Evaluation of Bio-Pesticides against the South American Tomato Leaf Miner, Tuta absoluta Meyrick (Lepidoptera: Gelechiidae) in India
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Colony
2.2. Bio-Pesticides
2.3. Laboratory Bioassays
2.4. Field Experiments
2.5. Treatment and Data Collection
2.6. Data Analysis for Field Experiments
3. Results
3.1. Laboratory Bioassays
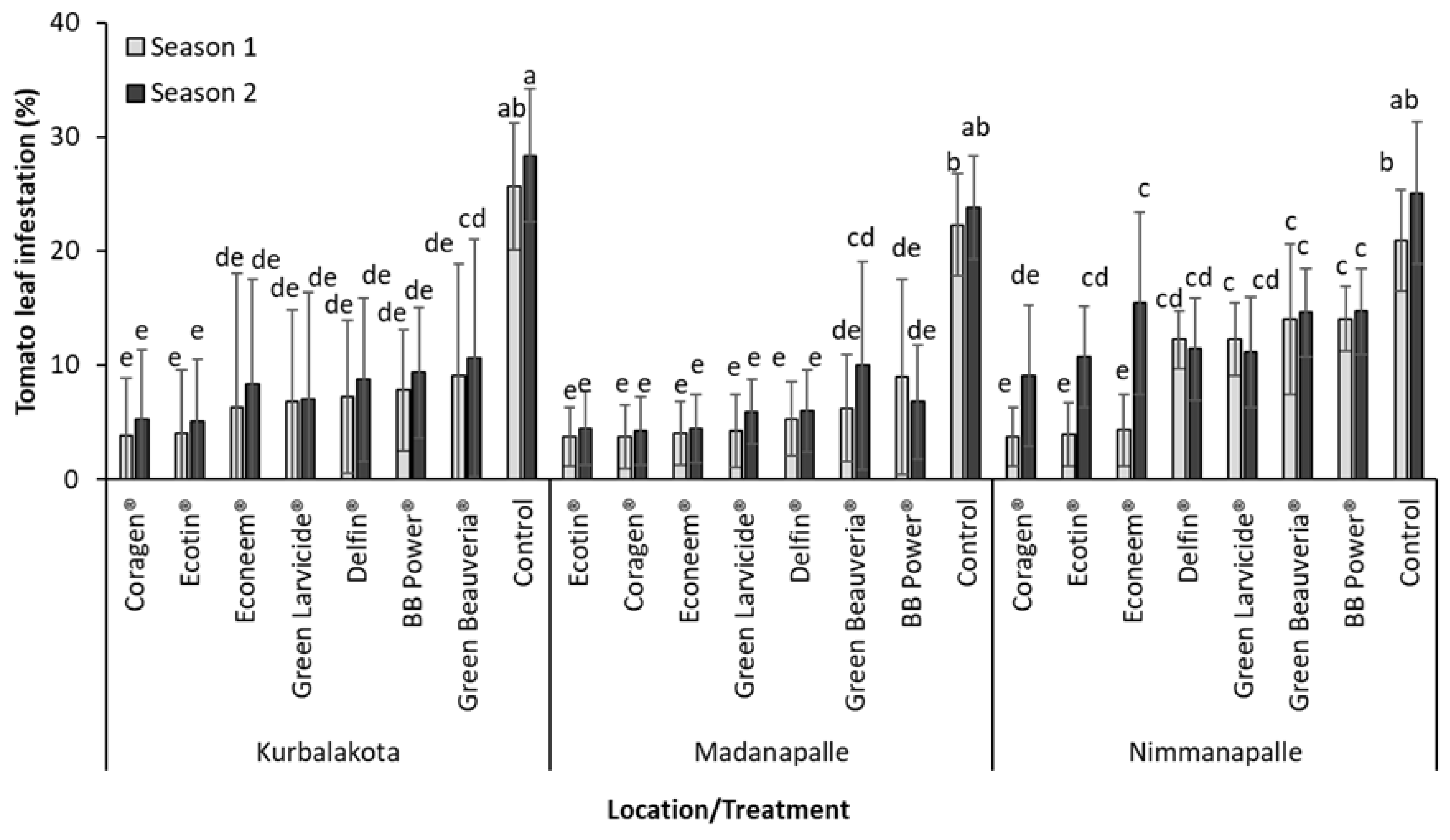
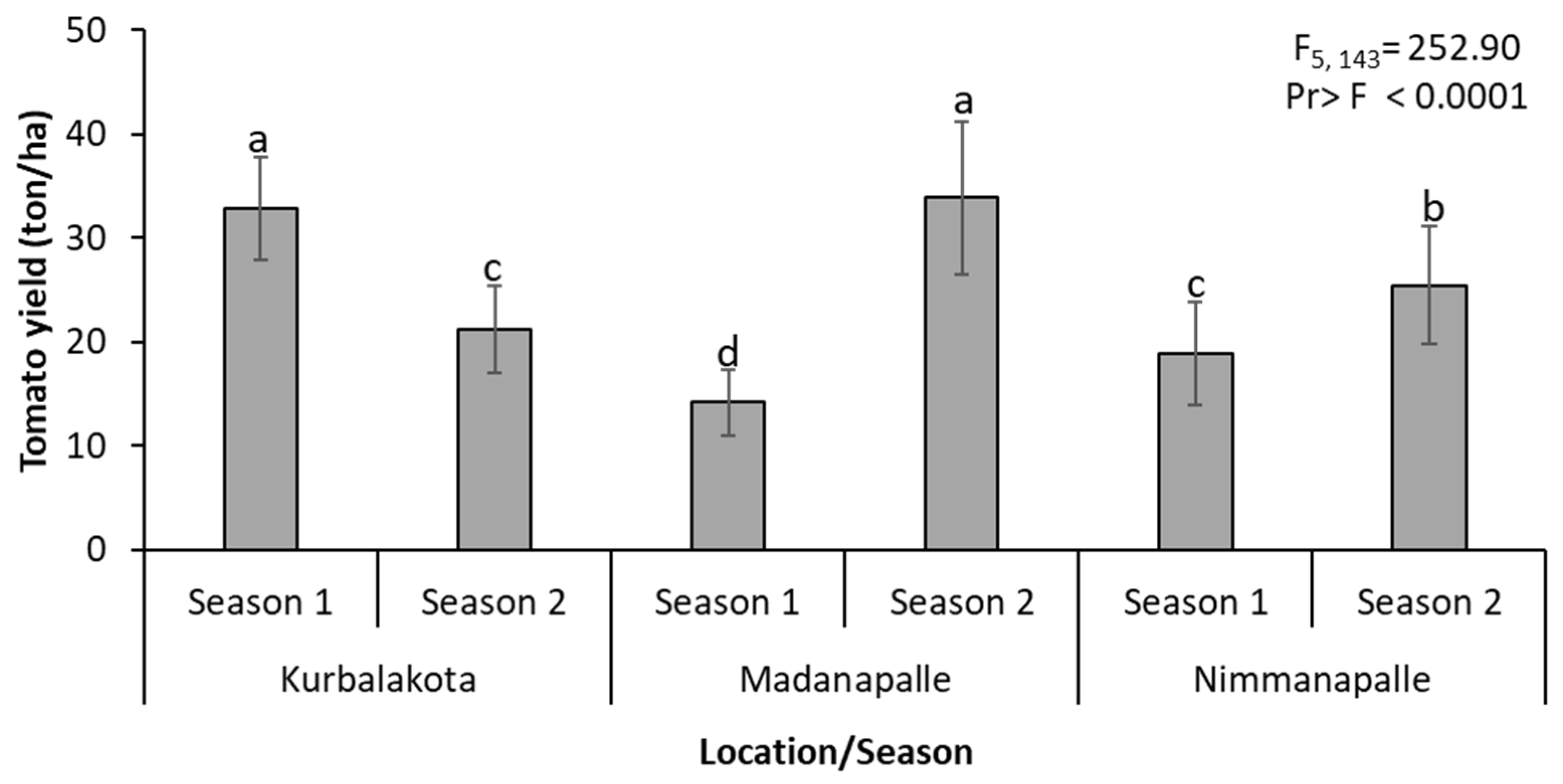
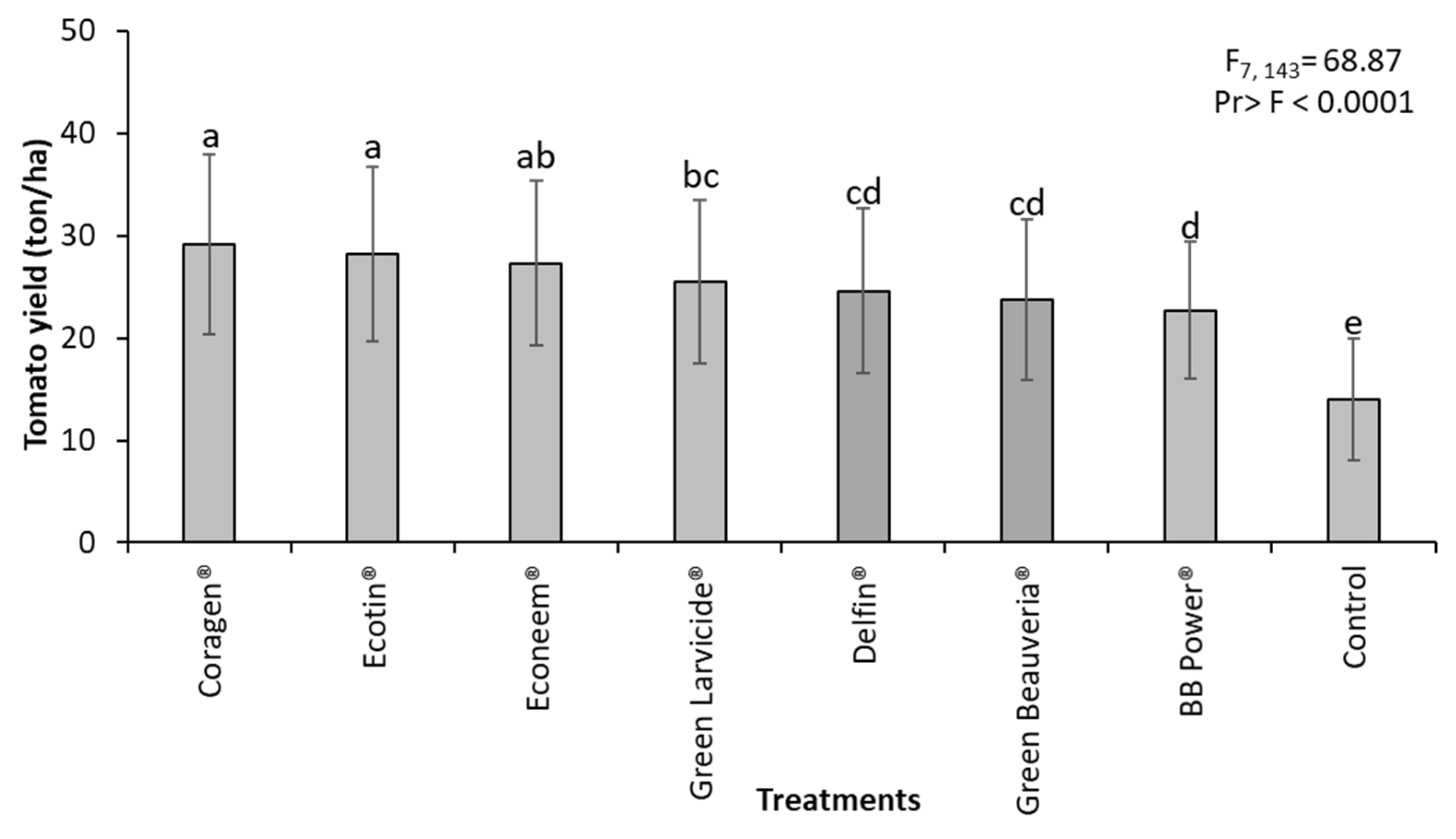
3.2. Field Experiments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agarwal, S.; Rao, A.O. Tomato lycopene and its role in human health and chronic diseases. Can. Med. Assoc. J. 2000, 163, 739–744. [Google Scholar]
- Raiola, A.; Tenore, G.C.; Barone, A.; Frusciante, L.; Rigano, M.M. Vitamin E content and composition in tomato fruits: Beneficial roles and bio-fortification. Int. J. Mol. Sci. 2015, 16, 29250–29264. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. FAOSTAT Statistical Database. 2018. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 1 November 2019).
