Configuration by Osmotic Eustress Agents of the Morphometric Characteristics and the Polyphenolic Content of Differently Pigmented Baby Lettuce Varieties in Two Successive Harvests
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Growing Condition, and Experimental Design
2.2. Morphological Measurements
2.3. Quantification of Polyphenols
2.4. Statistical Analysis
3. Results
3.1. Effects on the Morphological Parameters
3.2. Effect on Polyphenols and Anthocyanins
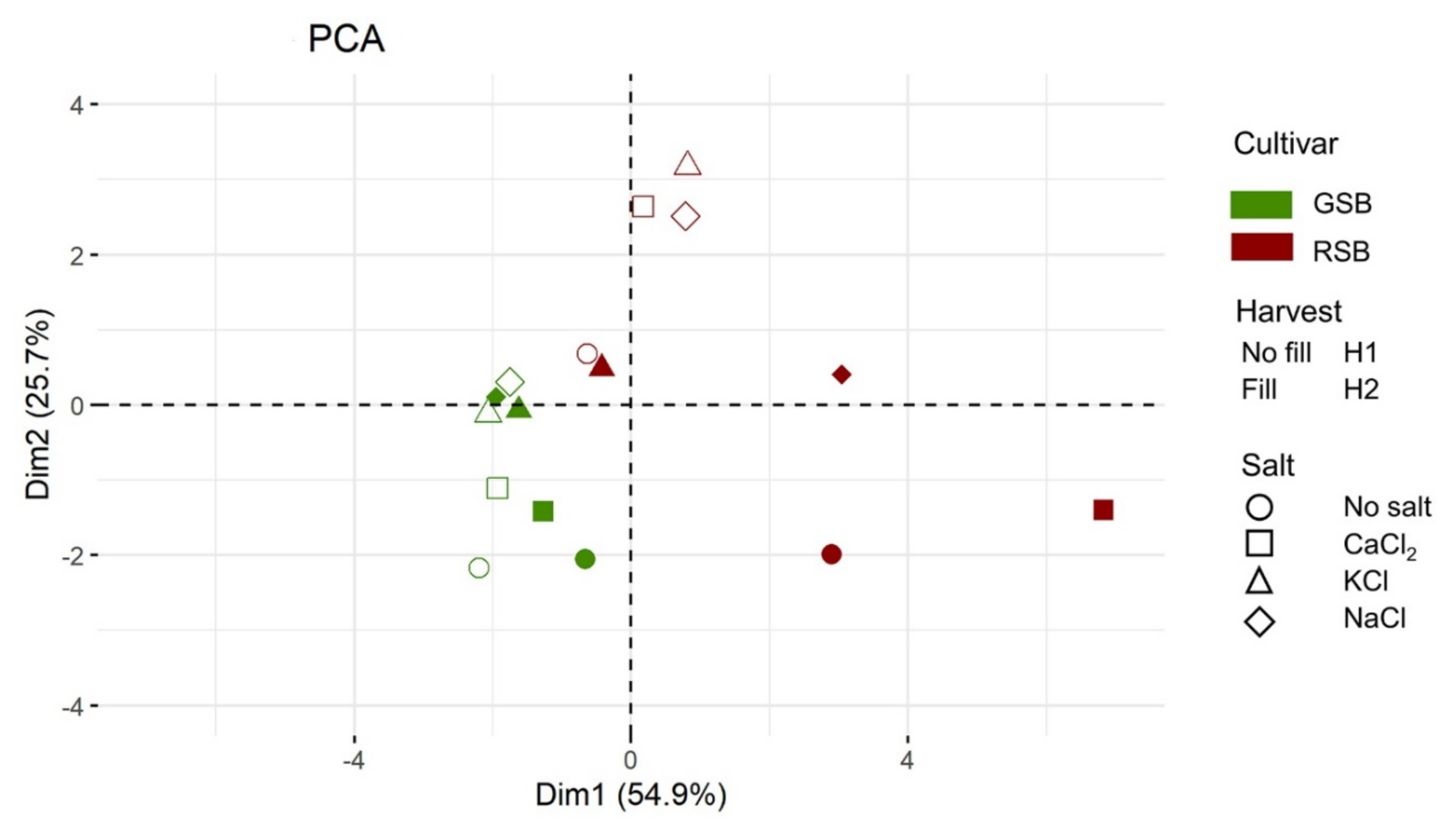
3.3. Multivariate Analysis of the Lettuce Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mou, B. Lettuce. Vegetables I; Springer: New York, NY, USA, 2008; pp. 75–116. [Google Scholar]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Martínez-Sánchez, A.; Luna, M.C.; Selma, M.V.; Tudela, J.A.; Abad, J.; Gil, M.I. Baby-leaf and multi-leaf of green and red lettuces are suitable raw materials for the fresh-cut industry. Postharvest Biol. Technol. 2012, 63, 1–10. [Google Scholar] [CrossRef]
- Sharma, N.; Acharya, S.; Kumar, K.; Singh, N.; Chaurasia, O. Hydroponics as an advanced technique for vegetable production: An overview. J. Soil Water Conserv. 2018, 17, 364–371. [Google Scholar] [CrossRef]
- Tudela, J.A.; Marín, A.; Martínez-Sánchez, A.; Luna, M.C.; Gil, M.I. Preharvest and postharvest factors related to off-odours of fresh-cut iceberg lettuce. Postharvest Biol. Technol. 2013, 86, 463–471. [Google Scholar] [CrossRef]
- Turner, E.R.; Luo, Y.; Buchanan, R.L. Microgreen nutrition, food safety, and shelf life: A review. J. Food Sci. 2020, 85, 870–882. [Google Scholar] [CrossRef] [PubMed]
- Choe, U.; Yu, L.L.; Wang, T.T. The science behind microgreens as an exciting new food for the 21st century. J. Agric. Food Chem. 2018, 66, 11519–11530. [Google Scholar] [CrossRef]
- Timmermann, B.N.; Steelink, C.; Loewus, F.A. Phytochemical Adaptations to Stress; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 18. [Google Scholar]
- Oh, M.-M.; Carey, E.E.; Rajashekar, C. Environmental stresses induce health-promoting phytochemicals in lettuce. Plant Physiol. Biochem. 2009, 47, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.A.; De Pascale, S.; Colla, G. Improving vegetable quality in controlled environments. Sci. Hortic. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C. Enhancing quality of fresh vegetables through salinity eustress and biofortification applications facilitated by soilless cultivation. Front. Plant Sci. 2018, 9, 1254. [Google Scholar] [CrossRef]
