Evaluation of Lettuce (Lactuca sativa L.) Production under Hydroponic System: Nutrient Solution Derived from Fish Waste vs. Inorganic Nutrient Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growth Conditions
2.2. Experimental Design
2.3. Growth Parameters
2.3.1. Leaf Area
2.3.2. Stomata Density
2.3.3. Total Chlorophyll and Carotene Analysis
2.3.4. Total Phenolics, Flavonoids Content and Antioxidant Activity
2.4. Nutrient Content of Lettuce Leaf
2.5. Statistical Analysis
3. Results and Discussion
3.1. Plant Height, Number of Leaves, and Leaf Area
3.2. Fresh and Dry Weights
3.3. Stomatal Density
3.4. Chlorophyll and Carotene Contents
3.5. Total Phenolic and Flavonoid Contents
3.6. Antioxidant Activity
3.7. Lettuce Nutrient Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sapkota, S.; Sapkota, S.; Liu, Z. Effects of nutrient composition and lettuce cultivar on crop production in hydroponic culture. Horticulturae 2019, 5, 72. [Google Scholar] [CrossRef] [Green Version]
- Mulabagal, V.; Ngouajio, M.; Nair, A.; Zhang, Y.; Gottumukkala, A.L.; Nair, M.G. In vitro evaluation of red and green lettuce (Lactuca sativa) for functional food properties. Food Chem. 2010, 118, 300–306. [Google Scholar] [CrossRef]
- Xylia, P.; Chrysargyris, A.; Tzortzakis, N. The combined and single effect of Marjoram essential oil, ascorbic acid, and chitosan on fresh-cut lettuce preservation. Foods 2021, 10, 575. [Google Scholar] [CrossRef]
- Kaiser, C.; Ernst, M. Hydroponic Lettuce CCDCP-63; Center for Crop Diversification, University of Kentucky College of Agriculture, Food and Environment: Lexington, KY, USA, 2012; Available online: http://www.uky.edu/ccd/sites/www.uky.edu.ccd/files/hydrolettuce.pdf (accessed on 20 January 2021).
- Qadeer, A.; Butt, S.J.; Asam, H.M.; Mehmood, T.; Nawaz, M.K.; Haidree, S.R. Hydroponics as an innovative technique for lettuce production in greenhouse environment. Pure Appl. Biol. 2020, 9, 20–26. [Google Scholar] [CrossRef]
- Moncada, A.; Miceli, A.; Vetrano, F. Use of plant growth-promoting rhizobacteria (PGPR) and organic fertilization for soilless cultivation of basil. Sci. Hortic. 2021, 275, 109733. [Google Scholar] [CrossRef]
- Koukounaras, A. Advanced greenhouse horticulture: New technologies and cultivation practices. Horticulturae 2021, 7, 1. [Google Scholar] [CrossRef]
- Singh, R.; Upadhyay, S.; Diwakar, A.; Sharma, I.; Affiliation, N. A study on hydroponic farming system of wheat, spinach and sword lily for sustainable development of agriculture. Bio Sci. Res. Bull. 2020, 35, 58–63. [Google Scholar] [CrossRef]
- Sardare, M.; Admane, S. A review on soil-less culture. Int. J. Res. Eng. Technol. 2013, 2, 299–304. [Google Scholar]
- Lommen, W.J.M. The canon of potato science: Hydroponics. Potato Res. 2008, 50, 315–318. [Google Scholar] [CrossRef] [Green Version]
- Gashgari, R.; Alharbi, K.; Mughrbil, K.; Jan, A.; Glolam, A. Comparison between growing plants in hydroponic system and soil based system. In Proceedings of the 4th World Congress on Mechanical, Chemical, and Material Engineering, ICMIE, Madrid, Spain, 16–18 August 2018; pp. 1–7. [Google Scholar]
- De Souza, P.F.; Borghezan, M.; Zappelini, J.; de Carvalho, L.R.; Ree, J.; Barcelos-Oliveira, J.L. Physiological differences of ‘Crocantela’ lettuce cultivated in conventional and hydroponic systems. Hortic. Bras. 2019, 37, 101–105. [Google Scholar] [CrossRef]
- Massa, D.; Magán, J.J.; Montesano, F.F.; Tzortzakis, N. Minimizing water and nutrient losses from soilless cropping in southern Europe. Agric. Water Manag. 2020, 241, 106395. [Google Scholar] [CrossRef]