- Government of India. Horticultural Statistics at a Glance. 2018. Available online: http://agricoop.nic.in/sites/default/files/Horticulture%20Statistics%20at%20a%20Glance-2018.pdf (accessed on 1 November 2019).
- Arora, S.; Kanojia, A.K.; Kumar, A.; Mogha, N.; Sahu, V. Bio-pesticide formulation to control tomato lepidopteran pest menace. Asian Agrihist. 2014, 18, 283–293. [Google Scholar]
- Mondal, B.; Mondal, P.; Das, A.; Bhattyacharyya, K. Seasonal incidence of different insect pests of tomato (Lycopersicon esculentum Mill.) and their correlation with abiotic factor in lateritic zone of West Bengal. J. Entomol. Zool. 2019, 7, 1426–1430. [Google Scholar]
- Shashank, P.R.; Chandrashekar, K.; Meshram, N.M.; Sreedevi, K. Occurrence of Tuta absoluta (Lepidoptera Gelechiidae), an invasive pest from India. Indian J. Entomol. 2015, 77, 323. [Google Scholar] [CrossRef]
- Sridhar, V.; Chakravarthy, A.K.; Asokan, R.; Vinesh, L.S.; Rebijith, K.B.; Vennila, S. New record of invasive South American tomato leaf miner, Tuta absoluta (Meyrick) (Lepidoptera Gelechiidae) in India. Pest Manag. Hort. Ecosyst. 2014, 20, 148–154. [Google Scholar]
- Biondi, A.; Guedes, R.N.C.; Wan, F.H.; Desneux, N. Ecology, world- wide spread, and management of the invasive South American tomato pinworm, Tuta absoluta: Past, present, and future. Annu. Rev. Entomol. 2018, 63, 239–258. [Google Scholar] [CrossRef]