- Wang, Y.; Frei, M. Stressed food–the impact of abiotic environmental stresses on crop quality. Agric. Ecosyst. Environ. 2011, 141, 271–286. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant growth-promoting bacteria: Biological tools for the mitigation of salinity stress in plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef] [PubMed]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.; Franken, P.; Krumbein, A.; Kläring, H.-P.; Bar-Yosef, B. Nutrient Management in Soilless Culture in the Conflict of Plant, Microorganism, Consumer and Environmental Demands. In Proceedings of the International Symposium on Soilless Culture and Hydroponics 843, Lima, Peru, 25–28 August 2008; pp. 27–34. [Google Scholar]
- Tomasi, N.; Pinton, R.; Dalla Costa, L.; Cortella, G.; Terzano, R.; Mimmo, T.; Scampicchio, M.; Cesco, S. New ‘solutions’ for floating cultivation system of ready-to-eat salad: A review. Trends Food Sci. Technol. 2015, 46, 267–276. [Google Scholar] [CrossRef]
- Rouphael, Y.; Petropoulos, S.A.; Cardarelli, M.; Colla, G. Salinity as eustressor for enhancing quality of vegetables. Sci. Hortic. 2018, 234, 361–369. [Google Scholar] [CrossRef]
- Ghoname, A.; Abou-Hussein, S.; El-Tohamy, W. Eustress (positive stress) salinity as an enhancement tool for bioactive ingredients and quality characteristics of vegetables. A review. Sciences 2019, 9, 456–463. [Google Scholar]
- Zushi, K.; Matsuzoe, N. Metabolic profile of organoleptic and health-promoting qualities in two tomato cultivars subjected to salt stress and their interactions using correlation network analysis. Sci. Hortic. 2015, 184, 8–17. [Google Scholar] [CrossRef]
- Moya, C.; Oyanedel, E.; Verdugo, G.; Flores, M.F.; Urrestarazu, M.; Álvaro, J.E. Increased electrical conductivity in nutrient solution management enhances dietary and organoleptic qualities in soilless culture tomato. HortScience 2017, 52, 868–872. [Google Scholar] [CrossRef]
- Marin, A.; Rubio, J.S.; Martinez, V.; Gil, M.I. Antioxidant compounds in green and red peppers as affected by irrigation frequency, salinity and nutrient solution composition. J. Sci. Food Agric. 2009, 89, 1352–1359. [Google Scholar] [CrossRef]
- Giuffrida, F.; Cassaniti, C.; Malvuccio, A.; Leonardi, C. Effects of salt stress imposed during two growth phases on cauliflower production and quality. J. Sci. Food Agric. 2017, 97, 1552–1560. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Massa, D.; Salerno, A.; Rea, E. Yield, fruit quality and mineral composition of grafted melon plants grown under saline conditions. J. Hortic. Sci. Biotechnol. 2006, 81, 146–152. [Google Scholar] [CrossRef]