- Williams, K.A.; Nelson, J.S. Challenges of using organic fertilizers in hydroponic production systems. Acta Hortic. 2016, 1112, 365–370. [Google Scholar] [CrossRef]
- Gorenjak, A.H.; Koležnik, U.R.; Cencič, A. Nitrate content in dandelion (Taraxacum officinale) and lettuce (Lactuca sativa) from organic and conventional origin: Intake assessment. Food Addit. Contam. Part B Surveill. 2012, 5, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Chrysargyris, A.; Xylia, P.; Anastasiou, M.; Pantelides, I.; Tzortzakis, N. Effects of Ascophyllum nodosum seaweed extracts on lettuce growth, physiology and fresh-cut salad storage under potassium deficiency. J. Sci. Food Agric. 2018, 98, 5861–5872. [Google Scholar] [CrossRef]
- Burnett, S.E.; Mattson, N.S.; Williams, K.A. Substrates and fertilizers for organic container production of herbs, vegetables, and herbaceous ornamental plants grown in greenhouses in the United States. Sci. Hortic. 2016, 208, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Burnett, S.E.; Stack, L.B. Survey of the Research needs of the potential organic ornamental bedding plant industry in maine. HortTechnology 2009, 19, 743. [Google Scholar] [CrossRef] [Green Version]
- Treadwell, D.D.; Hochmuth, G.J.; Hochmuth, R.C.; Simonne, E.H.; Davis, L.L.; Laughlin, W.L. Nutrient management in organic greenhouse herb production: Where are we now? HortTechnology 2007, 17, 461–466. [Google Scholar] [CrossRef] [Green Version]
- Shubha; Mukherjee, A.; Dubey, A.; Koley, T. Bioponics—A new way to grow soilless vegetable cultivation. Agric. Food Mag. 2019, 1. [Google Scholar] [CrossRef]
- Bi, G.; Evans, W.B.; Spiers, J.M.; Witcher, A.L. Effects of organic and inorganic fertilizers on marigold growth and flowering. HortScience 2010, 45, 1373–1377. [Google Scholar] [CrossRef]
- Di Gioia, F.; Rosskopf, E. Organic hydroponics: A U.S. reality challenging the traditional concept of “Organic” and “Soilless” cultivation. Acta Hortic. in press.
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. Circ. 1950, 347, 1–32. [Google Scholar]
- Miyazawa, S.I.; Livingston, N.J.; Turpin, D.H. Stomatal development in new leaves is related to the stomatal conductance of mature leaves in poplar (Populus trichocarpa × P. deltoides). . J. Exp. Bot. 2005, 57, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Viacava, G.E.; Roura, S.I.; Agüero, M.V. Optimization of critical parameters during antioxidants extraction from butterhead lettuce to simultaneously enhance polyphenols and antioxidant activity. Chemom. Intell. Lab. Syst. 2015, 146, 47–54. [Google Scholar] [CrossRef]
- Ahmed, Z.F.R.; Alblooshi, S.S.N.A.; Kaur, N.; Maqsood, S.; Schmeda-Hirschmann, G. Synergistic effect of preharvest spray application of natural elicitors on storage life and bioactive compounds of date palm (Phoenix dactylifera L., cv. Khesab). Horticulturae 2021, 7, 145. [Google Scholar] [CrossRef]
- Hseu, Z.Y.; Chen, Z.S.; Tsai, C.C.; Tsui, C.C.; Cheng, S.F.; Liu, C.L.; Lin, H.T. Digestion methods for total heavy metals in sediments and soils. Water Air Soil Pollut. 2002, 141, 189–205. [Google Scholar] [CrossRef]
- Bremner, J.; Mulvaney, C. Total Nitrogen; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Atkin, K.; Nichols, M. Organic hydroponics. Acta Hortic. 2004, 648, 121–127. [Google Scholar] [CrossRef]
- Ahmed, Z.F.R.; Askri, A.; Alnuaimi, A.; Altamimi, A.; Alnaqbi, M. Liquid fertilizer as a potential alternative nutrient solution for strawberry production under greenhouse condition. Acta Hortic. in press.