- Desneux, N.; Luna, M.G.; Guillemaud, T.; Urbaneja, A. The invasive South American tomato pinworm, Tuta absoluta, continues to spread in Afro-Eurasia and beyond: The new threat to tomato world production. J. Pest Sci. 2011, 84, 403–408. [Google Scholar] [CrossRef]
- El Hajj, A.K.; Rizk, H.; Gharib, M.; Houssein, M.; Talej, V.; Taha, N.; Aleik, S.; Mousa, Z. Management of Tuta absoluta Meyrick (Lepidoptera Gelechiidae) using bio pesticides on tomato crop under Greenhouse conditions. J. Agric. Sci. 2017, 9, 123–129. [Google Scholar] [CrossRef][Green Version]
- Guedes, P.; Picanço, M.C. The tomato borer Tuta absoluta in South America: Pest status, management and insecticide resistance. Bull. OEPP 2012, 42, 211–216. [Google Scholar] [CrossRef]
- The Insecticide Resistance Action Committee. Tuta absoluta—The Tomato Leaf Miner or Tomato Borer. Recommendations for Sustainable and Effective Resistance Management. 2011. Available online: https://irac-online.org/content/uploads/2009/12/Tuta_brochure_print-version_11Oct11.pdf (accessed on 1 September 2017).