- Hegazi, A.M.; El-Shraiy, A.M.; Ghoname, A. Alleviation of salt stress adverse effect and enhancing phenolic anti-oxidant content of eggplant by seaweed extract. Gesunde Pflanz. 2015, 67, 21–31. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Levizou, E.; Ntatsi, G.; Fernandes, A.; Petrotos, K.; Akoumianakis, K.; Barros, L.; Ferreira, I.C. Salinity effect on nutritional value, chemical composition and bioactive compounds content of Cichorium spinosum L. Food Chem. 2017, 214, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Fonseca, J.M.; Choi, J.-H.; Kubota, C.; Kwon, D.Y. Salt in irrigation water affects the nutritional and visual properties of romaine lettuce (Lactuca sativa L.). J. Agric. Food Chem. 2008, 56, 3772–3776. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Berenguer, C.; Martínez-Ballesta, M.A.D.C.; Moreno, D.A.; Carvajal, M.; Garcia-Viguera, C. Growing hardier crops for better health: Salinity tolerance and the nutritional value of broccoli. J. Agric. Food Chem. 2009, 57, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Kogi, M.; Yanagisawa, T. Effects of salinity and nutrients in seawater on hydroponic culture of red leaf lettuce. Environ. Control Biol. 2014, 52, 189–195. [Google Scholar] [CrossRef][Green Version]
- Borghesi, E.; González-Miret, M.L.; Escudero-Gilete, M.L.; Malorgio, F.; Heredia, F.J.; Meléndez-Martínez, A.J. Effects of salinity stress on carotenoids, anthocyanins, and color of diverse tomato genotypes. J. Agric. Food Chem. 2011, 59, 11676–11682. [Google Scholar] [CrossRef]
- Fallovo, C.; Rouphael, Y.; Rea, E.; Battistelli, A.; Colla, G. Nutrient solution concentration and growing season affect yield and quality of Lactuca sativa L. var. Acephala in floating raft culture. J. Sci. Food Agric. 2009, 89, 1682–1689. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Svecova, E.; Rea, E.; Lucini, L. Effects of saline stress on mineral composition, phenolic acids and flavonoids in leaves of artichoke and cardoon genotypes grown in floating system. J. Sci. Food Agric. 2013, 93, 1119–1127. [Google Scholar] [CrossRef]
- Dominguez-Perles, R.; Martinez-Ballesta, M.C.; Riquelme, F.; Carvajal, M.; Garcia-Viguera, C.; Moreno, D.A. Novel varieties of broccoli for optimal bioactive components under saline stress. J. Sci. Food Agric. 2011, 91, 1638–1647. [Google Scholar] [CrossRef]
- Waśkiewicz, A.; Muzolf-Panek, M.; Goliński, P. Phenolic content changes in plants under salt stress. In Ecophysiology and Responses of Plants under Salt Stress; Springer: New York, NY, USA, 2013; pp. 283–314. [Google Scholar]
- Carillo, P.; Giordano, M.; Raimondi, G.; Napolitano, F.; Di Stasio, E.; Kyriacou, M.C.; Sifola, M.I.; Rouphael, Y. Physiological and nutraceutical quality of green and red pigmented lettuce in response to NaCl concentration in two successive harvests. Agronomy 2020, 10, 1358. [Google Scholar] [CrossRef]
