assay is applicable to hydrophilic and lipophilic antioxidant systems, whereas DPPH● is only applicable to hydrophobic systems [45].

Table 6.
Antioxidant capacity of methanolic extracts of Vaccinium stenophyllum Steud. fruits during ripening.
3.7.2. HPTLC-DPPH● Antioxidant Assay
The HPTLC-DPPH● technique allows one to observe which bands in the chromatogram have antioxidant potential and decolorizes DPPH● from purple to yellow (Figure 5). All the extracts exhibited antioxidant bands at Rf = 0.80, 0.60, and 0.50, with the stage 4 extract additionally showing antioxidant bands at Rf = 0.44 and 0.34. One of the main antioxidant bands in the chromatogram was that corresponding to chlorogenic acid (Rf = 0.50). There were also other bands with antioxidant power that could correspond to cyanidin-3-glucoside (Rf = 0.34), and one at Rf = 0.42 that probably corresponds to a glycosylated cyanidin other than cyanidin-3-glucoside or cyanidin-3-rutoside. According to previous reports, the bands present at Rf = 0.60 correspond to hyperoside [24], which is another band with antioxidant activity. Hyperoside is one of the main flavonoids reported in species of the genus Vaccinium [43,46,47]. In addition, other antioxidant compounds were present in stage 4 extracts (Rf = 0.42) that were not identified.
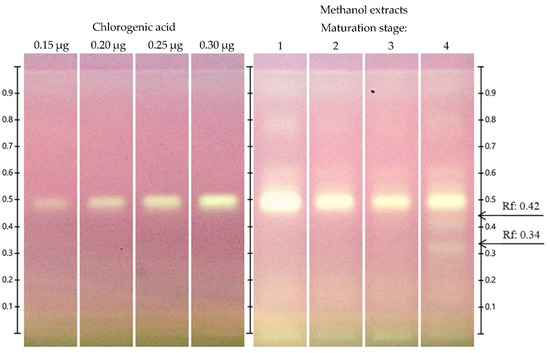
Figure 5.
Detection of HPTLC-DPPH● antioxidant compounds in extracts of Vaccinium stenophyllum Steud. fruits under white light illumination, after derivatization with NP reagent. Chlorogenic acid was applicated on different volumes as a 4-point calibrated (3, 6, 9 and 12 µL, equivalent to 0.3, 0.6, 0.9 and 1.2 µg; y = 2.745 × 10−4x − 5.225 × 10−2, R2 = 0.9900). Vaccinium stenophyllum Steud. fruit extract (stages 1–4) were applied in triplicate, volume = 2 μL (20 mg DW/mL, w/v). Bands from extract not identified with standard (Rf: 0.42). Bands from extract identified as cyanidin-3-glucoside (Rf: 0.34).
Among the polyphenolic compounds, phenolic acids have the highest antioxidant capacity, which is greater than that of flavonoids; however, among the flavonoids, anthocyanins exhibited the highest antioxidant activity [17]. The antioxidant action of cyanidin-3-glucoside is usually attributed to its chemical structure, which is highly reactive because the electron deficiency of the C-ring is modulated by the pattern of hydroxyl (OH) substitutions, the degree of methylations on the aromatic β-ring, and the number of sugars attached to the molecule [48]. Chlorogenic acid is a potent electron-donating antioxidant, which is able to scavenge the nitrogen-centered stable free radical of DPPH● [49]. Previous studies on blueberries grown by liquid chromatography integrated mass spectrometry report m-coumaric as one of the antioxidant compounds in the fruit [50].
3.8. MIC and MIB of Vaccinium stenophyllum Steud. Fruit Extracts
The highest MIC and MBC values (Table 7) were found in stage 1 extracts, being 1.5 times higher than the rest of the extracts. The MIC and MBC for E. coli, S. flexneri, and S. choleraesuis were identical. This phenomenon was previously reported in berry extracts of Prunus spinosa L., which exhibited the same MIC of 250 µg/mL for Escherichia coli ATCC 25922 and Shigella sonnei ATCC 25931 [51]. Contrarily, Cerezo et al. [52] found different MICs for Escherichia coli ATCC 25922 (3.74 mg/mL) and Salmonella enteritidis ATCC 13076 (2.16 mg/mL) in Vaccinium corymbosum extracts.