- Rao, J.U. Andhra Pradesh and Telangana Suffer from High Pesticide Residues. Deccan Chronicle. 2016. Available online: https://www.deccanchronicle.com/nation/current-affairs/181016/andhra-pradesh-and-telangana-suffer-from-high-pesticide-residues.html (accessed on 26 July 2021).
- Domínguez, A.; López, S.; Bernabé, A.; Guerrero, Á.; Quero, C. Influence of age, host plant and mating status in pheromone production and new insights on perception plasticity in Tuta absoluta. Insects 2019, 10, 256. [Google Scholar] [CrossRef]
- Lietti, M.M.M.; Botto, E.; Alzogaray, R.A. Insecticide resistance in Argentine populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Neotrop. Entomol. 2005, 34, 113–119. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Landgren, O.; Kyle, R.A.; Hoppin, J.A.; Beane Freeman, L.E.; Cerhan, J.R.; Katzmann, J.A.; Rajkumar, S.V.; Alavanja, M.C. Pesticide exposure and risk of monoclonal gammopathy of undetermined significance in the Agricultural Health Study. Blood 2009, 113, 6386–6391. [Google Scholar] [CrossRef]
- Khater, H.M. Prospects of botanical bio-pesticides in insect pest management. Pharmacologia 2012, 3, 641–656. [Google Scholar]
- Hosseinzadeh, A.; Aramideh, S.; Ghassemi-Kahrizeh, A. Efficacy of bio-insecticides on Tuta absoluta (Meyrick) (Lep. Gelechiidae) in laboratory and field conditions. AgricEngInt: CIGR J. 2019, 21, 164–170. [Google Scholar]
- Soares, M.A.; Campos, M.R.; Passos, L.C.; Carvalho, G.A.; Haro, M.M.; Lavoir, A.V.; Biondi, A.; Zappala, L.; Desneux, N. Botanical insecticide and natural enemies: A potential combination for pest management against Tuta absoluta. J. Pest Sci. 2019, 92, 1433–1443. [Google Scholar] [CrossRef]
- Abd El-Ghany, N.M.; Abdel-Razek, A.S.; Djelouah, K.; Moussa, A. Efficacy of bio-rational insecticides against Tuta absoluta (Meyrick) (Lepidoptera Gelechiidae) on tomatoes. Biosci. Res. 2018, 15, 28–40. [Google Scholar]
- Hossain, M.S.; Das, A.K.; Akhter, S.; Mian, M.Y.; Muniappan, R. Development of biorational management for tomato leaf miner, Tuta absoluta. J. Biol. Control 2019, 33, 132–136. [Google Scholar] [CrossRef]
- IRAC. Tuta absoluta Susceptibility Test Method #022. 2012. Available online: http://www.irac-online.org/methods/tuta-absoluta-larvae/ (accessed on 4 March 2017).