- Carillo, P.; Soteriou, G.A.; Kyriacou, M.C.; Giordano, M.; Raimondi, G.; Napolitano, F.; Di Stasio, E.; Mola, I.D.; Mori, M.; Rouphael, Y. Regulated salinity eustress in a floating hydroponic module of sequentially harvested lettuce modulates phytochemical constitution, plant resilience, and post-harvest nutraceutical quality. Agronomy 2021, 11, 1040. [Google Scholar] [CrossRef]
- Neocleous, D.; Koukounaras, A.; Siomos, A.; Vasilakakis, M. Assessing the salinity effects on mineral composition and nutritional quality of green and red “baby” lettuce. J. Food Qual. 2014, 37, 1–8. [Google Scholar] [CrossRef]
- Gary, C.; Jones, J.; Longuenesse, J. Modelling Daily Changes in Specific Leaf Area of Tomato: The Contribution of the Leaf Assimilate Pool. In Proceedings of the International Workshop on Greenhouse Crop Models 328, Saumane, France, 25 August 1991; pp. 205–210. [Google Scholar]
- Barrs, H.; Weatherley, P. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Llorach, R.; Martínez-Sánchez, A.; Tomás-Barberán, F.A.; Gil, M.I.; Ferreres, F. Characterisation of polyphenols and antioxidant properties of five lettuce varieties and escarole. Food Chem. 2008, 108, 1028–1038. [Google Scholar] [CrossRef]
- Corrado, G.; De Micco, V.; Lucini, L.; Miras-Moreno, B.; Senizza, B.; Zengin, G.; El-Nakhel, C.; De Pascale, S.; Rouphael, Y. Isosmotic macrocation variation modulates mineral efficiency, morpho-physiological traits, and functional properties in hydroponically grown lettuce varieties (Lactuca sativa L.). Front. Plant Sci. 2021, 12, 1010. [Google Scholar] [CrossRef] [PubMed]
- Matesanz, S.; Milla, R. Differential plasticity to water and nutrients between crops and their wild progenitors. Environ. Exp. Bot. 2018, 145, 54–63. [Google Scholar] [CrossRef]
- Läuchli, A.; Grattan, S. Plant growth and development under salinity stress. In Advances in Molecular Breeding toward Drought and Salt Tolerant Crops; Springer: Dordrecht, The Netherlands, 2007; pp. 1–32. [Google Scholar]
- Scagel, C.F.; Bryla, D.R.; Lee, J. Salt exclusion and mycorrhizal symbiosis increase tolerance to NaCl and CaCl2 salinity in ‘Siam Queen’ basil. HortScience 2017, 52, 278–287. [Google Scholar] [CrossRef]
- Hu, Y.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Franco-Navarro, J.D.; Rosales, M.A.; Cubero-Font, P.; Calvo, P.; Álvarez, R.; Diaz-Espejo, A.; Colmenero-Flores, J.M. Chloride as a macronutrient increases water-use efficiency by anatomically driven reduced stomatal conductance and increased mesophyll diffusion to CO2. Plant J. 2019, 99, 815–831. [Google Scholar]
- Trajkova, F.; Papadantonakis, N.; Savvas, D. Comparative effects of NaCl and CaCl2 salinity on cucumber grown in a closed hydroponic system. HortScience 2006, 41, 437–441. [Google Scholar] [CrossRef]