- Gaskell, M.; Smith, R. Nitrogen sources for organic vegetable crops. HortTechnology 2007, 17, 431–441. [Google Scholar] [CrossRef] [Green Version]
- Lau, V.; Mattson, N. Effects of hydrogen peroxide on organically fertilized hydroponic lettuce (Lactuca sativa L.). Horticulturae 2021, 7, 106. [Google Scholar] [CrossRef]
- Fallovo, C.; Rouphael, Y.; Cardarelli, M.; Rea, E.; Battistelli, A.; Colla, G. Yield and quality of leafy lettuce in response to nutrient solution composition and growing season. J. Food Agric. Environ. 2009, 7, 456–462. [Google Scholar]
- Ellis, B.; Foth, H. Soil Fertility; Lewis CRC Press LLC: Boca Raton, FL, USA, 1996. [Google Scholar]
- Bertolino, L.T.; Caine, R.S.; Gray, J.E. Impact of stomatal density and morphology on water-use efficiency in a changing world. Front. Plant Sci. 2019, 10, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakoda, K.; Yamori, W.; Shimada, T.; Sugano, S.S.; Hara-Nishimura, I.; Tanaka, Y. Higher stomatal density improves photosynthetic induction and biomass production in arabidopsis under fluctuating light. Front. Plant Sci. 2020, 11, 1609. [Google Scholar] [CrossRef] [PubMed]
- Broadley, M.R.; Escobar-Gutiérrez, A.J.; Burns, A.; Burns, I.G. Nitrogen-limited growth of lettuce is associated with lower stomatal conductance. New Phytol. 2001, 152, 97–106. [Google Scholar] [CrossRef]
- Büssis, D.; von Groll, U.; Fisahn, J.; Altmann, T. Stomatal aperture can compensate altered stomatal density in Arabidopsis thaliana at growth light conditions. Funct. Plant Biol. 2006, 33, 1037–1043. [Google Scholar] [CrossRef]