Table 7.
Antimicrobial activity of methanolic extracts of Vaccinium stenophyllum Steud. fruits during maturation for E. coli, S. flexneri, and S. choleraesuis.
Notably, the methanol stage 1 extract had the highest chlorogenic acid content, but not the best MIC and MBC. However, the antimicrobial effect observed could be due to the toxicity of phenolic compounds to microorganisms. The mechanisms involved in this toxicity include enzyme inhibition by oxidized compounds, possibly through a reaction with sulfhydryl groups or through more nonspecific interactions with proteins that lead to their inactivation and loss of function [53,54].
3.9. Correlation of the Variables under Study
Table 8 shows the correlation of the variables in this study. The minimum values of MIC and MBC were mainly correlated with the cyanidin-3-glucoside content. Furthermore, the best antioxidant capacity according to the DPPH● method was mainly correlated with the of chlorogenic acid, phenol, and total flavonoid contents. The ABTS●+ method’s results were strongly correlated with the total phenol and flavonoid values.

Table 8.
Correlation coefficient between quantifications and biological activity of Vaccinium stenophyllum Steud. fruit extracts.
In Vaccinium corymbosum L. fruits, the content of total phenols, have been the main metabolites correlated with antioxidant activity [17], as it was observed in the present study. On the other hand, it has been reported a higher correlation of total anthocyanin content with antioxidant activity than that showed by total phenols content, contrary to what was found in this work [55]. Moreover, in fruits of Vaccinium ashei Reade cultivar, the best antioxidant activities obtained by the DPPH method were correlated with the content of chlorogenic acid, being similar to the results showed in this study. This could be attributed to the genetic load, which plays an important role both in the composition of phenols, flavonoids and anthocyanins, and in the biological activities of its extracts, as it has been reported for different blueberry varieties [38].
The fruits of Vaccinium stenophyllum Steud. showed a content of total phenols, total flavonoids, total anthocyanins, chlorogenic acid and cyanidin-3-glucoside close to that reported for other wild and cultivated species of Vaccinum [35,37,39,41,56]. Similarly, the antioxidant and antimicrobial activities showed values close to those reported in both wild and cultivated species [48,49,54,57]. It should be noted that the genetics of each species or variety of Vaccinium play an important role in its composition and content of bioactive compounds, which confer different potentials in biological activities [38].
This becomes important due to the growing interest in the use of natural antioxidants in food products [58], as well as natural and effective antimicrobial agents as an alternative to antibiotics or chemical additives [59].
4. Conclusions
This study is the first to report on the phenolic composition of Vaccinium stenophyllum Steud. fruits during ripening, and the antioxidant and antimicrobial potential of the fruits. Among the antioxidant compounds found in the extracts were chlorogenic acid and cyanidin-3-glucoside. In addition, the extracts exhibited an antimicrobial effect against enterobacteria of public health concern. Based on the results, Vaccinium stenophyllum Steud. could become a plant material of interest for plant breeding focused on fruit nutraceutical quality, in addition, the fruit has great potential as a natural source of metabolites with biological activities relevant for the development of nutritional supplements, food additives, among other products of plant origin that benefit health.