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Moore, J.K.; Dixon, P.M. Analysis of combined experiments revisited. Agron. J. 2015, 107, 763–771. [Google Scholar] [CrossRef]
- Peterson, R.G. Agriculture Field Experiments. Design and Analysis; Marcel Dekker: New York, NY, USA, 1994. [Google Scholar]
- Jallow, M.F.A.; Dahab, A.A.; Albaho, M.S.; Devi, V.Y. Efficacy of some biorational insecticides against Tuta absoluta (Meyrick) (Lepidoptera Gelechiidae) under laboratory and greenhouse conditions in Kuwait. J. Appl. Entomol. 2018, 143, 187–195. [Google Scholar] [CrossRef]
- Rodriguez, M.; Gerding, M.; France, A. Effectivity of entomopathogenic fungus strains on tomato moth Tuta absoluta Meyrick (Lepidoptera Gelechiidae) larvae. Agric. Tec. 2006, 66, 159–165. [Google Scholar]
- Gonzalez-Cabrera, J.; Molla, O.; Monton, H.; Urbaneja, A. Efficacy of Bacillus thuringiensis (Berliner) in controlling the tomato borer, Tuta absoluta (Meyrick) (Lepidoptera Gelechiidae). BioControl 2011, 56, 71–80. [Google Scholar] [CrossRef]
- Pires, L.M.; Marques, E.J.; de Olivieira, J.V.; Alves, S.B. Selection of isolates of entomopathogenic fungi for controlling Tuta absoluta (Meyrick) (Lepidoptera Gelechiidae) and their compatibility with insecticides used in tomato crop. Neotrop. Entomol. 2010, 39, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Theoduloz, C.; Vega, A.; Salazar, M.; González, E.; Meza-Basso, L. Expression of a Bacillus thuringiensis δ-endotoxin cry1Ab gene in Bacillus subtilis and Bacillus licheniformis strains that naturally colonize the phylloplane of tomato plants (Lycopersicon esculentum Mills). J. Appl. Microbiol. 2003, 94, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Kona, N.E.M.; Taha, A.K.; Mahmoud, M.E.E. Effects of botanical extracts of neem (Azadirachta indica) and Jatropha (Jatropha curcus) on eggs and larvae of tomato leaf miner, Tuta absoluta (Meyrick) (Lepidoptera Gelechiidae). Persian Gulf Crop Protec. 2014, 3, 41–46. [Google Scholar]
- Abd El-Ghany, N.M.; Abdel-Razek, A.S.; Ebadah, I.M.A.; Mahmoud, Y.A. Evaluation of some microbial agents, natural and chemical compounds for controlling tomato leaf miner, Tuta absoluta (Meyrick) (Lepidoptera Gelechiidae). J. Plant Prot. Res. 2016, 56, 372–379. [Google Scholar] [CrossRef]
- Tome, H.V.V.; Martins, J.C.; Correa, A.S.; Galdino, T.V.S.; Picanco, M.C.; Guedes, R.N.C. Azadirachtin avoidance by larvae and adult females of the tomato leafminer Tuta absoluta. Crop Prot. 2013, 46, 63–69. [Google Scholar] [CrossRef]
- Martinez, S.; van Emden, H.F. Growth disruption, abnormalities and mortality of Spodoptera littoralis (Boisduval) (Lepidoptera Noctuidae) caused by Azadirachtin. Neotrop. Entomol. 2001, 30, 113–125. [Google Scholar] [CrossRef]
- Berxolli, A.; Shahini, S. Azadirachtin, a useful alternative for controlling Tuta absoluta (Myerick). EJPAS. 2017, 5, 41–45. [Google Scholar]
- Srinivasan, G.; Dilipsundar, N. Evaluation of bio-efficacy of botanicals against tomato pinworm, Tuta absoluta (Meyrick, 1917). Pest Manag. Hort. Ecosyst. 2019, 25, 44–50. [Google Scholar]
- Yankova, V.; Valchev, N.; Markova, D. Effectiveness of phytopesticide neem azal T/S ® against tomato leaf miner (Tuta absoluta Meyrick) in greenhouse tomato. Bulg. J. Agric. Sci. 2014, 20, 1116–1118. [Google Scholar]
- Shiberu, T.; Getu, E. Evaluation of bio-pesticides on integrated management of tomato leafminer, Tuta absoluta (Meyrick) (Gelechiidae Lepidoptera) on tomato crops in Western Shewa of Central Ethiopia. Entomol. Ornithol. Herpetol. 2018, 07, 1–8. [Google Scholar] [CrossRef]
- Cunha, U.S.; Vendramim, J.D.; Rocha, W.C.; Vieira, P.C. Fractions of Trichilia pallens with insecticidal activity against Tuta absoluta. Pesq. agropec. Bras. 2006, 41, 1579–1585. [Google Scholar] [CrossRef][Green Version]
- Sabbour, M.M.; Soliman, N. Evaluations of three Bacillus thuringiensis against Tuta absoluta (Meyrick) (Lepidoptera Gelechiidae) in Egypt. Int. J. Sci. Res. 2014, 3, 2067–2073. [Google Scholar]