- Doheny-Adams, T.; Hunt, L.; Franks, P.J.; Beerling, D.J.; Gray, J.E. Genetic manipulation of stomatal density influences stomatal size, plant growth and tolerance to restricted water supply across a growth carbon dioxide gradient. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 547–555. [Google Scholar] [CrossRef]
- Phibunwatthanawong, T.; Riddech, N. Liquid organic fertilizer production for growing vegetables under hydroponic condition. Int. J. Recycl. Org. Waste Agric. 2019, 8, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Gamon, J.A.; Surfus, J.S. Assessing leaf pigment content and activity with a reflectometer. New Phytol. 1999, 143, 105–117. [Google Scholar] [CrossRef]
- Carter, G.A.; Spiering, B.A. Optical properties of intact leaves for estimating chlorophyll concentration. J. Environ. Qual. 2002, 31, 1424–1432. [Google Scholar] [CrossRef]
- Rambo, L.; Ma, B.L.; Xiong, Y.; da Silvia, R.F.P. Leaf and canopy optical characteristics as crop-N-status indicators for field nitrogen management in corn. J. Plant Nutr. Soil Sci. 2010, 173, 434–443. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viacava, G.E.; Goyeneche, R.; Goñi, M.G.; Roura, S.I.; Agüero, M.V. Natural elicitors as preharvest treatments to improve postharvest quality of Butterhead lettuce. Sci. Hortic. 2018, 228, 145–152. [Google Scholar] [CrossRef]
- Nicolle, C.; Carnat, A.; Fraisse, D.; Lamaison, J.L.; Rock, E.; Michel, H.; Amouroux, P.; Remesy, C. Characterisation and variation of antioxidant micronutrients in lettuce (Lactuca sativa folium). J. Sci. Food Agric. 2004, 84, 2061–2069. [Google Scholar] [CrossRef]
- Zandvakili, O.R.; Barker, A.V.; Hashemi, M.; Etemadi, F.; Autio, W.R. Comparisons of commercial organic and chemical fertilizer solutions on growth and composition of lettuce. J. Plant Nutr. 2019, 42, 990–1000. [Google Scholar] [CrossRef]