Author Contributions
Conceptualization, H.G.M.-V., J.M.-T., M.V.A.-P. and I.G.-R.; methodology, J.G.C.-V., H.G.M.-V. and I.G.-R.; validation, J.O.B.-G.; formal analysis, H.G.M.-V.; investigation, J.O.B.-G.; resources, H.G.M.-V.; data curation, J.A.C.-D.; writing—original draft preparation, J.O.B.-G.; writing—review and editing, H.G.M.-V., J.M.-T. and M.V.A.-P.; visualization, J.M.-T.; supervision, J.M.-T.; project administration, H.G.M.-V.; funding acquisition, H.G.M.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The first author thanks the Consejo Nacional de Ciencia y Tecnología (CONACYT) in México for the economic support through national scholarship 746315.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Zapata, L.M.; Castagnini, J.M.; Quintero, C.F.; Malleret, A.D. Desarrollo de productos de arándanos con propiedades antioxidantes y probióticas. Cienc. Docencia y Tecnol. Supl. 2019, 9, 134–155. [Google Scholar]
- Song, G.-Q.; Hancock, J.F. Vaccinium. In Wild Crop Relatives: Genomic and Breeding Resources: Temperate Fruits; Springer: Berlin/Heidelberg, Germany, 2011; pp. 197–247. ISBN 9783642143878. [Google Scholar]
- Folta, K.M.; Kole, C. Genetics Genomics and Breeding of Berries; Folta, K.M., Kole, C., Eds.; CRC Press: Clemson, SC, USA, 2016; ISBN 9781439856604. [Google Scholar]
- Jiménez-Bonilla, V.; Abdelnour-Esquivel, A. Protocolo de micropropagación de arándano nativo de Costa Rica (Vaccinium consanguinium). Rev. Tecnol. Marcha 2018, 31, 146. [Google Scholar] [CrossRef]
- Totad, M.G.; Sharma, R.R.; Sethi, S.; Joshi, A.; Verma, M.K.; Sharma, V.K.; Singh, S.; Dhiman, M.R.; Jaiswal, S. Genotypic variability in nutritional and functional attributes of blueberry varieties grown in northern-western Himalayas. J. Food Sci. Technol. 2020, 57, 2251–2258. [Google Scholar] [CrossRef] [PubMed]
- Correa-Betanzo, J.; Allen-Vercoe, E.; McDonald, J.; Schroeter, K.; Corredig, M.; Paliyath, G. Stability and biological activity of wild blueberry (Vaccinium angustifolium) polyphenols during simulated in vitro gastrointestinal digestion. Food Chem. 2014, 165, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, N.H.; Charfuelan, C. Contribución a la Caracterización y Evaluación de la Actividad Antioxidante de las Antocianinas del Fruto de Ivilan (Monnina obtusifolia H.B.K). Inf. Technol. 2019, 30, 81–90. [Google Scholar] [CrossRef]
- Zárate, N.B.; Alavez, A.Y.; Domínguez, V.J.M. Manejo agronómico del cultivo de arándano (Vaccinium corymbosum L.) en la Sierra Norte de Oaxaca. Univ. Cienc. 2017, 6, 138–155. [Google Scholar]
- Johnson, J.B.; Steicke, M.; Mani, J.S.; Rao, S.; Anderson, S.; Wakeling, L. Changes in Anthocyanin and Antioxidant Contents during Maturation of Australian Highbush Blueberry (Vaccinium corymbosum L.) Cultivars. Eng. Proc. 2021, 11, 6. [Google Scholar] [CrossRef]
- Vargas-Ramella, M.; Lorenzo, J.M.; Zamuz, S.; Valdés, M.E.; Moreno, D.; Guamán Balcázar, M.C.; Fernández-Arias, J.M.; Reyes, J.F.; Franco, D. The antioxidant effect of colombian berry (Vaccinium meridionale sw.) extracts to prevent lipid oxidation during pork patties shelf-life. Antioxidants 2021, 10, 1290. [Google Scholar] [CrossRef]
- Baenas, N.; Ruales, J.; Moreno, D.A.; Barrio, D.A.; Stinco, C.M.; Martínez-Cifuentes, G.; Meléndez-Martínez, A.J.; García-Ruiz, A. Characterization of andean blueberry in bioactive compounds, evaluation of biological properties, and in vitro bioaccessibility. Foods 2020, 9, 1483. [Google Scholar] [CrossRef]
- Shen, X.; Sun, X.; Xie, Q.; Liu, H.; Zhao, Y.; Pan, Y.; Hwang, C.-A.; Wu, V.C.H. Antimicrobial effect of blueberry (Vaccinium corymbosum L.) extracts against the growth of Listeria monocytogenes and Salmonella enteritidis. Food Control. 2014, 35, 159–165. [Google Scholar] [CrossRef]