- Sabbour, M.M.; Singer, S.M. Observations of the effect of two isolated nano Bacillus thuringiensis on Tuta absoluta infestation under laboratory and field condition. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1891–1897. [Google Scholar] [CrossRef]
- Magda, S.; Moharam, M.E. Comparing the effect of seven isolated Bacillus thuringiensis against Tuta absoluta infesting in laboratory and field condition. Int. J. Sci. Res. 2015, 4, 458–462. [Google Scholar] [CrossRef]
- Rezaei, A.A.; Talaei-Hassanlous, R. The Use of Bacillus thuringiensis-based Products in Biocontrol of Tomato Leaf Miner, Tuta absoluta (Lepidoptera Gelechiidae). Int. J. Agric. Innov. Res. 2016, 4, 814–817. [Google Scholar]
- Harizia, A.; Benguerai, A.; Boukhari, Y. Larvicidal activity of Bacillus thuringiensis kurstaki against Tuta absoluta (Lepidoptera Gelechiidae). LSJ 2019, 20, 352–362. [Google Scholar] [CrossRef]
- Hilbeck, A.; Otto, M. Specificity and combinatorial effects of Bacillus thuringiensis Cry toxins in the context of GMO environmental risk assessment. Front. Environ. Sci. 2015, 3, 71. [Google Scholar] [CrossRef]
- Pang, A.S.; Gringorten, J.L. Degradation of Bacillus thuringiensis δ-endotoxin in host insect gut juice. FEMS Microbiol. Lett. 1998, 167, 281–285. [Google Scholar] [CrossRef]
- Broderick, N.A.; Robinson, C.J.; McMahon, M.D.; Holt, J.; Handelsman, J.; Raffa, K.F. Contributions of gut bacteria to Bacillus thuringiensis-induced mortality vary across a range of Lepidoptera. BMC Biol. 2009, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.; Zamudio, F.Z.; Bravo, A. Processing of Cry1Ab δ-endotoxin from Bacillus thuringiensis by Manduca sexta and Spodoptera frugiperda midgut proteases: Role in protoxin activation and toxin inactivation. Insect Biochem. Mol. Biol. 2001, 31, 1155–1163. [Google Scholar] [CrossRef]
- Bue, P.L.O.; Abbas, S.; Peri, E.; Colazza, S. Use of biorational insecticides for the control of Tuta absoluta (Meyrick) infestations on open field tomato. New Medit. 2012, 4, 39–41. [Google Scholar]
- Nazarpour, L.; Yarahmadi, F.; Saber, M.; Rajabpour, A. Short and long term effects of some bio-insecticides on Tuta absoluta Meyrick (Lepidoptera Gelechiidae) and its coexisting generalist predators in tomato fields. J. Crop Prot. 2016, 5, 331–342. [Google Scholar] [CrossRef]
- Youssef, N.A.; Hassan, G.M. Bioinsecticide activity of Bacillus thuringiensis isolates on tomato borer, Tuta absoluta (Meyrick) and their molecular identification. Afr. J. Biotechnol. 2013, 12, 3699–3709. [Google Scholar]
- Shalaby, H.H.; Faragalla, F.H.; El-Saadany, H.M.; Ibrahim, A.A. Efficacy of three entomopathogenic agents for control the tomato borer, Tuta absoluta (Meyrick) (Lepidoptera Gelechiidae). Nat. Sci. 2013, 11, 63–72. [Google Scholar]
- Klieber, J.; Reineke, A. The entomopathogen Beauveria bassiana has epiphytic and endophytic activity against the tomato leaf miner Tuta absoluta. J. Appl. Entomol. 2016, 140, 580–589. [Google Scholar] [CrossRef]
- Ndereyimana, A.; Nyalala, S.; Murerwa, P.; Gaidashova, S. Pathogenicity of some commercial formulations of entomopathogenic fungi on the tomato leaf miner, Tuta absoluta (Meyrick) (Lepidoptera Gelechiidae). Egypt J. Biol. Pest Co. 2019, 29, 1–5. [Google Scholar] [CrossRef]
- Marrone, P.G. The market and potential for biopesticides. In Biopesticides: State of the Art and Future Opportunities; Gross, A.D., Coats, J.R., Duke, S.O., Seiber, J.N., Eds.; American Chemical Society: Washington, DC, USA, 2014; pp. 245–258. [Google Scholar]
- Schrank, A.; Vainstein, M.H. Metarhizium anisopliae enzymes and toxins. Toxicon 2010, 56, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Fabrice, D.H.; Elie, D.A.; Kobi, D.O.; Valerien, A.; Thomas, H.A.; Joelle, T.; Maurille, E.I.A.T.; Denis, O.B.; Manuele, T. Toward the efficient use of Beauveria bassiana in integrated cotton insect pest management. J. Cotton Res. 2020, 3, 1–21. [Google Scholar]