- Hichem, H.; Mounir, D. Differential responses of two maize (Zea mays L.) varieties to salt stress: Changes on polyphenols composition of foliage and oxidative damages. Ind. Crop. Prod. 2009, 30, 144–151. [Google Scholar] [CrossRef]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A.; Ghazanfar, A. Possible involvement of some secondary metabolites in salt tolerance of sugarcane. J. Plant Physiol. 2006, 163, 723–730. [Google Scholar] [CrossRef]
- Chisari, M.; Todaro, A.; Barbagallo, R.N.; Spagna, G. Salinity effects on enzymatic browning and antioxidant capacity of fresh-cut baby romaine lettuce (Lactuca sativa L. Cv. Duende). Food Chem. 2010, 119, 1502–1506. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Huang, J.; Gruber, M.Y.; Kaddour, R.; Lachaal, M.; Ouerghi, Z.; Hannoufa, A. The impact of genotype and salinity on physiological function, secondary metabolite accumulation, and antioxidative responses in lettuce. J. Agric. Food Chem. 2010, 58, 5122–5130. [Google Scholar] [CrossRef]
- Zhang, L.; Su, W.; Tao, R.; Zhang, W.; Chen, J.; Wu, P.; Yan, C.; Jia, Y.; Larkin, R.M.; Lavelle, D. RNA sequencing provides insights into the evolution of lettuce and the regulation of flavonoid biosynthesis. Nat. Commun. 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
| Source of Variance | Height (cm) | Leaves (No.) | LFW (g fw/Plant) | SLA (cm2/g fw) | LSU (g/cm2) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cultivar (C) | ||||||||||
| GSB | 14.83 ± 0.36 | a | 6.12 ± 0.08 | a | 3.73 ± 0.12 | a | 536.32 ± 15.56 | b | 35.62 ± 0.69 | a |
| RSB | 14.18 ± 0.39 | b | 5.30 ± 0.08 | b | 3.18 ± 0.14 | b | 608.66 ± 18.02 | a | 33.28 ± 0.45 | b |
| Salt (S) | ||||||||||
| Control | 16.49 ± 0.61 | a | 5.77 ± 0.12 | 3.90 ± 0.22 | a | 544.52 ± 22.58 | 34.70 ± 0.45 | a | ||
| CaCl2 | 13.90 ± 0.32 | b | 5.63 ± 0.18 | 3.54 ± 0.20 | ab | 563.04 ± 27.82 | 36.30 ± 1.25 | ab | ||
| KCl | 13.77 ± 0.39 | b | 5.81 ± 0.16 | 3.18 ± 0.17 | b | 592.68 ± 28.28 | 33.69 ± 0.89 | ab | ||
| NaCl | 13.86 ± 0.32 | b | 5.63 ± 0.19 | 3.20 ± 0.13 | b | 589.72 ± 24.89 | 33.11 ± 0.50 | b | ||
| Harvest (H) | ||||||||||
| 1 | 14.49 ± 0.22 | 5.58 ± 0.11 | b | 3.29 ± 0.15 | b | 612.58 ± 14.16 | a | 35.09 ± 0.81 | ||
| 2 | 14.51 ± 0.49 | 5.85 ± 0.12 | a | 3.62 ± 0.13 | a | 532.40 ± 18.45 | b | 33.81 ± 0.33 | ||
| C × S | ||||||||||
| Control, GSB | 16.57 ± 0.63 | 5.97 ± 0.17 | 4.09 ± 0.30 | 502.71 ± 19.01 | 37.78 ± 2.35 | |||||
| CaCl2, GSB | 14.36 ± 0.56 | 6.17 ± 0.12 | 3.94 ± 0.18 | 505.22 ± 31.55 | 35.68 ± 0.51 | |||||
| KCl, GSB | 14.06 ± 0.76 | 6.20 ± 0.15 | 3.49 ± 0.18 | 563.52 ± 32.90 | 34.95 ± 0.86 | |||||
| NaCl, GSB | 14.33 ± 0.51 | 6.13 ± 0.21 | 3.41 ± 0.17 | 573.82 ± 34.14 | 34.06 ± 0.85 | |||||
| Control, RSB | 16.41 ± 1.12 | 5.57 ± 0.15 | 3.71 ± 0.32 | 586.32 ± 34.39 | 34.82 ± 0.68 | |||||