- Veberic, R.; Slatnar, A.; Bizjak, J.; Stampar, F.; Mikulic-Petkovsek, M. Anthocyanin composition of different wild and cultivated berry species. LWT-Food Sci. Technol. 2015, 60, 509–517. [Google Scholar] [CrossRef]
- Jiménez-Bonilla, V.; Abdelnour-Esquivel, A. Identificación y valor nutricional de algunos materiales nativos de arándano (Vaccinium spp.). Tecnol. En Marcha 2013, 26, 3. [Google Scholar] [CrossRef]
- Ocete, R.; López, M.Á.; Gallardo, A.; Arnold, C. Comparative analysis of wild and cultivated grapevine (Vitis vinifera) in the Basque Region of Spain and France. Agric. Ecosyst. Environ. 2008, 123. [Google Scholar] [CrossRef]
- Georgieva, M.; Badjakov, I.; Dincheva, I.; Yancheva, S.; Kondakova, V. In vitro propagation of wild Bulgarian small berry fruits (Bilberry, lingonberry, raspberry and strawberry). Bulg. J. Agric. Sci. 2016, 22, 46–51. [Google Scholar]
- Castrejón, A.D.R.; Eichholz, I.; Rohn, S.; Kroh, L.W.; Huyskens-Keil, S. Phenolic profile and antioxidant activity of highbush blueberry (Vaccinium corymbosum L.) during fruit maturation and ripening. Food Chem. 2008, 109, 564–572. [Google Scholar] [CrossRef]
- Kaushik, P.; Andújar, I.; Vilanova, S.; Plazas, M.; Gramazio, P.; Herraiz, F.J.; Brar, N.S.; Prohens, J. Breeding vegetables with increased content in bioactive phenolic acids. Molecules 2015, 20, 18464–18481. [Google Scholar] [CrossRef]
- González-Elizondo, M.S.; González-Elizondo, M. Flora del Bajío y de Regiones Adyacentes. Fascículo 183, Familia Ericaceae; Instituto de Ecología A.C.: Pátzcuaro, Mexico, 2014. [Google Scholar]
- Villaseñor, J.L. Checklist of the native vascular plants of Mexico/Catálogo de las plantas vasculares nativas de Mexico. Rev. Mex. Biodivers. 2016, 87, 559–902. [Google Scholar] [CrossRef]
- CONABIO. Catálogo de Autoridades Taxonómicas de Especies de Flora y Fauna Con Distribución En México; Base de Datos SNIB-CONABIO; CONABIO: Ciudad de Mexico, Mexico, 2021.
- Arteaga Dalgo, M.; Andrade Cuvi, M.J.; Moreno Guerrero, C. Relación del desarrollo del color con el contenido de antocianinas y clorofila en diferentes grados de madurez de mortiño (Vaccinium floribundum). Enfoque UTE 2014, 5, 14–28. [Google Scholar] [CrossRef]
- Santos, R.O.; Trindade, S.C.; Maurer, L.H.; Bersch, A.M.; Sautter, C.K.; Penna, N.G. Physicochemical, antioxidant and sensory quality of Brazilian Blueberry Wine. An. Acad. Bras. Cienc. 2016, 88, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- Cretu, G.; Totu, E.E.; Miron, A.R.; Nechifor, A.C. Development of a quantitative high performance thin layer chromatographic method for analysis of caffeic acid and quercetin from cranberry extract. Rom. Biotechnol. Lett. 2013, 18, 8271–8278. [Google Scholar]
- Spinardi, A.; Cola, G.; Gardana, C.S.; Mignani, I. Variation of Anthocyanin Content and Profile Throughout Fruit Development and Ripening of Highbush Blueberry Cultivars Grown at Two Different Altitudes. Front. Plant Sci. 2019, 10, 1045. [Google Scholar] [CrossRef]
- Woisky, R.G.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Hucl, P. A rapid method for quantifying total anthocyanins in blue aleurone and purple pericarp wheats. Cereal Chem. 1999, 76, 350–354. [Google Scholar] [CrossRef]
- Hosu, A.; Cimpoiu, C.; David, L.; Moldovan, B. Study of the Antioxidant Property Variation of Cornelian Cherry Fruits during Storage Using HPTLC and Spectrophotometric Assays. J. Anal. Methods Chem. 2016, 2016, 5. [Google Scholar] [CrossRef]
- Untea, A.; Lupu, A.; Saracila, M.; Panaite, T. Comparison of ABTS, DPPH, Phosphomolybdenum Assays for Estimating Antioxidant Activity and Phenolic Compounds in Five Different Plant Extracts. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Anim. Sci. Biotechnol. 2018, 75, 110. [Google Scholar] [CrossRef]