- Bryson, G.; Mills, H.; Sasseville, D.; Jones, J.B., Jr.; Barker, A. Plant Analysis Handbook III; Micro-Macro Publishing: Athens, GA, USA, 2014. [Google Scholar]
- Etemadi, F.; Hashemi, M.; Randhir, R.; ZandVakili, O.; Ebadi, A. Accumulation of l-DOPA in various organs of faba bean and influence of drought, nitrogen stress, and processing methods on l-DOPA yield. Crop J. 2018, 6, 426–434. [Google Scholar] [CrossRef]
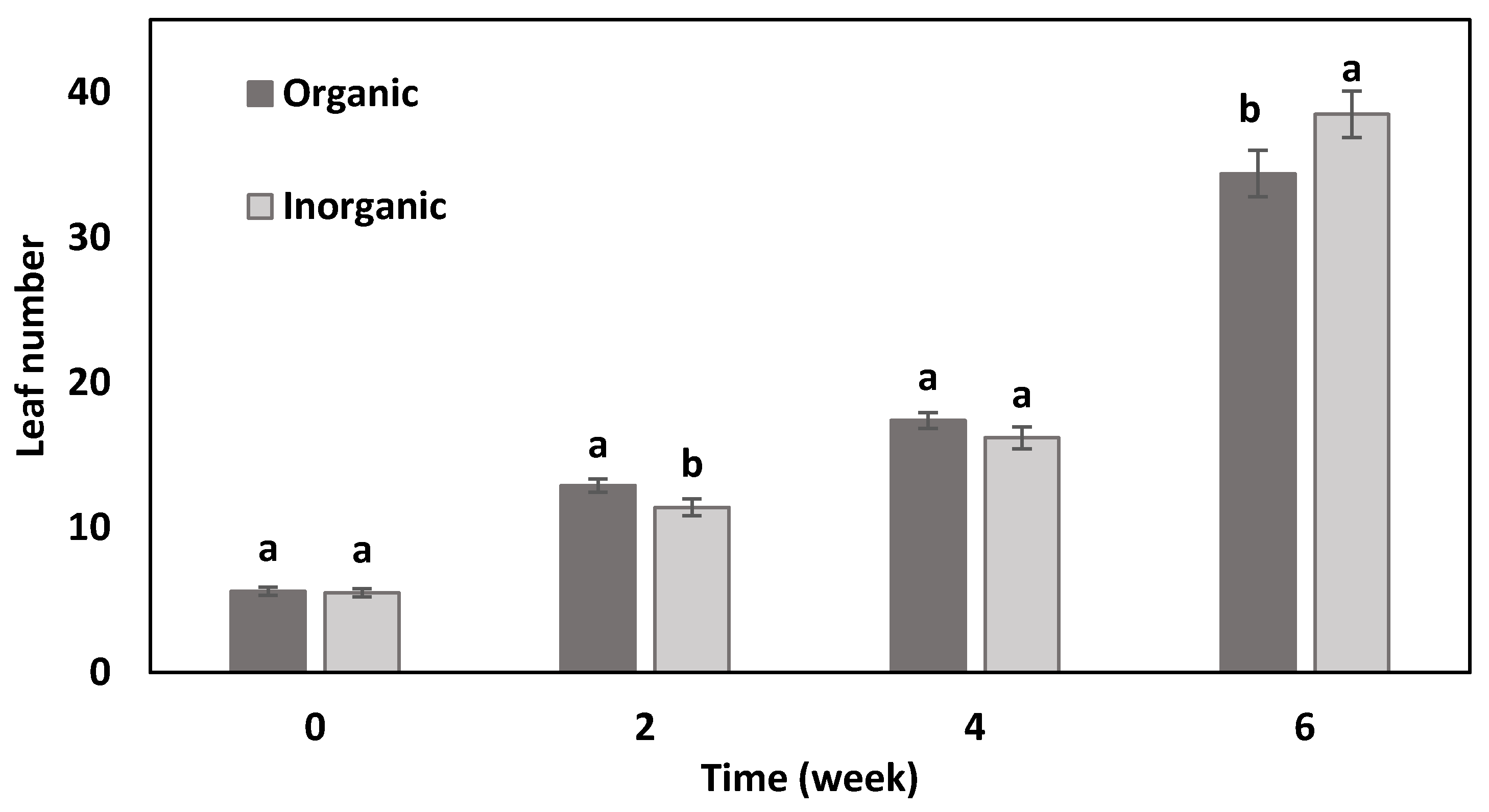
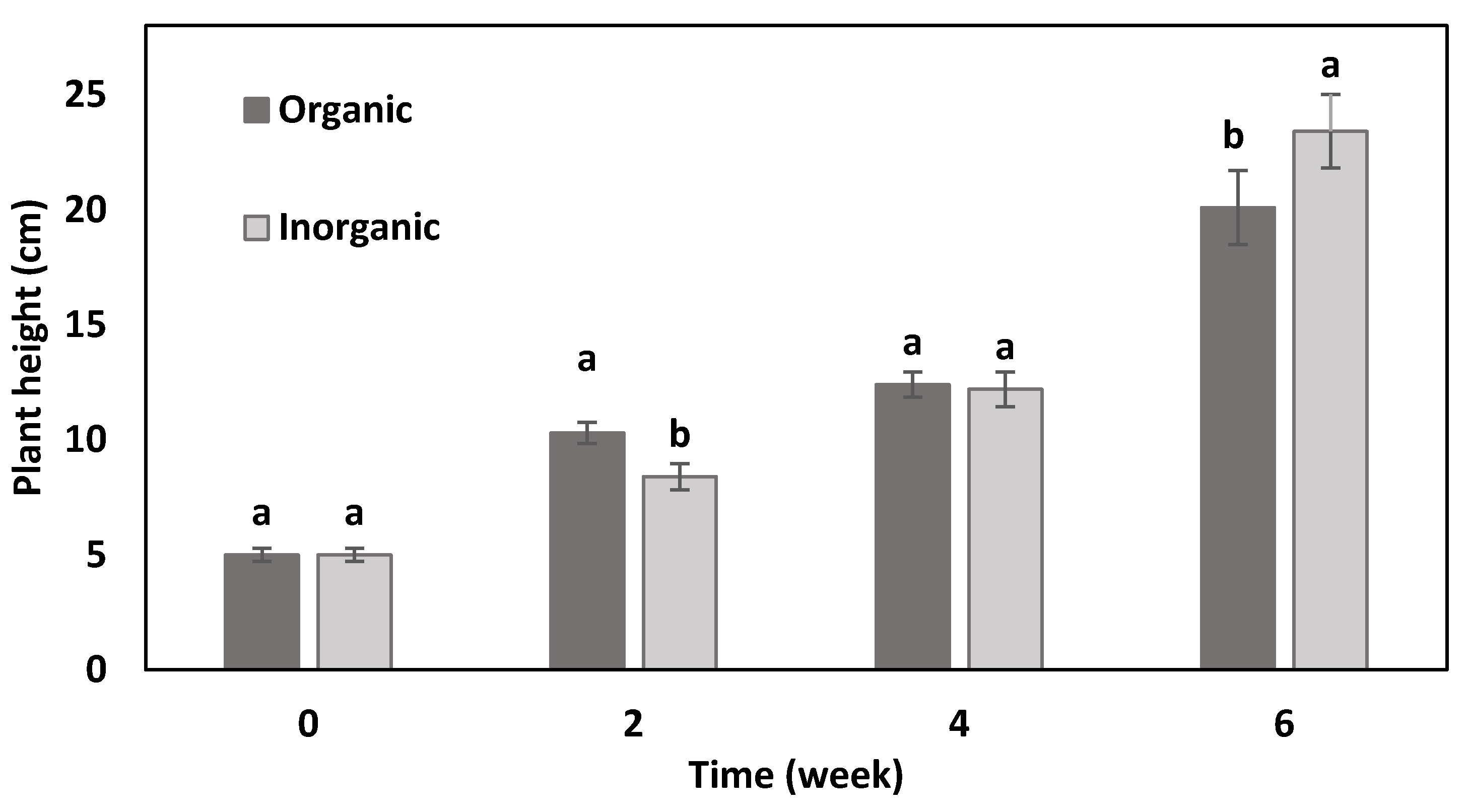
| Treatment | Yield (g/Plant) | Root Fresh Mass (g/Plant) | Shoot Dry Mass (%) | Root Dry Mass (%) | Leaf Area (cm2) | Stomata Density/mm2 disc |
|---|---|---|---|---|---|---|
| Organic | 163.9 ± 2.7 b | 36.1 ± 1.7 b | 7.01 ± 0.22 a | 17.3 ± 0.32 a | 172.32 ± 1.25 b | 28.6 ± 0.85 b |
| Inorganic | 182.3 ± 2.2 a | 37.9 ± 1.9 a | 7.4 ± 0.46 a | 17.1 ± 0.37 a | 181.37 ± 1.36 a | 36.3 ± 0.77 a |
| Time (Week) | Treatment | Leaf Pigment (mg/g FW) | |||
|---|---|---|---|---|---|
| Total Ch | Ch a | Ch b | Carotene | ||
| Week 4 | Organic | 1.060 ± 0.03 a | 0.811 ± 0.08 b | 0.252 ± 0.012 a | 0.443 ± 0.04 a |
| Inorganic | 1.040 ± 0.05 a | 0.833 ± 0.06 b | 0.211 ± 0.02 b | 0.432 ± 0.03 a | |
| Week 6 | Organic | 1.181 ± 0.04 a | 0.891 ± 0.04 a | 0.293 ± 0.02 a | 0.223 ± 0.02 a |
| Inorganic | 1.040 ± 0.03 b | 0.731 ± 0.02 b | 0.313 ± 0.02 a | 0.162 ± 0.01 b | |
| Organic | Inorganic | |
|---|---|---|
| Total phenolics (mg/100 g) | 3168.6 ± 61.5 a | 2715.3 ± 49.6 b |
| Total flavonoids (mg/100 g) | 714.7 ± 42.4 a | 538.6 ± 25.6 b |
| ABTS (mg/100 g) | 810.7 ± 10.1 a | 723.6 ± 12.7 b |
| IC50 (ug/mL) | 153.1 ± 1.8 b | 157.0 ± 2.1 a |
| Element (mg/kg) | Organic | Inorganic |