| CaCl2, RSB | 13.45 ± 0.22 | 5.10 ± 0.10 | 3.13 ± 0.28 | 620.86 ± 32.74 | 33.73 ± 0.51 | |||||
| KCl, RSB | 13.47 ± 0.21 | 5.42 ± 0.17 | 2.87 ± 0.25 | 621.84 ± 45.78 | 32.42 ± 1.44 | |||||
| NaCl, RSB | 13.39 ± 0.30 | 5.13 ± 0.12 | 3.00 ± 0.19 | 605.62 ± 38.21 | 32.15 ± 0.11 | |||||
| S × H | ||||||||||
| Control, 1 | 14.85 ± 0.28 | b | 5.72 ± 0.12 | 3.68 ± 0.34 | 548.04 ± 21.76 | 38.44 ± 2.15 | ||||
| CaCl2, 2 | 13.56 ± 0.29 | bc | 5.73 ± 0.24 | 3.79 ± 0.15 | 526.88 ± 50.71 | 34.53 ± 0.51 | ||||
| KCl, 2 | 13.05 ± 0.43 | c | 6.03 ± 0.22 | 3.35 ± 0.22 | 535.43 ± 33.53 | 33.93 ± 0.53 | ||||
| NaCl, 1 | 14.39 ± 0.41 | bc | 5.47 ± 0.26 | 3.18 ± 0.18 | 653.14 ± 18.64 | 33.58 ± 0.64 | ||||
| Control, 2 | 18.12 ± 0.71 | a | 5.82 ± 0.23 | 4.12 ± 0.27 | 540.99 ± 42.01 | 34.16 ± 0.65 | ||||
| CaCl2, 1 | 14.25 ± 0.56 | bc | 5.53 ± 0.28 | 3.28 ± 0.36 | 599.19 ± 17.62 | 34.88 ± 0.79 | ||||
| KCl, 1 | 14.48 ± 0.52 | bc | 5.58 ± 0.21 | 3.01 ± 0.27 | 649.94 ± 32.91 | 33.44 ± 1.77 | ||||
| NaCl, 2 | 13.33 ± 0.40 | bc | 5.80 ± 0.28 | 3.23 ± 0.21 | 526.30 ± 27.75 | 32.63 ± 0.78 | ||||
| C × H | ||||||||||
| GSB, 1 | 15.44 ± 0.10 | a | 6.00 ± 0.08 | 3.80 ± 0.17 | a | 575.66 ± 17.65 | 37.14 ± 1.16 | a | ||
| GSB, 2 | 14.22 ± 0.68 | bc | 6.23 ± 0.13 | 3.66 ± 0.16 | a | 496.98 ± 20.48 | 34.10 ± 0.47 | b | ||
| RSB, 1 | 13.55 ± 0.15 | c | 5.15 ± 0.09 | 2.77 ± 0.12 | b | 649.50 ± 16.72 | 33.04 ± 0.79 | b | ||
| RSB, 2 | 14.81 ± 0.73 | ab | 5.46 ± 0.11 | 3.59 ± 0.20 | a | 567.82 ± 27.85 | 33.52 ± 0.46 | b | ||
| Cultivar (C) | * | *** | ** | *** | ** | |||||
| Salt (S) | *** | n.s. | ** | n.s. | * | |||||
| Harvest (H) | n.s. | * | * | *** | n.s. | |||||
| C × S | n.s. | n.s. | n.s. | n.s. | n.s. | |||||
| S × H | *** | n.s. | n.s. | n.s. | n.s. | |||||
| C × H | *** | n.s. | ** | n.s. | * | |||||
| C × S × H | n.s. | n.s. | n.s. | n.s. | n.s. | |||||
| Source of Variance | CTA (mg/100 g dw) | 5-CQA (mg/100 g dw) | DCTA (mg/100 g dw) | m-DCTA (mg/100 g dw) | 3,5-CQA (mg/100 g dw) | Sum Phenolic Acids (mg/100 g dw) | CMG (mg/100 g dw) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cultivar (C) | ||||||||||||||
| GSB | 50.18 ± 3.73 | b | 32.98 ± 3.69 | b | 452.77 ± 52.01 | b | 14.57 ± 1.28 | b | 20.65 ± 1.87 | b | 571.15 ± 59.81 | b | n.a. | |
| RSB | 70.04 ± 8.97 | a | 200.22 ± 28.16 | a | 1049.32 ± 161.60 | a | 50.51 ± 8.56 | a | 90.3 ± 8.77 | a | 1460.39 ± 213.64 | a | 8.42 ± 1.21 | |
| Salt (S) | ||||||||||||||
| Control | 57.16 ± 7.09 | b | 134.39 ± 38.18 | ab | 776.68 ± 153.31 | ab | 30.91 ± 7.38 | ab | 62.16 ± 14.14 | 1061.30 ± 213.45 | ab | 8.51 ± 2.26 | ab | |
| CaCl2 | 81.45 ± 15.72 | a | 149.17 ± 53.43 | a | 1066.36 ± 288.34 | a | 48.67 ± 16.57 | a | 62.45 ± 17.29 | 1408.09 ± 387.60 | a | 12.81 ± 3.34 | a | |