- Orsini, F.; Vovk, I.; Glavnik, V.; Jug, U.; Corradini, D. HPTLC, HPTLC-MS/MS and HPTLC-DPPH methods for analyses of flavonoids and their antioxidant activity in Cyclanthera pedata leaves, fruits and dietary supplement. J. Liq. Chromatogr. Relat. Technol. 2019, 42, 290–301. [Google Scholar] [CrossRef]
- Sun, X.-H.; Hao, L.-R.; Xie, Q.-C.; Lan, W.-Q.; Zhao, Y.; Pan, Y.-J.; Wu, V.C.H. Antimicrobial effects and membrane damage mechanism of blueberry (Vaccinium corymbosum L.) extract against Vibrio parahaemolyticus. Food Control. 2020, 111, 107020. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, G.; Zhang, Q.; Wang, Y.; Dia, V.P.; Meng, X. Ripening affects the physicochemical properties, phytochemicals and antioxidant capacities of two blueberry cultivars. Postharvest Biol. Technol. 2020, 162, 111097. [Google Scholar] [CrossRef]
- Lobos, T.E.; Retamales, J.B.; Ortega-Farías, S.; Hanson, E.J.; López-Olivari, R.; Mora, M.L. Regulated deficit irrigation effects on physiological parameters, yield, fruit quality and antioxidants of Vaccinium corymbosum plants cv. Brigitta. Irrig. Sci. 2018, 36, 49–60. [Google Scholar] [CrossRef]
- Ordóñez-Díaz, J.L.; Pereira-Caro, G.; Cardeñosa, V.; Muriel, J.L.; Moreno-Rojas, J.M. Study of the quality attributes of selected blueberry (Vaccinium corymbosum L.) varieties grown under different irrigation regimes and cultivation systems. Appl. Sci. 2020, 10, 8459. [Google Scholar] [CrossRef]
- Kalt, W.; Lawand, C.; Ryan, D.A.J.; McDonald, J.E.; Donner, H.; Forney, C.F. Oxygen Radical Absorbing Capacity, Anthocyanin and Phenolic Content of Highbush Blueberries (Vaccinium corymbosum L.) during Ripening and Storage. J. Am. Soc. Hortic. Sci. 2003, 128, 917–923. [Google Scholar] [CrossRef]
- Yang, J.; Li, B.; Shi, W.; Gong, Z.; Chen, L.; Hou, Z. Transcriptional Activation of Anthocyanin Biosynthesis in Developing Fruit of Blueberries (Vaccinium corymbosum L.) by Preharvest and Postharvest UV Irradiation. J. Agric. Food Chem. 2018, 66, 10931–10942. [Google Scholar] [CrossRef]
- Jurca, T.; Vicaș, L.; Tóth, I.; Braun, M.; Marian, E.; Teusdea, A.; Vicaș, S.; Mureșan, M. Mineral elements profile, bioactive compounds and antioxidant capacity of wild blueberry and of pharmaceutical preparations from blueberry (Vaccinium myrtillus). Farmacia 2016, 64, 581–587. [Google Scholar]
- Guofang, X.; Xiaoyan, X.; Xiaoli, Z.; Yongling, L.; Zhibing, Z. Changes in phenolic profiles and antioxidant activity in rabbiteye blueberries during ripening. Int. J. Food Prop. 2019, 22, 320–329. [Google Scholar] [CrossRef]
- Okan, O.T.; Deniz, I.; Yayli, N.; Şat, I.G.; Öz, M.; Serdar, G.H. Antioxidant activity, sugar content and phenolic profiling of blueberries cultivars: A comprehensive comparison. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 639–652. [Google Scholar] [CrossRef]
- Subbiah, V.; Zhong, B.; Nawaz, M.A.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Screening of Phenolic Compounds in Australian Grown Berries by LC-ESI-QTOF-MS/MS and Determination of Their Antioxidant Potential. Antioxidants 2021, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.W.; Yu, D.J.; Lee, H.J. Changes in anthocyanidin and anthocyanin pigments in highbush blueberry (Vaccinium corymbosum cv. Bluecrop) fruits during ripening. Hortic. Environ. Biotechnol. 2016, 57, 424–430. [Google Scholar] [CrossRef]
- Chaves, J.O.; de Souza, M.C.; da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; da Fonseca Machado, A.P.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids From Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef]