- Neves, P.J.; Alves, S.B. Selection of Beauveria bassiana (Bals.) Vuill. and Metarhizium anisopliae (Metsch.) Sorok. strains for control of Cornitermes cumulans (Kollar). Braz Arch Biol Technol 2000, 43, 319–328. [Google Scholar] [CrossRef][Green Version]
- Kleespies, R.G.; Zimmermann, G. Effect of additives on the production, viability and virulence of blastospores of Metarhizium anisopliae. Biochem. Sci. Technol. 1998, 8, 207–214. [Google Scholar] [CrossRef]
- Borisade, O.A.; Magan, N. Growth and sporulation of entomopathogenic Beauveria bassiana, Metarhizium anisopliae, Isaria farinosa and Isaria fumosorosea strains in relation to water activity and temperature interactions. Biocontrol Sci. Technol. 2014, 24, 999–1011. [Google Scholar] [CrossRef]
- Agbessenou, A.; Akutse, K.S.; Yusuf, A.A.; Ekesi, S.; Subramanian, S.; Khamis, F.M. Endophytic fungi protect tomato and nightshade plants against Tuta absoluta (Lepidoptera Gelechiidae) through a hidden friendship and cryptic battle. Sci. Rep. 2020, 10, 1–13. [Google Scholar]
- Zappalà, L.; Bernardo, U.; Biondi, A.; Cocco, A.; Deliperi, S.; Delrio, G.; Giorgini, M.; Pedata, P.; Rapisarda, C.; Tropea Garzia, G.; et al. Recruitment of native parasitoids by the exotic pest Tuta absoluta in southern Italy. Bull Insectol. 2012, 65, 51–61. [Google Scholar]
- Nozad-Bonab, Z.; Hejazi, M.J.; Iranipour, S.; Arzanlou, M.; Biondi, A. Lethal and sublethal effects of synthetic and bio-insecticides on Trichogramma brassicae parasitizing Tuta absoluta. PLoS ONE 2021, 16, e243334. [Google Scholar] [CrossRef] [PubMed]
- Contreras, J.; Mendoza, J.E.; Martınez-Aguirre, M.R.; Garcıa-Vidal, J.; Izquierdo and Bielza, P. Efficacy of entomopathogenic fungus Metarhizium anisopliae against Tuta absoluta (Lepidoptera Gelechiidae). J. Eco. Entomol. 2014, 107, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Olson, S. An analysis of the biopesticide market now and where is going. Outlooks Pest Manag. 2015, 26, 203–206. [Google Scholar] [CrossRef]
- Das, S.K. Recent development and future of botanical pesticides in India. Popular Kheti 2014, 2, 93–99. [Google Scholar]
- Sharma, K.R.; Raju, S.V.S.; Jaiswal, D.K.; Thakur, S. Biopesticides: An effective tool for insect pest management and current scenario in India. IJAAS 2018, 4, 59–62. [Google Scholar]
- Zhu, F.; Lavine, L.; Neal, S.O.; Lavine, M.; Foss, C.; Walsh, D. Insecticide Resistance and Management Strategies in Urban Ecosystems. Insects 2016, 7, 2. [Google Scholar] [CrossRef]
- Huang, F.; Buschman, L.L.; Higgins, R.A.; McGaughey, W.H. Inheritance of resistance to Bacillus thuringiensis toxin (Dipel ES) in the European corn borer. Science 1999, 284, 965–967. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Aguda, R.M.; Cohen, M.B.; Gould, F.L.; Dean, D.H. Determination of binding of Bacillus thuringiensis δ-endotoxin receptors to rice stem borer midguts. Appl. Environ. Microbiol. 1997, 63, 1453–1459. [Google Scholar] [CrossRef]
- Srinivasan, R. Susceptibility of legume pod borer (LPB), Maruca vitrata to δ-endotoxins of Bacillus thuringiensis (Bt) in Taiwan. J. Invertebr. Pathol. 2008, 97, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Buragohain, P.; Saikia, D.K.; Sotelo-Cardona, P.; Ramasamy, S. Development and validation of an integrated pest management strategy against the invasive South American tomato leaf miner, Tuta absoluta in South India. Crop. Prot. 2021, 139, 1–6. [Google Scholar] [CrossRef]
- Shahini, S.; Bërxolli, A.; Kokojka, F. Effectiveness of bio-insecticides and mass trapping based on population fluctuations for controlling Tuta absoluta under greenhouse conditions in Albania. Heliyon 2021, 7, e05753. [Google Scholar] [CrossRef]

| Bio-Pesticides | Trade Name | Dosage a | Source of Bio-Pesticides |
|---|---|---|---|