| KCl | 46.67 ± 4.38 | b | 74.27 ± 16.50 | b | 478.03 ± 55.09 | b | 19.81 ± 2.67 | b | 43.16 ± 7.30 | 661.93 ± 79.93 | b | 4.24 ± 0.36 | b | |
| NaCl | 55.16 ± 7.30 | b | 108.57 ± 32.22 | ab | 683.10 ± 163.99 | b | 30.78 ± 7.47 | ab | 54.14 ± 14.22 | 931.76 ± 222.07 | b | 8.12 ± 1.82 | ab | |
| Harvest (H) | ||||||||||||||
| 1 | 39.79 ± 2.00 | b | 75.26 ± 11.49 | b | 423.10 ± 40.64 | b | 21.1 ± 2.32 | b | 46.61 ± 5.34 | b | 605.85 ± 60.12 | b | 4.89 ± 0.34 | b |
| 2 | 80.43 ± 7.94 | a | 157.95 ± 33.68 | a | 1078.98 ± 159.85 | a | 43.98 ± 9.29 | a | 64.35 ± 12.27 | a | 1425.69 ± 219.50 | a | 11.95 ± 1.94 | a |
| C × S | ||||||||||||||
| Control, GSB | 57.94 ± 8.45 | b | 50.98 ± 10.27 | 595.71 ± 140.96 | bc | 17.77 ± 3.42 | bc | 27.95 ± 3.19 | 750.34 ± 161.82 | bcd | n.a. | |||
| CaCl2, GSB | 57.18 ± 7.94 | b | 31.92 ± 4.36 | 550.13 ± 106.44 | bc | 16.04 ± 2.38 | c | 19.05 ± 4.13 | 674.33 ± 116.71 | cd | n.a. | |||
| KCl, GSB | 43.86 ± 7.10 | b | 24.62 ± 3.49 | 351.93 ± 68.19 | c | 12.48 ± 1.10 | c | 20.93 ± 2.82 | 453.82 ± 77.64 | d | n.a. | |||
| NaCl, GSB | 41.73 ± 5.16 | b | 24.40 ± 4.49 | 313.29 ± 44.87 | c | 12.00 ± 2.66 | c | 14.67 ± 3.30 | 406.10 ± 56.81 | d | n.a. | |||
| Control, RSB | 56.39 ± 12.23 | b | 217.79 ± 59.37 | 957.65 ± 265.42 | b | 44.04 ± 12.61 | bc | 96.37 ± 20.03 | 1372.25 ± 368.25 | bc | n.a. | |||
| CaCl2, RSB | 105.71 ± 28.09 | a | 266.42 ± 83.91 | 1582.59 ± 497.89 | a | 81.29 ± 27.86 | a | 105.84 ± 23.36 | 2141.86 ± 657.31 | a | n.a. | |||
| KCl, RSB | 49.47 ± 5.54 | b | 123.92 ± 14.13 | 604.12 ± 48.42 | bc | 27.14 ± 2.93 | bc | 65.38 ± 5.40 | 870.04 ± 68.98 | bcd | n.a. | |||
| NaCl, RSB | 68.58 ± 11.65 | b | 192.75 ± 41.38 | 1052.92 ± 248.23 | b | 49.56 ± 9.87 | ab | 93.61 ± 16.00 | 1457.42 ± 321.28 | ab | n.a. | |||
| S × H | ||||||||||||||
| Control, 1 | 38.96 ± 4.89 | d | 69.11 ± 22.89 | b | 391.68 ± 91.61 | d | 17.47 ± 4.87 | b | 46.95 ± 8.41 | ab | 564.17 ± 127.58 | c | 4.10 ± 0.14 | c |
| CaCl2, 1 | 42.49 ± 3.37 | cd | 69.43 ± 18.81 | b | 428.43 ± 68.24 | cd | 20.65 ± 4.26 | b | 41.36 ± 9.83 | b | 602.37 ± 101.88 | c | 6.12 ± 0.76 | bc |
| KCl, 1 | 37.29 ± 3.87 | d | 81.61 ± 29.43 | b | 419.23 ± 99.29 | cd | 21.36 ± 4.68 | b | 49.98 ± 12.00 | ab | 609.47 ± 147.96 | c | 4.18 ± 0.26 | bc |
| NaCl, 1 | 40.42 ± 4.51 | d | 80.87 ± 25.60 | b | 453.05 ± 84.20 | cd | 24.91 ± 5.42 | b | 48.13 ± 14.29 | ab | 647.39 ± 131.37 | c | 5.17 ± 0.73 | bc |
| Control, 2 | 75.37 ± 8.04 | b | 199.66 ± 64.69 | a | 1161.67 ± 189.04 | b | 44.34 ± 11.99 | ab | 77.37 ± 26.76 | ab | 1558.42 ± 292.13 | ab | 12.93 ± 2.47 | ab |
| CaCl2, 2 | 120.40 ± 21.67 | a | 228.91 ± 98.30 | a | 1704.29 ± 445.40 | a | 76.69 ± 29.59 | a | 83.53 ± 32.27 | a | 2213.81 ± 625.29 | a | 19.50 ± 3.23 | a |