- Gudžinskaitė, I.; Stackevičienė, E.; Liaudanskas, M.; Zymonė, K.; Žvikas, V.; Viškelis, J.; Urbštaitė, R.; Janulis, V. Variability in the qualitative and quantitative composition and content of phenolic compounds in the fruit of introduced american cranberry (Vaccinium macrocarpon Aiton). Plants 2020, 9, 1379. [Google Scholar] [CrossRef]
- Chauca Aguilar, M.A.; Chávez Quintana, S.G. Fenoles y capacidad antioxidante de Psidium guajava, Vaccinium myrtillus, Selenicereus megalanthus y Physalis peruviana de diferentes procedencias. Bioagro 2020, 32, 225–230. [Google Scholar]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Hu, J.; Wang, J.; Li, S.; Yang, B.; Gong, M.; Li, X.; Zhang, L.; Tian, J. Phytochemical compositions, antioxidant and antimicrobial activities analysis of extracts from Vaccinium bracteatum Thunb. Leaves. J. Appl. Bot. Food Qual. 2016, 89, 150–155. [Google Scholar] [CrossRef]
- Hicks, J.M.; Muhammad, A.; Ferrier, J.; Saleem, A.; Cuerrier, A.; Arnason, J.T.; Colson, K.L. Quantification of chlorogenic acid and hyperoside directly from crude blueberry (Vaccinium angustifolium) leaf extract by NMR spectroscopy analysis: Single-laboratory validation. J. AOAC Int. 2012, 95, 1406–1411. [Google Scholar] [CrossRef]
- Moreno, Y.S.; Salinas, C.G.; Estrada, B.C.; Vidal Martínez, V.A. Variabilidad en contenido y tipos de antocianinas en granos de color azul/morado de poblaciones mexicanas de maíz. Rev. Fitotec. Mex. 2013, 36, 285–294. [Google Scholar] [CrossRef]
- Kono, Y.; Kobayashi, K.; Tagawa, S.; Adachi, K.; Ueda, A.; Sawa, Y.; Shibata, H. Antioxidant activity of polyphenolics in diets. Rate constants of reactions of chlorogenic acid and caffeic acid with reactive species of oxygen and nitrogen. Biochim. Biophys. Acta Gen. Subj. 1997, 1335, 335–342. [Google Scholar] [CrossRef]
- Wang, H.; Guo, X.; Hu, X.; Li, T.; Fu, X.; Liu, R.H. Comparison of phytochemical profiles, antioxidant and cellular antioxidant activities of different varieties of blueberry (Vaccinium spp.). Food Chem. 2017, 217, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Radovanović, B.C.; Andelković, A.S.M.; Radovanović, A.B.; Andelković, M.Z. Antioxidant and antimicrobial activity of polyphenol extracts from wild berry fruits grown in Southeast Serbia. Trop. J. Pharm. Res. 2013, 12, 813–819. [Google Scholar] [CrossRef]
- Cerezo, A.B.; Cătunescu, G.M.; González, M.M.P.; Hornedo-Ortega, R.; Pop, C.R.; Rusu, C.C.; Chirilă, F.; Rotar, A.M.; Carmen Garcia-Parrilla, M.; Troncoso, A.M. Anthocyanins in blueberries grown in hot climate exert strong antioxidant activity and may be effective against urinary tract bacteria. Antioxidants 2020, 9, 478. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, A.A.; Al-Askar, A.A.; Almaary, K.S.; Dawoud, T.M.; Sholkamy, E.N.; Bakri, M.M. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J. Biol. Sci. 2018, 25, 361–366. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial activity of polyphenols and alkaloids in middle eastern plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Costa, E.M.; Mendes, M.; Morais, R.M.; Calhau, C.; Pintado, M.M. Antimicrobial, antiadhesive and antibiofilm activity of an ethanolic, anthocyanin-rich blueberry extract purified by solid phase extraction. J. Appl. Microbiol. 2016, 121, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Costa, E.M.; Coelho, M.C.; Morais, R.M.; Pintado, M.E. Variation of anthocyanins and other major phenolic compounds throughout the ripening of four Portuguese blueberry (Vaccinium corymbosum L.) cultivars. Nat. Prod. Res. 2017, 31, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Manuel Franco, C.; Vázquez, B.I. Natural compounds as antimicrobial agents. Antibiotics 2020, 9, 217. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).