| Bacillus thuringiensis kurstaki (2 × 1011 CFU/mL) | Green Larvicide® | 5 mL/L of water. | Greenlife Biotech Laboratory, Coimbatore, Tamil Nadu, India |
| Bacillus thuringiensis kurstaki (6 × 107 spores/mg) | Delfin® WG | 1 gm/L of water | Margo Biocontrols Private Limited, Bangalore, Karnataka, India |
| Beauveria bassiana (2 × 108 spores/mL) | Green Beauveria® | 10 mL/L of water | Greenlife Biotech Laboratory, Coimbatore, Tamil Nadu, India |
| Beauveria bassiana (1 × 108 CFU/gram) | BB Power® | 5 gm/L of water | K N Biosciences (India) Pvt. Ltd., Hyderabad, Telangana, India |
| Azadirachtin 10,000 ppm | Econeem Plus ® | 3 mL/L of water | Margo Biocontrols Private Limited, Bangalore, Karnataka, India |
| Azadirachtin 50,000 ppm | Ecotin® | 1 mL/L of water | Margo Biocontrols Private Limited, Bangalore, Karnataka, India |
| Chlorantraniliprole | Coragen 20 SC | 0.4 mL/L of water | Agriplex Private Limited, Bangalore, Karnataka, India |
| Bio-Pesticide | Concentration |
|---|---|
| Green Larvicide (Bacillus thuringiensis kurstaki) (2 × 1011 CFU/mL) | 1 × 107, 1 × 108, 1 × 109, 1 × 1010, 1 × 1011 |
| Delfin (Bacillus thuringiensis kurstaki) (6 × 107 spores/mg) | 6 × 106, 1.8 × 107, 3 × 107, 4.2 × 107, 6 × 107 |
| Green Beauveria (Beuveria bassiana) (2 × 108 spores/mL) | 1 × 107, 2 × 107, 4 × 107, 6 × 107, 8 × 107 |
| BB Power (Beauveria bassiana) (1 × 108 CFU/g) | 1 × 106, 1 × 107, 2 × 107, 4 × 107, 5 × 107 |
| Econeem Plus—Azadirachtin 10,000 ppm | 50, 500, 1000, 1500, 2000, 2500 |
| Ecotin—Azadirachtin 50,000 ppm | 50, 500, 1000, 2500, 5000 |
| Bio-Pesticide | LC50 | Fiducial Limit | Slope | X2 |
|---|---|---|---|---|
| Green Larvicide (Bacillus thuringiensis kurstaki) (2 × 1011 CFU/mL) | 4.10 × 109 | 1.67 × 109–1.25 × 1010 | 0.293 ± 0.042 | 1.054 |
| Delfin (Bacillus thuringiensis kurstaki) (6 × 107 spores/mg) | 8.06 × 106 | 6.35 × 106–9.68 × 106 | 2.163 ± 0.197 | 2.368 |
| Green Beauveria (Beuveria bassiana) (2 × 108 spores/mL) | 4.473 × 107 | 3.648 × 107–5.638 × 107 | 1.361 ± 0.185 | 1.756 |
| BB Power (Beauveria bassiana) (1 × 108 CFU/g) | 1.367 × 107 | 8.597 × 106–2.158 × 107 | 0.595 ± 0.098 | 6.05 |
| Econeem Plus–Azadirachtin 10,000 ppm | 212.676 | 118.352–319.565 | 0.703 ± 0.094 | 8.855 |
| Ecotin–Azadirachtin 50,000 ppm | 91.866 | 14.163–220.892 | 0.373 ± 0.084 | 6.959 |
| Source | df | Leaf Infestation (%) | Fruit Infestation (%) | Yield | |||
|---|---|---|---|---|---|---|---|
| F | Pr > F | F | Pr > F | F | Pr > F | ||
| Location | 5 | 56.39 | <0.0001 | 47.70 | <0.0001 | 252.9 | <0.0001 |
| Block | 12 | 0.71 | 0.7435 | 0.86 | 0.5902 | 0.98 | 0.4719 |
| Treatment | 7 | 205.69 | <0.0001 | 113.85 | <0.0001 | 68.87 | <0.0001 |
| LocationXTreatment | 35 | 3.93 | <0.0001 | 5.86 | <0.0001 | 1.48 | 0.0756 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buragohain, P.; Saikia, D.K.; Sotelo-Cardona, P.; Srinivasan, R. Evaluation of Bio-Pesticides against the South American Tomato Leaf Miner, Tuta absoluta Meyrick (Lepidoptera: Gelechiidae) in India. Horticulturae 2021, 7, 325. https://doi.org/10.3390/horticulturae7090325
Buragohain P, Saikia DK, Sotelo-Cardona P, Srinivasan R. Evaluation of Bio-Pesticides against the South American Tomato Leaf Miner, Tuta absoluta Meyrick (Lepidoptera: Gelechiidae) in India. Horticulturae. 2021; 7(9):325. https://doi.org/10.3390/horticulturae7090325
Chicago/Turabian StyleBuragohain, Priyakshi, Dilip Kumar Saikia, Paola Sotelo-Cardona, and Ramasamy Srinivasan. 2021. "Evaluation of Bio-Pesticides against the South American Tomato Leaf Miner, Tuta absoluta Meyrick (Lepidoptera: Gelechiidae) in India" Horticulturae 7, no. 9: 325. https://doi.org/10.3390/horticulturae7090325
APA StyleBuragohain, P., Saikia, D. K., Sotelo-Cardona, P., & Srinivasan, R. (2021). Evaluation of Bio-Pesticides against the South American Tomato Leaf Miner, Tuta absoluta Meyrick (Lepidoptera: Gelechiidae) in India. Horticulturae, 7(9), 325. https://doi.org/10.3390/horticulturae7090325