|---|---|---|
| N | 33,300.2 ± 87.4 a | 28,200.4 ± 93.3 b |
| K | 10,400.3 ± 66.4b | 66,500.2 ± 68.1 a |
| P | 6822.4 ± 58.5 a | 2631.2 ± 37.9 b |
| Ca | 13,009.4 ± 71.2 b | 7630.0 ± 53.2 a |
| Mg | 2852.5 ± 31.1 b | 3626.49 ± 21.3 a |
| Na | 23,280.2 ± 47.1 a | 11,104.2 ± 55.6 b |
| S | 2129.5 ± 36.7 a | 2539.5 ± 47.3 a |
| Mn | 30.90 ± 1.4 b | 57.14 ± 3.8 a |
| Fe | 169.8 ± 2.5 a | 119.5 ± 6.4 b |
| Zn | 39.38 ± 1.12 b | 105.79 ± 5.3 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, Z.F.R.; Alnuaimi, A.K.H.; Askri, A.; Tzortzakis, N. Evaluation of Lettuce (Lactuca sativa L.) Production under Hydroponic System: Nutrient Solution Derived from Fish Waste vs. Inorganic Nutrient Solution. Horticulturae 2021, 7, 292. https://doi.org/10.3390/horticulturae7090292
Ahmed ZFR, Alnuaimi AKH, Askri A, Tzortzakis N. Evaluation of Lettuce (Lactuca sativa L.) Production under Hydroponic System: Nutrient Solution Derived from Fish Waste vs. Inorganic Nutrient Solution. Horticulturae. 2021; 7(9):292. https://doi.org/10.3390/horticulturae7090292
Chicago/Turabian StyleAhmed, Zienab F. R., Alghazal K. H. Alnuaimi, Amira Askri, and Nikolaos Tzortzakis. 2021. "Evaluation of Lettuce (Lactuca sativa L.) Production under Hydroponic System: Nutrient Solution Derived from Fish Waste vs. Inorganic Nutrient Solution" Horticulturae 7, no. 9: 292. https://doi.org/10.3390/horticulturae7090292
APA StyleAhmed, Z. F. R., Alnuaimi, A. K. H., Askri, A., & Tzortzakis, N. (2021). Evaluation of Lettuce (Lactuca sativa L.) Production under Hydroponic System: Nutrient Solution Derived from Fish Waste vs. Inorganic Nutrient Solution. Horticulturae, 7(9), 292. https://doi.org/10.3390/horticulturae7090292