| KCl, 2 | 56.04 ± 5.84 | bcd | 66.93 ± 17.62 | b | 536.82 ± 45.96 | cd | 18.25 ± 2.90 | b | 36.33 ± 8.49 | b | 714.39 ± 71.53 | c | 4.29 ± 0.76 | bc |
| NaCl, 2 | 69.89 ± 11.28 | bc | 136.28 ± 60.04 | ab | 913.15 ± 300.13 | bc | 36.65 ± 14.23 | b | 60.16 ± 25.91 | ab | 1216.13 ± 409.11 | bc | 11.07 ± 2.72 | abc |
| C × H | ||||||||||||||
| GSB, 1 | 36.39 ± 2.70 | c | 27.63 ± 4.57 | c | 289.00 ± 39.17 | c | 13.18 ± 1.69 | b | 24.30 ± 2.86 | c | 390.50 ± 49.77 | c | n.a. | |
| GSB, 2 | 63.96 ± 4.03 | b | 38.33 ± 5.56 | c | 616.54 ± 70.00 | b | 15.96 ± 1.92 | b | 17.00 ± 2.01 | c | 751.79 ± 80.92 | bc | n.a. | |
| RSB, 1 | 43.19 ± 2.70 | c | 122.89 ± 10.88 | b | 557.20 ± 45.84 | bc | 29.01 ± 2.87 | b | 68.91 ± 4.52 | b | 821.2 ± 64.85 | b | n.a. | |
| RSB, 2 | 96.89 ± 14.09 | a | 277.56 ± 45.94 | a | 1541.43 ± 251.14 | a | 72.00 ± 14.64 | a | 111.69 ± 14.76 | a | 2099.58 ± 335.21 | a | n.a. | |
| Cultivar (C) | *** | *** | *** | *** | *** | *** | n.d. | |||||||
| Salt (S) | *** | * | *** | ** | n.s. | *** | ** | |||||||
| Harvest (H) | *** | *** | *** | *** | ** | *** | *** | |||||||
| C × S | *** | n.s. | ** | ** | n.s. | ** | n.d. | |||||||
| S × H | *** | ** | *** | ** | * | *** | * | |||||||
| C × H | ** | *** | *** | *** | *** | *** | n.d. | |||||||
| C × S × H | *** | ** | ** | ** | * | ** | n.d. | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrado, G.; Vitaglione, P.; Soteriou, G.A.; Kyriacou, M.C.; Rouphael, Y. Configuration by Osmotic Eustress Agents of the Morphometric Characteristics and the Polyphenolic Content of Differently Pigmented Baby Lettuce Varieties in Two Successive Harvests. Horticulturae 2021, 7, 264. https://doi.org/10.3390/horticulturae7090264
Corrado G, Vitaglione P, Soteriou GA, Kyriacou MC, Rouphael Y. Configuration by Osmotic Eustress Agents of the Morphometric Characteristics and the Polyphenolic Content of Differently Pigmented Baby Lettuce Varieties in Two Successive Harvests. Horticulturae. 2021; 7(9):264. https://doi.org/10.3390/horticulturae7090264
Chicago/Turabian StyleCorrado, Giandomenico, Paola Vitaglione, Georgios A. Soteriou, Marios C. Kyriacou, and Youssef Rouphael. 2021. "Configuration by Osmotic Eustress Agents of the Morphometric Characteristics and the Polyphenolic Content of Differently Pigmented Baby Lettuce Varieties in Two Successive Harvests" Horticulturae 7, no. 9: 264. https://doi.org/10.3390/horticulturae7090264
APA StyleCorrado, G., Vitaglione, P., Soteriou, G. A., Kyriacou, M. C., & Rouphael, Y. (2021). Configuration by Osmotic Eustress Agents of the Morphometric Characteristics and the Polyphenolic Content of Differently Pigmented Baby Lettuce Varieties in Two Successive Harvests. Horticulturae, 7(9), 264. https://doi.org/10.3390/horticulturae7090264









